Lithium salts have been in widespread use since the 1960s for the treatment of several psychiatric disorders, including bipolar disorders and depression (Reference Muller-Oerlinghausen, Berghofer and BauerMuller-Oerlinghausen et al, 2002). Since the introduction of lithium therapy a variety of studies have investigated its side-effects, such as cutaneous reactions, thyroid disorders and urine-concentration abnormalities. Polyuria has been estimated to occur in 15-40% of all patients treated with lithium. Clinically established nephrogenic diabetes insipidus has been found in about 12% of all patients treated with lithium (Reference Boton, Gaviria and BattleBoton et al, 1987).
Early studies found that 24-h urine volumes were increased and urinary osmolality was decreased in patients using lithium concurrently with psychotropic medication (Reference Bone, Roose and DunnerBone et al, 1980; Reference Bendz, Andersch and AurellBendz et al, 1983). However, these studies were conducted before the introduction of many new psychotropic drugs, especially the second-generation antidepressants. More recently, it has been found that patients treated with both lithium and unspecified psychotropic drugs had a lower urinary concentrating capacity and glomerular filtration rate than patients taking lithium alone (Reference Bendz, Aurell and BalldinBendz et al, 1994).
The objectives of our study were to estimate the prevalence of polyuria associated with the use of lithium and to identify additional risk factors, especially concurrently used medication, that predispose patients on lithium to develop polyuria.
METHOD
Setting and study population
A prospective follow-up study was designed to assess the prevalence and determinants of polyuria among lithium users. The source population of our study was all psychiatric patients with a diagnosis of affective disorder attending the lithium clinic of a psychiatry department within the period September 2001 to January 2002. All patients using lithium for at least 1 month were asked to participate in this study during their routine visits (scheduled once every 3 months) to the out-patient lithium clinic in the psychiatry department. All data were anonymously processed and all participants gave written informed consent for inclusion in this study.
Outcome
The primary end-point of this study was the presence of polyuria, defined quantitively as a daily (24-h) urine volume greater than 3 litres (Reference Baylis, Weatherall, Ledingham and WarrelBaylis, 1996). Patients were given clear instructions (both verbal and written) regarding the collection of the urine samples and were encouraged to contact us when there were any questions. The volume of the urine was determined and creatinine concentrations and osmolality were measured by standard laboratory methods. If the urinary creatinine content was less than 6.0 mmol per day, the 24-h urine collection was considered inaccurate. Fluid intake was estimated by means of a written questionnaire, asking patients to report fluid consumption during the 24-h urine sampling period.
Determinants
Serum lithium concentrations were measured in the routine laboratory therapeutic drug monitoring programme. The cumulative amount of ingested lithium was expressed as the product of time on lithium and the average daily dosage, determined from medical records. Patients were defined as current drug users if the prescription lasted until the day of the visit to the lithium clinic. A psychiatrist or nurse practitioner obtained drug prescription data during the patient's visit, and the community pharmacy was consulted. Data on comorbidity and smoking behaviour were obtained from medical records.
Antidepressant drugs were classified into two groups. The first group consisted of antidepressants predominantly acting on the serotonergic system (serotonergic antidepressants), which included clomipramine, fluoxetine, paroxetine, sertraline, trazodone and venlafaxine. The second group consisted of antidepressants that have less potency to inhibit the serotonin reuptake mechanism, including amitriptyline, imipramine, maprotiline and nortriptyline. Clomipramine, trazodone and venlafaxine were included in the first group because these compounds are known to be potent antagonists of the serotonin reuptake mechanism (Reference Tatsumi, Groshan and BlakelyTatsumi et al, 1997).
Data analysis
For all patients the prevalence of each characteristic on the inclusion date was determined. An analysis of variance (Student's t-test) was used to compare frequencies of continuous variables for participants with and without polyuria. Differences in proportions of categorical variables were assessed for significance by a chi-squared test.
To estimate a possible association between potential risk factors and polyuria, crude and adjusted odds ratios were calculated as measures of relative risk using logistic regression and were presented with a 95% confidence interval. The final logistic regression model included age and gender and all univariately associated (at P ≤ 0.1) risk factors for polyuria. In order to study and adjust for potential confounding factors, additional data on concomitant medication (antidepressants, diuretics, antihypertensives, neuroleptics) and comorbidity (diabetes mellitus, hypertension, thyroid disease) were collected. All calculations were carried out using the Statistical Package for the Social Sciences, version 10.0.
RESULTS
The final study population consisted of 75 patients. All these patients gave informed consent and collected adequate 24-h urine samples. Six patients were excluded for various reasons. Table 1 summarises the characteristics of the patients, stratified according to presence or absence of polyuria. The mean age of the patients was 52 years; the women were slightly older (54 years v. 49 years) but this was not statistically significant (P=0.069). In the group as a whole, 46 patients (61%) were female and the average lithium treatment duration was 4.7 years. For 44 patients (59%) the primary psychiatric indication for lithium was bipolar affective disorder and for 31 patients (41%) it was major depressive disorder. There was no diagnosis of schizo-affective disorder. In the group with major depressive disorder, lithium was always added to an antidepressant as an augmentation strategy. In the group of patients with bipolar (I and II) disorder an antidepressant was added when lithium alone did not prevent depression. The lithium serum concentrations were not statistically different between the two patient groups (P=0.258). Twenty-eight patients (37%) were identified as having polyuria. Outcome parameters for patients with and without polyuria are presented in Table 2. There was no difference between the two groups in 24-h creatinine excretion. In both patient groups, the intake volume equalled the 24-h urine output volume. Half of the patients with polyuria were current users of serotonergic antidepressants, whereas less than a quarter of the non-polyuria patients (23%) were current users of these drugs. Users of serotonergic antidepressants had a three times higher risk (unadjusted relative risk 3.06, 95% CI 1.04-9.00) for polyuria compared with non-recipients of these agents (Table 3). Concurrent use of benzodiazepines, hypertension, lithium dosing frequency and smoking were not significantly associated with polyuria. Thyroid disorders were inversely associated with polyuria, but this was not statistically significant.
Table 1 Baseline demographic and medical characteristics of the study participants

| All lithium users (n=75) | With polyuria (n=28) | Without polyuria (n=47) | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years): mean (s.d.) | 52 (12) | 51 (13) | 54 (9) |
| Gender: n (%) | |||
| Male | 29 (39) | 11 (39) | 18 (38) |
| Female | 46 (61) | 17 (61) | 29 (62) |
| Diagnosis: n (%) | |||
| Major depressive disorder | 31 (41) | 9 (32) | 22 (47) |
| Bipolar I | 38 (51) | 16 (57) | 22 (47) |
| Bipolar II | 6 (8) | 3 (11) | 3 (6) |
| Lithium use (years) | |||
| Mean (range) | 4.7 (0.4-30.7) | 6.0 (0.6-30.7) | 3.9 (0.4-14.6) |
| <3 years: n (%) | 35 (47) | 10 (36) | 25 (53) |
| ≥ 3 years: n (%) | 40 (53) | 18 (64) | 22 (47) |
| Dosage (mg1): mean (range) | 919 (400-1800) | 958 (400-1800) | 895 (400-1400) |
| Lithium dosing once daily: n (%) | 53 (71) | 19 (68) | 34 (72) |
| Serum lithium (mmol/l): mean (s.d.) | 0.78 (0.17) | 0.81 (0.15) | 0.76 (0.18) |
| BMI (kg/m2): mean (s.d.) | 26.1 (4.2) | 25.9 (4.3) | 26.2 (4.2) |
| Smoking | 23 (31) | 10 (36) | 13 (28) |
| Drug use and comorbidity | |||
| Antidepressants: n (%) | |||
| Serotonergic | 25 (33) | 14 (50) | 11 (23) |
| Other | 16 (21) | 4 (14) | 12 (26) |
| Antipsychotics: n (%) | 6 (8) | 0 (0) | 6 (13) |
| Benzodiazepines: n (%) | 15 (20) | 6 (21) | 9 (19) |
| Diuretics: n (%) | 0 (0) | 0 (0) | 0 (0) |
| Diabetes mellitus: n (%) | 2 (3) | 0 (0) | 2 (4) |
| Hypertension: n (%) | 9 (12) | 3 (11) | 6 (13) |
| Thyroid disorder: n (%) | 16 (21) | 3 (11) | 13 (28) |
Table 2 Outcome characteristics
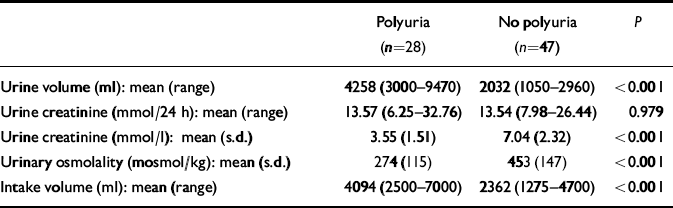
| Polyuria (n=28) | No polyuria (n=47) | P | |
|---|---|---|---|
| Urine volume (ml): mean (range) | 4258 (3000-9470) | 2032 (1050-2960) | < 0.001 |
| Urine creatinine (mmol/24 h): mean (range) | 13.57 (6.25-32.76) | 13.54 (7.98-26.44) | 0.979 |
| Urine creatinine (mmol/l): mean (s.d.) | 3.55 (1.51) | 7.04 (2.32) | < 0.001 |
| Urinary osmolality (mosmol/kg): mean (s.d.) | 274 (115) | 453 (147) | < 0.001 |
| Intake volume (ml): mean (range) | 4094 (2500-7000) | 2362 (1275-4700) | < 0.001 |
Table 3 Risk factors for polyuria among lithium users
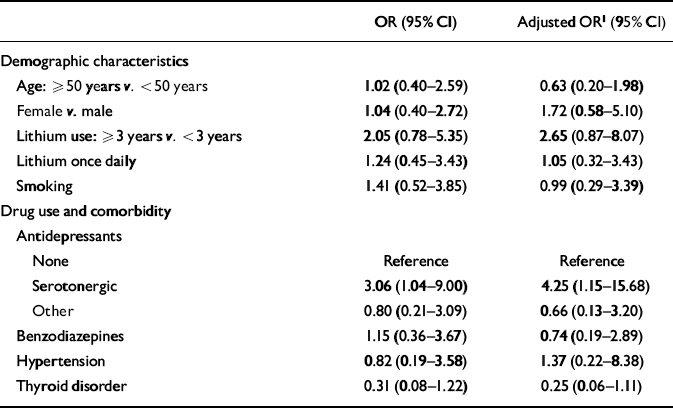
| OR (95% CI) | Adjusted OR1 (95% CI) | |
|---|---|---|
| Demographic characteristics | ||
| Age: ≥ 50 years v. < 50 years | 1.02 (0.40-2.59) | 0.63 (0.20-1.98) |
| Female v. male | 1.04 (0.40-2.72) | 1.72 (0.58-5.10) |
| Lithium use: ≥ 3 years v. < 3 years | 2.05 (0.78-5.35) | 2.65 (0.87-8.07) |
| Lithium once daily | 1.24 (0.45-3.43) | 1.05 (0.32-3.43) |
| Smoking | 1.41 (0.52-3.85) | 0.99 (0.29-3.39) |
| Drug use and comorbidity | ||
| Antidepressants | ||
| None | Reference | Reference |
| Serotonergic | 3.06 (1.04-9.00) | 4.25 (1.15-15.68) |
| Other | 0.80 (0.21-3.09) | 0.66 (0.13-3.20) |
| Benzodiazepines | 1.15 (0.36-3.67) | 0.74 (0.19-2.89) |
| Hypertension | 0.82 (0.19-3.58) | 1.37 (0.22-8.38) |
| Thyroid disorder | 0.31 (0.08-1.22) | 0.25 (0.06-1.11) |
After adjustment for potential confounding factors, the clear association between the use of serotonergic antidepressants and polyuria in this cohort of lithium users remains: adjusted relative risk 4.25 (95% CI 1.15-15.68) compared with non-recipients. The most frequently used serotonergic antidepressants were clomipramine and paroxetine. Stratified analysis showed that patients using lithium for 3 years or more were at a higher risk (OR=2.65, 95% CI 0.87-8.07) of polyuria compared with patients exposed for a shorter period, but again that was not statistically significant.
DISCUSSION
Major findings
In this prospective study we found an overall prevalence of relevant polyuria of 37% in patients treated with lithium. The most important finding was that patients on lithium concurrently using serotonergic antidepressants were four times more likely to develop polyuria than non-recipients of these drugs. Long-term lithium treatment was also associated with a higher risk of polyuria, but this was not statistically significant.
Context of the results
Polyuria is a frequent complication in patients receiving lithium. The prevalence found in this study is in line with other published values, although reported rates of lithium-associated polyuria vary widely (Reference Boton, Gaviria and BattleBoton et al, 1987). Prior evidence identifying serotonergic agents as potential risk factors for polyuria during lithium therapy is lacking. Our findings are similar to those of Bendz et al (Reference Bendz, Aurell and Balldin1994), who reported that patients treated with both lithium and psychotropic agents had a decreased urinary concentration capacity and increased 24-h urinary volume. In their study, however, the psychotropic agents were not assessed separately and no relative risk estimate was given.
High serum levels of lithium have been reportedly associated with significantly more cases of polyuria than have low serum levels (Reference Maj, Starace and NolfeMaj et al, 1986). However, our study found no statistically significant relationship between serum lithium levels and polyuria. The small number of cases might be a reason for this observation. It has been suggested that lithium administration in a single daily dose rather than multiple daily doses might accelerate the tubular regeneration process and decrease the risk of polyuria (Reference Bowen, Grof and GrofBowen et al, 1991). However, researchers are not unanimous. O'Donovan et al (Reference O'Donovan, Hawkes and Bowen1993) found that switching to a single daily dose of lithium did not reduce the 24-h urine volume. In our study urine volume was not significantly related to the dosing regimen. Both thiazides and potassium-sparing diuretics have been shown to ameliorate lithium-induced polyuria (Reference MartinMartin, 1993). Diuretics were not used in our patient population.
Smoking impairs urine excretion by increasing endogenous arginine vasopressin (AVP) secretion (Reference Allon, Allen and DeckAllon et al, 1990). However, in our study smoking was not found to be associated with a decreased urinary volume. Patients with thyroid disorder seemingly had a lower risk of polyuria. No possible pharmacological explanation was found in the research literature.
Pharmacological explanation
The mechanism of lithium-induced polyuria is biologically explicable. Under normal physiological circumstances AVP binds to vasopressin type 2 receptors located in the basolateral membrane of the renal collecting-duct cells. This binding initiates a cascade, triggering the insertion of vesicles containing the aquaporin-2 water channel into the normally watertight apical membrane, to cause increased water permeability followed by water resorption, leading to concentrated urine production (Reference Deen and KnoersDeen & Knoers, 1998). In rat models lithium appeared to cause polyuria through downregulation of aquaporin-2 expression (Reference Marples, Christensen and ChristensenMarples et al, 1995), which has been confirmed in humans in healthy individuals under lithium treatment (Reference Baumgarten, van de Pol and DeenBaumgarten et al, 2000). It has been suggested that lithium acts by inhibiting adenylate cyclase activity in the collecting-duct cells, preventing the production of cyclic adenosine monophosphate (cAMP), the second messenger for vasopressin.
A pharmacological explanation for serotonergically induced polyuria has not been explored. So far, it has been accepted that antidepressants are effective through interaction with certain key receptors in serotonergic, noradrenergic and/or dopaminergic neurotransmission systems (Reference StahlStahl, 1998). It is thought that lithium enhances antidepressant efficacy by increasing serotonergic transmission. The value of lithium augmentation has been demonstrated with a wide range of antidepressants, including serotonergic agents (Reference Zullino and BaumannZullino & Baumann, 2001). No clinically relevant pharmacokinetic interaction has been found between lithium and serotonin reuptake inhibitors (Reference MitchellMitchell, 1997). Double-blind trials have not demonstrated that adverse effects occurred frequently using the combination of lithium and serotonergic antidepressants (Reference Fava, Rosenbaum and McGrathFava et al, 1994); however, these trials were not designed to estimate the prevalence of such adverse effects, including polyuria.
Serotonergic antidepressants are associated with urinary problems in daily clinical practice. It has been recorded that serotonergic drugs can induce hyponatraemia (Reference Movig, Egberts and LenderinkMovig et al, 2002), supposedly by inappropriate secretion of antidiuretic hormone, which eventually leads to a decrease in urine production. Since we found a statistically significant additional effect of serotonergic antidepressants on lithium-induced polyuria, it is likely that the effect of serotonergic medication on lithium-induced polyuria is explained by a drug interaction at the tubular level.
Limitations of the study design
Our study has some potential limitations. Selection bias is a potential threat to study validity and occurs whenever the inclusion of patients is in some way associated with the outcome of interest. However, the psychiatrists were not informed about the primary objective of this study; they only recruited the patients and instructed them in the urine collection protocol. It is therefore unlikely that users of serotonergic agents were more frequently included than patients using other (or no) antidepressants. A second concern is the possibility of observer bias. This was minimised by medical record review after the patient had visited the lithium clinic to verify the study forms filled in by the psychiatrist.
Although the study was sufficiently powerful to detect a number of risk factors, it was somewhat limited by its small size. The wide confidence intervals around some of the odds ratios are a reflection of this limitation.
A dichotomous cut-off point of 3 litres of urine per day for polyuria has been used in the logistic regression model. Such a cut-off level is widely accepted for research purposes (Reference Baylis, Weatherall, Ledingham and WarrelBaylis, 1996), but it still might be arbitrary. Therefore, we also performed analyses using continuous measures of 24-h urine output as the dependent variable, i.e. linear regression analysis. We found that patients who had been treated with selective serotonin reuptake inhibitors (SSRIs) and lithium salts concurrently had significantly greater urinary output (mean difference 941 ml, 95% CI 280-1601; P=0.006) than patients treated with lithium only. The logistic regression model provided a comparable statistically significant P value, and in essence the same result, for the association between polyuria and treatment with both lithium and SSRIs compared with lithium use only.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Polyuria is a well-known and frequently occurring side-effect of lithium therapy.
-
▪ Physicians should be aware of the increased risk of lithium-induced polyuria in patients concomitantly using serotonergic antidepressants.
-
▪ These findings are in themselves insufficient reason to discontinue treatment.
LIMITATIONS
-
▪ The number of patients in this study was small.
-
▪ Selection bias might have occurred, albeit minimally.
-
▪ Generalisability may be limited because recruitment was restricted to a single clinic.






eLetters
No eLetters have been published for this article.