Cognition, memory, and depression symptomatology are important determinants to maintain quality of life and independence in older people. In the literature, moderate-to-high heritability has been estimated for cognitive skill, memory, and depression symptomatology by multiple twin studies in Western countries (Finkel et al., Reference Finkel, Pedersen and McGue1995; Johansson et al., Reference Johansson, Whitfield, Pedersen, Hofer, Ahern and McClearn1999; Lee et al., Reference Lee, Henry, Trollor and Sachdev2010; Reference Lee, Mosing, Henry, Trollor, Ames and Martin2012; McGue & Christensen, Reference McGue and Christensen2001; Reference McGue and Christensen2002; Sachdev et al., Reference Sachdev, Lammel, Trollor, Lee, Wright and Ames2009; Skytthe et al., Reference Skytthe, Kyvik, Bathum, Holm, Vaupel and Christensen2006; Tambs et al., Reference Tambs, Ronning, Prescott, Kendler, Reichborn-Kjennerud, Torgersen and Harris2009), confirming the genetic regulation of aging-related phenotypes. As the three phenotypes represent important domains of aging-associated impairment, it would be interesting to study the genetic mechanisms in the regulation of these phenotypes. Results from such analysis could help with our understanding of the biological mechanism of mental aging and provide useful information for the development of efficient strategies for promoting mental health and healthy aging.
In the literature of twin studies, both univariate and multivariate analyses (Rijsdijk & Sham, Reference Rijsdijk and Sham2002) have been applied to a variety of disease phenotypes or traits. Different from univariate twin models, multivariate twin models explore the common genetic background shared by multiple phenotypes to infer pleiotropic genetic effects underlying the covariance of multiple phenotypes. For example, based on different assumptions, the common factor common pathway and the common factor independent pathway models have been applied to infer if there are common or independent genetic mechanisms in the regulation of metabolic phenotypes (Pang et al., Reference Pang, Zhang, Li, Duan, Hjelmborg, Kruse and Tan2010), behavior (Dawood et al., Reference Dawood, Kirk, Bailey, Andrews and Martin2005) or psychological and psychiatric traits (Kendler et al., Reference Kendler, Aggen and Patrick2012). In aging studies, twin-based multivariate modeling has been conducted on different aging-related phenotypes using bivariate or multivariate analysis, but predominantly in Western populations (Finkel et al., Reference Finkel, Pedersen and Harris2000; Kremen et al., Reference Kremen, Jacobsen, Xian, Eisen, Eaves, Tsuang and Lyons2007; Ogata et al., Reference Ogata, Kato, Honda and Hayakawa2014; Robitaille et al., Reference Robitaille, Muniz, Piccinin, Johansson and Hofer2012; Svedberg et al., Reference Svedberg, Gatz and Pedersen2009; Tucker-Drob et al., Reference Tucker-Drob, Reynolds, Finkel and Pedersen2014); for example, the bivariate study of self-rated health and cognitive abilities (Svedberg et al., Reference Svedberg, Gatz and Pedersen2009), the multivariate genetic analysis of adult memory (Finkel et al., Reference Finkel, Pedersen and McGue1995), and the common genetic influence on hand strength, processing speed, and working memory (Ogata et al., Reference Ogata, Kato, Honda and Hayakawa2014). Interestingly, their results, based on Western populations, indicated that common genetic factors may influence memory and processing speed and that memory, processing speed and, visuospatial ability could represent different aspects of cognitive function.
The Chinese population is the world's largest and most rapidly aging population. The aging Chinese population is potentially creating a growing burden to a society under economic transition. Studying and promoting healthy aging in the Chinese population may hold the key to improving public health in China. This is especially important considering that the current findings from Western populations may not apply to the Chinese population, who live in a different social and physical environment. This article reports results from the first twin-based multivariate modeling of cognition, memory, and depression symptomatology as part of a comprehensive investigation of genetic and environmental regulation on aging-related phenotypes in middle-aged and older Chinese twins. Results from different multivariate models will be presented, with the aim of testing our hypothesis of a common genetic and environmental background for the three phenotypes. The article ends with discussion on findings from our analysis, together with their implications.
Methods
Study Participants
The study was based at the Qingdao Twin Registry, established in 1998 at the Qingdao Center for Disease Control and Prevention (Qingdao CDC; Duan et al., Reference Duan, Ning, Zhang, Wang, Zhang, Tan and Pang2013), the first population-based twin registry in China (Li et al., Reference Li, Gao, Lv, Cao, Zhan, Yang and Hu2006; Reference Li, Gao, Yu, Lv, Cao, Zhan and Hu2013). Different from Western countries with well-established registration systems, recruitment of Chinese twins is through multiple channels, especially given the lower twin birth rate (0.76%; Gan & Zheng, Reference Gan and Zheng2002). In this study, twins were recruited through the network of the Qingdao CDC in residential communities of the Qingdao municipality, using the residence registry, medical records, and media announcements. Complete pairs of middle-aged and older twins were recruited in 2012 and 2013. Twins who were unconscious, unable or unwilling to cooperate were excluded from sampling. The final sample consisted of 384 pairs, including 244 monozygotic (MZ, 116 male pairs, 128 female pairs) and 140 dizygotic (DZ, 42 male pairs, 39 female pairs, 59 opposite sex pairs) twin pairs aged from 33 to 80 years with a median age of 50. Altogether, there were 375 male twins with a mean age of 52.3 years and 393 female twins with a mean age of 50.9 years. Venous blood was drawn for zygosity determination for like-sex twin pairs using 16 multiple short tandem sequence repeat DNA markers (Becker et al., Reference Becker, Busjahn, Faulhaber, Bahring, Robertson, Schuster and Luft1997; Tomsey et al., Reference Tomsey, Kurtz, Kist, Hockensmith and Call2001) at the central laboratory of the Qingdao Blood Center, with correct zygosity assignment ascertained to be 99.9%.
The study was approved by the Regional Ethics Committee of the Qingdao CDC Institutional Review Boards and conducted according to the principles of the Helsinki Declaration. All participants signed a consent form and completed a questionnaire and health examination at the local service center of the Qingdao CDC or at community hospital/clinics.
Assessment of Cognition, Memory, and Depression Symptomatology
For each twin pair, face-to-face interviews with each twin were performed by the same well-trained and experienced interviewer. We used the MoCA (www.mocatest.org) scale to test the general cognitive performance of the participants. The MoCA is the most age-sensitive and effective test for assessing cognitive ability of healthy aging (Gluhm et al., Reference Gluhm, Goldstein, Loc, Colt, Liew and Corey-Bloom2013; Ismail et al., Reference Ismail, Rajji and Shulman2010; Reference Ismail, Mulsant, Herrmann, Rapoport, Nilsson and Shulman2013; Luis et al., Reference Luis, Keegan and Mullan2009; Nasreddine et al., Reference Nasreddine, Phillips, Bedirian, Charbonneau, Whitehead, Collin and Chertkow2005). The MoCA is a brief 0–30 point assessment of global cognition that evaluates a broad array of cognitive domains including attention, orientation, abstraction, linguistic skill, delayed recall and executive ability. A recent study reported that premorbid IQ influences MoCA in both healthy subjects and patients with cognitive impairments (Alves et al., Reference Alves, Simoes, Martins, Freitas and Santana2013). Here, we used the MoCA adjusted for education, with educational level quantified by totaling the number of years to complete the participant's highest of schooling, adding 1 score if it was less than 12 years of education. A higher cognitive score meant better cognition.
Memory was measured using the forward and backward digit span task from the Wechsler Adult Intelligence scale — Revised for China (WAIS-RC; Gong, Reference Gong1989). In the forward digit condition, the interviewer read aloud a series of numbers of increasing length, and the participant was instructed to repeat the numbers in the same order. For the backward digits, the interviewer also read aloud a series of numbers of increasing length, but the participant was instructed to repeat the numbers in the reverse order. The sequences were increased in length by 1 unit in each subsequent trial until the participant failed two trials in a row of the same sequence length. The forward digit battery consisted of sequences of 3–9 units, and the backward digits test consisted of sequences of 2–8 units. Forward and backward scores were based on the longest length of correct answers in each condition. Memory was measured as the sum of forward and backward digit span scores (range, 0–17). A higher digit span score indicated better memory performance.
Depression symptomatology was assessed by the self-reported GDS-30(Brink et al., Reference Brink, Yesavage, Lum, Heersema, Adey and Rose1982). All the items were transformed so that the higher the total score, the more severe the participant's mental condition.
Statistical Analysis
Data were entered and corrected by Epidata3.1 (www.epidata.dk). Considering the skewed distributions of cognition, memory, and depression symptomatology, all three measurements were transformed by Box–Cox power transformation (Box & Cox, Reference Box and Cox1964) to ensure approximately normal distribution using the free R package car (http://cran.r-project.org/web/packages/car/index.html). Data were analyzed to estimate the intra-pair correlation coefficients (ICCs) and test statistical significance for the effects of age and sex using twinlm function in the free R package mets (http://cran.r-project.org/web/packages/mets/index.html).
The classical twin models were applied to each of the three phenotypes to decompose the observed variance into the additive genetic (A), shared environmental (C), and unique environmental (E) components by fitting models encompassing the A, C, and E components (the full ACE model) or the A, D, and E components (the full ADE model) with the ADE model only considered when phenotype correlation in MZ twins was over two times that in the DZ twins. By dropping each variance component from the full model in turn, the nested models (AE, CE, DE, E) were fitted and statistical significance of the corresponding removed variance component was tested using the likelihood ratio test. This was followed by the multivariate analysis (Rijsdijk & Sham, Reference Rijsdijk and Sham2002) that fitted a full multivariate Cholesky model to all phenotypes and estimated genetic correlation (rG), common environmental correlation (rC) and unique environmental correlation (rE) among the three phenotypes. As a balance between model fit and parsimony in the number of variables, Akaike's Information Criterion (AIC; Akaike, Reference Akaike1987) was used to determine the suitability of models, with the lowest value indicating the best model.
In all model fitting, age and sex (1 for males and 2 for females) were included as the covariates to adjust for their effects on the three phenotypes. Robustness of analysis was assessed using bootstrap resampling to calculate empirical 95% CIs for estimated variables. All the multivariate twin modeling was performed by the free software Mx package (http://www.vcu.edu/mx).
Results
Descriptive Statistics
The descriptive statistics (median, percentiles) for the three phenotypes are shown in Table 1, together with results from fitting linear models to each measurement for the effects of age and sex. There was a strong pattern of decline in cognition and memory with increasing age, as indicated by the highly significant negative regression coefficients. For sex, memory was significantly lower in females than in males but there was a significant age–sex interaction in favor of females, meaning that with increasing age, females gradually outperform males in memory. Age and sex had no significant influence on depression symptomatology (p ˃ 0.05). Table 1 also displays the estimated ICCs for MZ and DZ twins separately. For cognition and memory, the ICC was significantly higher for MZ than for DZ twins, suggesting genetic influences. The ICC for depression symptomatology was also higher in MZ than in DZ twin pairs but the difference failed to reach statistical significance.
TABLE 1 Descriptive Statistics, Regression Outputs and ICCs for the Three Phenotypes

Bold type indicates statistical significance (p = 0.05).
Univariate Twin Models
In Table 1, the estimated ICCs for MZ twins were all lower than the doubled ICCs for DZ twins, thus no ADE model was considered in model fitting. Heritability first estimated by the univariate ACE models was low (0.22 for cognition, 95% CI: -0.13–0.57; 0.20 for depression symptomatology, 95% CI: -0.13–0.54) to moderate (0.48 for memory, 95% CI: 0.16–0.79; Table 2). Model comparison between the full ACE models and their nested models selected the best fitting models as the AE model for cognition and memory and the CE model for depression symptomatology. The best fitting AE models estimated moderate-to-high heritability for cognition (0.44, 95% CI: 0.34–0.53) and memory (0.56, 95% CI: 0.48–0.64) while the best-fitting CE model for depression symptomatology estimated a strong common environmental component (0.42, 95% CI: 0.33–0.50). In contrast to the genetic component, the unique environment had a relatively high contribution to the variation of the three phenotypes in all the fitted models.
TABLE 2 The Full and the Best Fitting Model for Cognition, Memory, and Depression Symptomatology Adjusted for Age and Gender

A = Additive genetic influence, C = Shared environmental influence, E = Unique/non-shared environment influence, 2LL = two times the log-likelihood; Δdf = difference in degree of freedom.
Multivariate Cholesky Models
We started multivariate analysis by fitting a full Cholesky decomposition model to the three phenotypes. In the full model, the 95% confidence intervals for the estimated common environmental contributions to total variance or covariance either overlapped with zero or were very close to zero. With this consideration, we fitted a reduced Cholesky decomposition model by fixing loadings for common environmental factors to zero. Comparison of the reduced (-2LLK = 5,811.526, df = 2,273) with the full (-2LLK = 5,818.894, df = 2,279) Cholesky model using likelihood ratio test gave a p-value of 0.29 (χ2 = 7.37, df = 6). As a result, the reduced Cholesky model was preferred with consideration of model parsimony and limited sample size. Figure 1 is the path diagram for the reduced Cholesky model, with each factor having one loading less than the previous one. Along the path, arrows are the standardized path coefficients for the additive genetic and unique environmental factors. The estimated path coefficients show strong factor loading from the genetic factor of cognition to memory. In contrast, the unique environmental factors for cognition and memory have only minor loading to depression symptomology. In Table 3, we show the estimated genetic and unique environmental correlation coefficients among the three phenotypes. As expected, the model estimated a high genetic correlation (rG = 0.69, 95% CI: 0.57–0.79) between cognition and memory. Depression had low but significant genetic correlation with cognition (rG = -0.31, 95% CI: -0.46– -0.14) and memory (rG = -0.28, 95% CI: -0.44– -0.11), suggesting their inverse genetic relationship with depression. The unique environmental correlation was only significant between cognition and memory (rE = 0.25, 95% CI: 0.14–0.36).
TABLE 3 Genetic and Environmental Correlation Coefficients for the Reduced Cholesky Decomposition Model

*p value .05.
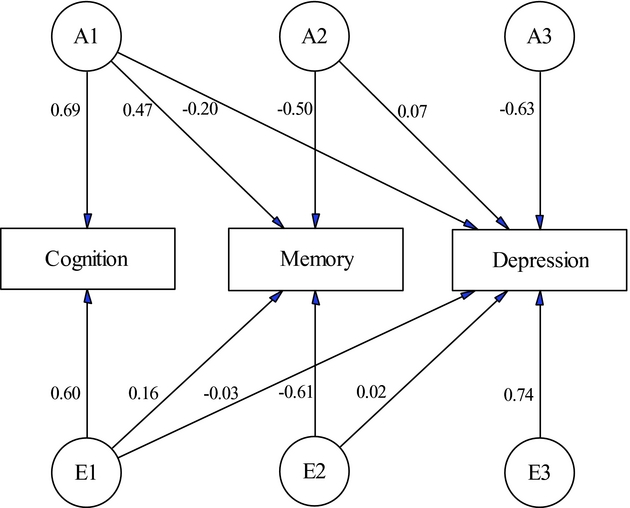
FIGURE 1. Path diagram of the reduced Cholesky decomposition model. The standardized path coefficients show strong factor loading from the genetic factor of cognition to memory. In contrast, the unique environmental factors for cognition and memory have only minor loading to depression symptomology.
Discussion
We have fitted both univariate and multivariate twin models to three important domains of aging phenotypes, that is, cognition, memory, and depression symptomatology. Our best fitting model estimated a moderate heritability for cognition (0.44; Table 2). This estimate is relative lower than some of the reported studies but is comparable with results from a Danish twin study (McGue & Christensen, Reference McGue and Christensen2001). In a recent study, Karlsgodt et al. estimated heritability for a subtest of the WAIS of 0.54 for forward digit span using family data (Karlsgodt et al., Reference Karlsgodt, Kochunov, Winkler, Laird, Almasy, Duggirala and Glahn2010). Interestingly, this is very close to our estimate of 0.56 by the best fitting model for memory. Different from cognition and memory, our best fitting model for depression estimated no genetic effect in depression symptomology. Based on a large sample of Danish twins, Johnson et al. (Reference Johnson, McGue, Gaist, Vaupel and Christensen2002) studied the additive genetic effects on depression symptoms with moderate heritability estimates (affective scale h2 = 0.27, somatic scale h2 = 0.26, total h2 = 0.29). Their estimates are, however, close to our estimate of 0.20 (95% CI: -0.13–0.54) although the latter is statistically insignificant. We think that the discrepancy in the estimated genetic effect in depression symptomology could be due to our limited power in estimating low heritability using a small twin sample, rather than population differences.
The focus of our multivariate analysis is to examine the common genetic and environmental background of the three phenotypes. Our estimates revealed a strong genetic correlation between cognition and memory, suggesting common genetic basis of the two phenotypes. In our assessment instrument, MoCA-based cognition is a composite measurement that evaluates a broad array of cognitive domains, including attention, orientation, abstraction, linguistic skill, delayed recall, and executive ability. The WAIS-RC (Finkel et al., Reference Finkel, Pedersen and McGue1995; Johansson et al., Reference Johansson, Whitfield, Pedersen, Hofer, Ahern and McClearn1999) measures memory with a backward and forward digit-span to assess an individual's intelligence in retrieving information. The WAIS backward and forward digit span test measures attention and short-term memory while the MoCA test includes items for delayed recall and attention. Thus, it is not surprising to see from our results that the two domains are highly genetically similar. Using results on twins, Finkel and McGue (Reference Finkel and McGue1993) reported that the relationship between cognition and memory was genetic in nature, a conclusion that is consistent with our finding from multivariate analysis. Overall, we conclude that memory and cognitive ability are highly genetically interrelated and if one becomes impaired, the other could also be affected.
As can be seen from Table 3, depression symptomatology has a weak but significant genetic correlation with cognition and memory. The negative and low genetic correlation coefficients imply that some genes that affect cognition and memory could also affect depression symptom but in an opposite direction. Although the GDS-30 is an assessment instrument to identify depression in the older people, different from cognition and memory, it does not seem to change by age in the age range of our samples (Table 1). Considering that the median age of our samples is 50 years, the reported high peak of depression risk in older subjects (80 years or older; Mirowsky & Ross, Reference Mirowsky and Ross1992) was actually missed. It would be interesting to study older aged Chinese twins, which could perhaps reveal a different picture of the multivariate models.
Indeed, our study was limited by its small sample size and relatively younger age. However, even with a relatively limited and middle-to-older aged samples, our study found interesting genetically correlated and uncorrelated patterns among the three important domains of aging-associated phenotypes, although with considerably large confidence intervals. The results have encouraged us to extend our study to older aged twins with the hope of capturing the dynamic genetic and environmental regulation patterns in mental aging. As shown in Table 1, both cognition and memory have a strong declining pattern with age in our middle-to-older aged twins. As a continuation of our current project, we are going to set up a longitudinal investigation to measure changes in aging phenotypes. The longitudinal study could provide important information concerning the genetic and environmental contribution to the rate of change over time, on top of the mean phenotype levels, and help to explain why and how people age differently.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 31371024). All authors contributed significantly to the study with Chunsheng Xu, Zengchang Pang, Dongfeng Zhang, Qihua Tan: experiment design, conduct of project, data analysis, drafting of manuscript; Jianping Sun, Fuling Ji, Xiaocao Tian, Haiping Duan, Yaoming Zhai, Shaojie Wang, Zhongtang Zhao: conduct of project, sample collection, data preparation; Shuxia Li, Jacob v.B. Hjelmborg, Kaare Christensen: analysis plan, interpretation, manuscript revision. We also thank the twins for their volunteer participation in this study.






