Take-Home Points
-
1. Drugs that bind D2 receptors are treatments for psychosis and mood; for many years they have been pharmacologically classified either as those that also block serotonin 5HT2A receptors or as those that do not.
-
2. A new classification of drugs for psychosis and mood that bind D2 receptors creates 3 different categories according to which dopamine receptors are bound: those that preferentially bind D2 receptors (most of these agents), those that also bind D3 receptors effectively (cariprazine>blonanserin), and those that also bind D1 receptors (asenapine, clozapine, and olanzapine>quetiapine and ziprasidone).
-
3. These 3 classes theoretically have functional differences with potential clinical implications, such as improvement in cognition, motivation, and mood from D3 antagonism/partial agonism and cognitive dysfunction from D1 antagonism.
Introduction
Classification of psychotropic drugs is an ever-evolving process, most recently culminating in neuroscience-based nomenclature (NbN), which is built upon pharmacological mechanism of action and not upon clinical indication.Reference Zohar, Stahl and Moller 1 , Reference Stahl 2 So-called “second-generation” or “atypical” antipsychotics have long been pharmacologically differentiated from “first-generation” or “conventional” antipsychotics by their potent binding to serotonin 2A receptors as well as to D2 dopamine receptors.Reference Stahl 3 Now these same agents that treat psychosis and mood can also be classified according to which dopamine receptors (D1, D2, D3) they bind (Figures 1–9 and Table 1).Reference Stahl 4 , Reference Roth 5 – Reference Tenjin, Miyamoto and Nonmiya 8 The presence or absence of actions at various dopamine receptor subtypes changes their neurobiological mechanisms and, potentially, their clinical effects.Reference Stahl 4

Figure 1 This figure shows the dopamine D1 receptor affinity relative to the dopamine D2 receptor affinity for drugs that treat psychosis and mood. No drug has a higher affinity for D1 than it does for D2, and some have very low affinities for D1 receptors relative to D2.

Figure 2 This figure shows the dopamine D3 receptor affinity relative to the dopamine D2 receptor affinity for drugs that treat psychosis and mood. Only one agent, cariprazine, has higher affinity for D3 receptors than for D2 receptors. All other agents are essentially equal or lower in affinity for D3 compared to D2.

Figure 3 This figure shows the relative affinities of drugs for psychosis and mood for D1 receptors compared to the affinity of dopamine itself for D1 receptors. All agents have equal or higher affinity for the D1 receptor than does dopamine itself.

Figure 4 This figure shows the relative affinities of drugs for psychosis and mood for D2 receptors compared to the affinity of dopamine itself for D2 receptors. All agents have equal or higher affinity for the D2 receptor than does dopamine itself. Many agents have higher or much higher affinities for D2 compared to dopamine.

Figure 5 This figure shows the relative affinities of drugs for psychosis and mood for D3 receptors compared to the affinity of dopamine itself for D3 receptors. All but 2 agents have affinities for D3 within 1 order of magnitude higher or lower than does dopamine itself. One agent has very much higher affinity for D3 than does dopamine, namely cariprazine; 1 agent has somewhat higher affinity, namely blonanserin.

Figure 6 Antagonist effects at D1 dopamine receptors are illustrated here. These include reducing dopamine neurotransmission in the prefrontal cortex, and theoretically causing cognitive dysfunction by “de-tuning” D1 receptor activity.

Figure 7 Antagonist/partial agonist effects at D2 dopamine receptors are illustrated here. These are well-known antipsychotic actions at D2 receptors in the nucleus accumbens, and also motor side effects at D2 receptors in the motor striatum.
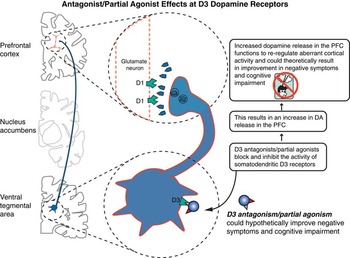
Figure 8 Antagonist/partial agonist effects at D3 receptors are illustrated here. Theoretically, D3 actions disinhibit dopamine release in the prefrontal cortex, which could improve negative symptoms, cognition, and mood.
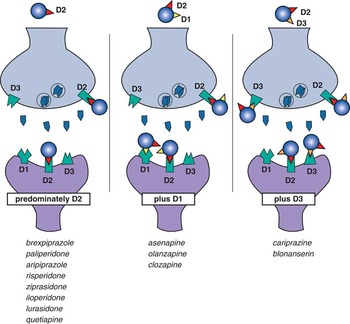
Figure 9 Three classes of drugs that bind to dopamine receptors. Eight agents bind predominantly to D2 receptors alone, 3 agents bind as well to D1 receptors, and 2 agents bind D3 receptors as well as D2 receptors.
Table 1 Three Classes of Dopamine Receptor Binding Drugs for Psychosis and Mood

All binding data from PDSP Ki database unless noted (5)
* Data from Frankel et al, 2017 (6); K. Meada (personal communication, Otsuka)
** Data from Greenberg et al, 2017 (7)
*** Data from N. Adham (personal communication, Allergan)
**** Data from Tenjin et al, 2013 (8)
A New Classification of Drugs Based on D1, D2, and D3 Binding
Here we categorize the so-called “second generation/atypical antipsychotics” in a new way: into 3 major categories based on their dopamine receptor subtype binding profiles (Table 1).Reference Stahl 4 – Reference Tenjin, Miyamoto and Nonmiya 8 By definition, all 13 of these agents bind to D2 receptors (Table 1 and Figure 4). However, some of these agents act effectively at D1 receptors as well as D2 receptors (Table 1 and Figures 1 and 3); others act effectively at D3 receptors as well as D2 receptors (Table 1 and Figures 2 and 5).Reference Roth 5 – Reference Tenjin, Miyamoto and Nonmiya 8 Clinically relevant binding at D1 and D3 receptors is based not only on how potently drugs bind to these receptors compared to how potently they bind to D2 receptors (Figures 1 and 2), but also how their binding potencies for D1 and D3 receptors compare to those of dopamine itself at these same receptors (Figures 3–5).
Drugs with Mostly D2 Antagonism/Partial Agonism
All drugs in Table 1 are more potent than dopamine for D2 receptors, and some are far more potent (Figure 4 and Table 1). This means that dopamine is less effective at competing for D2 receptors than are any of these drugs. At low levels of dopamine, D2 binding of all these drugs is therefore predominant. However, if dopamine floods the synapse during neurotransmission or when the stimulants methylphenidate or amphetamine are given, sufficiently large amounts of dopamine are released so that D2 binding by antipsychotics is reduced by mass action of dopamine predominating over its lower affinity.Reference Volkow, Wang and Fowler 9 – Reference Breier, Su and Saunders 11 Thus, the amount of D2 binding of drugs for psychosis will depend not only on binding affinity and dose of the drug, but also on the amount of dopamine present. In general, D2 antagonists/partial agonists are dosed so that the net binding to D2 receptors in the presence of whatever amount of dopamine is released in schizophrenia patients is 60% or more.Reference Stahl 3 , Reference Farde, Nordstrom, Wiesel, Pauli, Halldin and Sedvall 12
All drugs in Table 1 bind to all 3 dopamine receptors D1, D2, and D3 to some extent (Figures 1–5), but those that bind D1 and D3 receptors with much lower affinity than they bind D2 receptors will likely not have clinically relevant binding at D1 and D3 receptors. Clinically relevant binding at D1 and D3 receptors is especially unlikely if drug affinities for these receptors are also lower than dopamine’s own affinities for these receptors. That is, if dopamine’s affinity for a receptor is greater than that of the drug, dopamine would successfully compete with the drug for the receptor if dopamine and the drug are present at similar concentrations. Taking all these factors into consideration, most drugs for psychosis and mood that bind to D2 receptors are D2-preferring. This includes 8 of the 13 drugs in Table 1 (see also Figures 1–5 and 9).
The functional outcomes of antagonist/partial agonist actions at D2 dopamine receptors are the best characterized receptor actions for any drug that binds to any dopamine receptor. Namely, D2 antagonist/partial agonist actions in the nucleus accumbens are well known to reduce positive symptoms of psychosis, whereas these same D2 actions in the motor striatum can result in drug-induced parkinsonism in the short term and tardive dyskinesia in the long term (Figure 7).Reference Stahl 3
Drugs with Effective D1 Antagonism (Plus D2 Antagonism/Partial Agonism)
Some drugs in Table 1 bind to D1 receptors with affinities that suggest potentially clinically relevant occupancy at antipsychotic dose levels. That is, their D1 affinities are close to their D2 affinities (Figure 1), and greater than dopamine’s affinity for D1 receptors (which is relatively low) (Figure 3). Taken together, 3 agents—asenapine, olanzapine, and clozapine—are most likely to have clinically meaningful D1 antagonism at antipsychotic dosing levels (Table 1). Ziprasidone and quetiapine are next in order of having possible D1 binding at clinical doses because their affinities are within 1 one order of magnitude of their affinities for D2 receptors, and higher than dopamine’s affinity for D1 receptors (Figures 1 and 3). By the time a drug has binding that is 2 orders of magnitude (paliperidone, risperidone, and iloperidone) or higher affinity for D2 over D1 (Figure 1), there is diminishing probability of D1 binding.
Only relatively recently have the effects of D1 dopamine receptor blockade been characterized. In the prefrontal cortex, D1 receptors are the major postsynaptic dopamine receptor subtype, unlike the nucleus accumbens and motor striatum, which are rich in both D2 and D1 receptors.Reference Stahl 4 In animal models, including primates, D1 activity must be optimized in the prefrontal cortex for best cognitive effects (Figure 6).Reference Stahl 4 , Reference Cools and D’Esposito 13 – Reference Arnsten, Girgis, Gray and Mailman 16 Both too much as well as too little dopamine activity at cortical D1 receptors are associated with cognitive dysfunction (Figure 6).Reference Stahl 4 , Reference Cools and D’Esposito 13 – Reference Arnsten, Girgis, Gray and Mailman 16 Generally, drugs that block D1 receptors (or overstimulate them) would therefore theoretically dysregulate D1 receptors, and hypothetically this would lead to cognitive dysfunction.Reference Stahl 4 , Reference Cools and D’Esposito 13 – Reference Arnsten, Girgis, Gray and Mailman 16 Cognitive dysfunction would certainly be an undesired action of those agents that block D1 receptors, especially in patients with disorders already characterized by cognitive dysfunction, such as schizophrenia and bipolar disorder. Some of the drugs that potentially exhibit clinically relevant blockade of D1 receptors also block to varying degrees muscarinic cholinergic and histamine receptors,Reference Stahl 3 adding to their theoretical potential for cognitive dysfunction. Whether there are any potential benefits of D1 antagonism remains to be shown, although it is possible that blocking D1 receptors while blocking D2 receptors might diminish drug-induced parkinsonism.
Drugs with Effective D3 Antagonism/Partial Agonism (Plus D2 Antagonism/Partial Agonism)
Drug affinities for D3 receptors for many agents in Table 1 are about the same or higher than their affinities for D2 receptors (Figure 2), but they are mostly lower than dopamine’s affinity for the D3 receptor (Figure 5). This high affinity of endogenous dopamine for D3 receptors has been postulated to result in only minimal or no D3 receptor occupancy in dopamine-rich brain areas by any but those drugs with the most potent binding to D3, ie, those drugs with affinities that are even higher than that of dopamine itself for D3 receptors.Reference Nakajima, Gerretson and Tekeuchi 17 , Reference Gross and Drescher 18 Taken together, cariprazine and perhaps blonanserin (a drug for psychosis marketed in Japan)Reference Frankel and Schwartz 6 , Reference Tenjin, Miyamoto and Miyake 19 , Reference Hida, Mouri and Mori 20 may be the agents most likely to have clinically meaningful degrees of D3 receptor binding when dosed for psychosis. What are the implications of D3 binding?
Distribution of D3 receptors differs from that of both D1 and D2 receptors, with D3 receptors being localized predominantly in limbic areas.Reference Stahl 4 , Reference Gurevich and Joyce 21 , Reference Sokoloff and Le Foll 22 D3 receptors can be postsynaptic, presynaptic at axon terminals, or presynaptic at the somatodendritic area of certain dopamine neurons arising from the substantia nigra/ventral tegmentum. There are hardly any D3 receptors at all on dopamine terminals of the dorsal striatum (motor). Most areas of the brain that do express D3 receptors also express D2 receptors, so it can be difficult to determine which action to attribute to which receptor.
Since D3 receptors are unique among dopamine receptors in having a high affinity for dopamine, this suggests that dopamine may be occupying D3 receptors in the living brain for extended periods of time, leading to lots of activation of D3 autoreceptors, which would hold back the release of dopamine from nerve terminals.Reference Stahl 4 , Reference Nakajima, Gerretson and Tekeuchi 17 , Reference Gross and Drescher 18 Thus, due to their high affinity for dopamine, D3 receptors, unlike D1 or D2 receptors, appear to be stimulated by low levels of tonic dopamine release to attenuate the effects of dopamine fluctuation related to bursts of phasic dopamine release (see StahlReference Stahl 4 for discussion of tonic and phasic dopamine release).Reference Stahl 4 , Reference Nakajima, Gerretson and Tekeuchi 17 , Reference Gross and Drescher 18 Blocking this attenuation would disinhibit dopamine release and enhance neurotransmission. Theoretically, that would enhance dopaminergic neurotransmission, especially in brain areas such as the prefrontal cortex where dopamine release appears to be controlled by D3 receptors (Figure 8).Reference Nestler and Carlezon 23 – Reference Kiss, Horváth and Némethy 34 Also, blocking D3 receptors enhances acetylcholine release in the prefrontal cortex, which could also contribute to pro-cognitive actions.Reference Gobert, Rivet and Audinot 31 , Reference Lacroix, Ceolin and Zocchi 35
Current theories link a reduction of limbic dopamine release to anhedonia, depressive states, motivation, and pleasure.Reference Nestler and Carlezon 23 – Reference Kiss, Horváth and Némethy 34 Consistent with this notion, in animal models D3 antagonism improves cognition, mood, and motivation (Figure 8).Reference Nestler and Carlezon 23 – Reference Kiss, Horváth and Némethy 34 Early clinical results do suggest enhanced efficacy for negative symptoms of schizophrenia with the D3 preferring agent cariprazine.Reference Németh, Laszlovsky and Czobor 36 Much further research is required to follow up on these interesting therapeutic possibilities for D3 antagonist/partial agonist treatment of negative symptoms, mood, cognition, and even substance abuse.
Conclusions
Individualizing treatments for psychosis and mood involves finding the best therapeutic agent for a specific patient. Knowing the D1 and D3 dopamine receptor profiles of agents that act on D2 receptors—as well as the various additional receptors a given drug binds—can assist practitioners in matching the best therapeutic agent to the individual patient’s symptom profile.












