Introduction
Invasive pneumococcal disease (IPD), caused by infection with the bacterium Streptococcus pneumoniae, is one of the most common causes of bacteraemic pneumonia, septicaemia and meningitis worldwide, with a substantial global burden in young children, older adults and those with certain co-morbidities [Reference O'Brien1, Reference Drijkoningen and Rohde2]. The UK immunisation programme currently includes two pneumococcal vaccines [3]. The 13-valent pneumococcal conjugate vaccine (PCV13) was introduced in 2010 (2 + 1 schedule), directly replacing the seven-valent PCV introduced in 2006 for children. A single dose of 23-valent pneumococcal polysaccharide vaccine (PPV23) has been recommended for people at increased risk since 1992 and all individuals aged ⩾65 years since 2003. Immunisation coverage of PCV at 12 months has been consistently above 90% both nationally and across the North East of England (NEE) since 2009, whilst PPV coverage for persons ⩾65 years of age has consistently reached 70% (immunisation data available from www.gov.uk).
IPD incidence in NEE, and the UK as a whole, declined significantly after the introduction of PCV7 and PCV13 [Reference Houseman4, Reference Ladhani5]; reductions due to direct effects for those vaccinated and indirect (herd-level) effects for non-vaccinated age groups due to reduced carriage and transmission [Reference Flasche6, Reference van Hoek7]. However, the decline in PCV serotypes coincided with the emergence of non-PCV serotypes and the incidence of IPD increased for the first time in 2014/2015 and has done so consistently thereafter; suggesting the maximum benefit of the PCV programme may have been achieved [Reference Houseman4, Reference Ladhani5]. In addition to altered serotype dynamics, changes have been observed in the age profile of cases with increased incidence most pronounced in older age groups [Reference Houseman4, Reference Ladhani5].
Evidence suggests that case fatality rates (CFR) following IPD have declined following the introduction of PCVs [Reference Marrie8–Reference van Hoek14]. However, published studies have either included only specific age groups [Reference Oligbu9, Reference Grau12] or have not fully adjusted for individual-level risk factors and/or undertaken a full trend analysis [Reference Marrie8, Reference Becker-Dreps10, Reference Backhaus11, Reference Harboe13, Reference van Hoek14]. Using enhanced surveillance data from a region of England, we estimated the trend in 30-day all-cause CFR following IPD over a 10-year period after fully adjusting for known individual risk factors.
Methods
Study population
The NEE has a relatively stable population of about 2.6 million persons, which includes approximately 147 000 children aged <5 years and 457 000 adults aged over 64 years (population data available from: www.ons.gov.uk). The NEE covers an area of approximately 8600 km2 and comprises 12 local authorities.
Surveillance data and linkage to death data
Surveillance data for cases of IPD with a specimen date between 1 April 2006 and 31 March 2016 were obtained from the North East IPD Enhanced Surveillance System (IPDESS). This system has been in operation since April 2006; full details of the surveillance system are described elsewhere [Reference Chapman, Wilson and Gorton15]. In brief, all laboratory-confirmed cases of IPD are notified to the local health protection team and telephone interviews with laboratory and clinical staff are conducted to obtain details of risk factors and pneumococcal immunisation history. Typing is undertaken at the national reference laboratory. Cases are residents of NEE with S. pneumoniae detected from a normally-sterile site (e.g. blood, cerebrospinal fluid) and clinical diagnosis of IPD. Cases were linked to the Office for National Statistics registered deaths up to 30 December 2016 using unique National Health Service numbers.
Statistical analysis
A binary logistic regression model was used to estimate adjusted independent associations between epidemiological year of diagnosis (April–March, retained in the model throughout) and CFR (30-day all-cause) following IPD. A forward selection model-building approach was used, starting from the base model including epidemiological year. Additional variables were added into the model one at a time in order of decreasing statistical significance from single-variable analysis and the added covariate retained if the P value of the added variable and improvement in model fit using a likelihood ratio test (LRT) were both <0.05. Covariates considered were: age, sex, clinical diagnosis (bacteraemic pneumonia, septicaemia, meningitis, other), clinical risk factor (one or more of the following: chronic heart disease, chronic lung disease, chronic liver disease, chronic renal disease, diabetes, immunosuppression, asplenia, cochlear implant, cerebrospinal fluid leak), alcohol misuse, immunisation status, vaccine-type serotype subgroup (PCV7: 4, 6B, 9V, 14, 18C, 19F, 23F; PCV13-exclusive: 1, 3, 5, 6A, 7F, 19A; PPV23-exclusive: 2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, 33F; non-vaccine type, NVT: all other serotypes) and individual pneumococcal serotypes which each accounted for ⩾1% of cases (included as a single categorical variable). Where no risk factor data were available, cases were coded as having no clinical risk factors or alcohol abuse.
Goodness of fit of the final model (where all parameters had an associated P value <0.05) was assessed using the Hosmer–Lemeshow goodness of fit test (10 groups) and Receiver Operating Characteristic curve [Reference Hosmer and Lemeshow16]. A multivariable fractional polynomial model of the final logistic regression model was used to test the assumption of linearity for epidemiological year and age [Reference Sauerbrei17]. Adjusted CFR was estimated using predictions from the final model with mean values for other covariates.
Statistical software
All analyses were performed using Stata v13.1 (StataCorp., College Station, Texas, USA).
Results
Surveillance data and linkage to death data
After exclusion of four cases who were either not permanent residents of NEE (n = 2) or where data within IPDESS were inconsistent with that retrieved during linkage to death data (n = 2), a total of 2510 IPD cases were included in the analysis (Table 1).
Table 1. Characteristics of invasive pneumococcal disease cases and 30-day all-cause case fatality rate in North East England, April 2006–March 2016
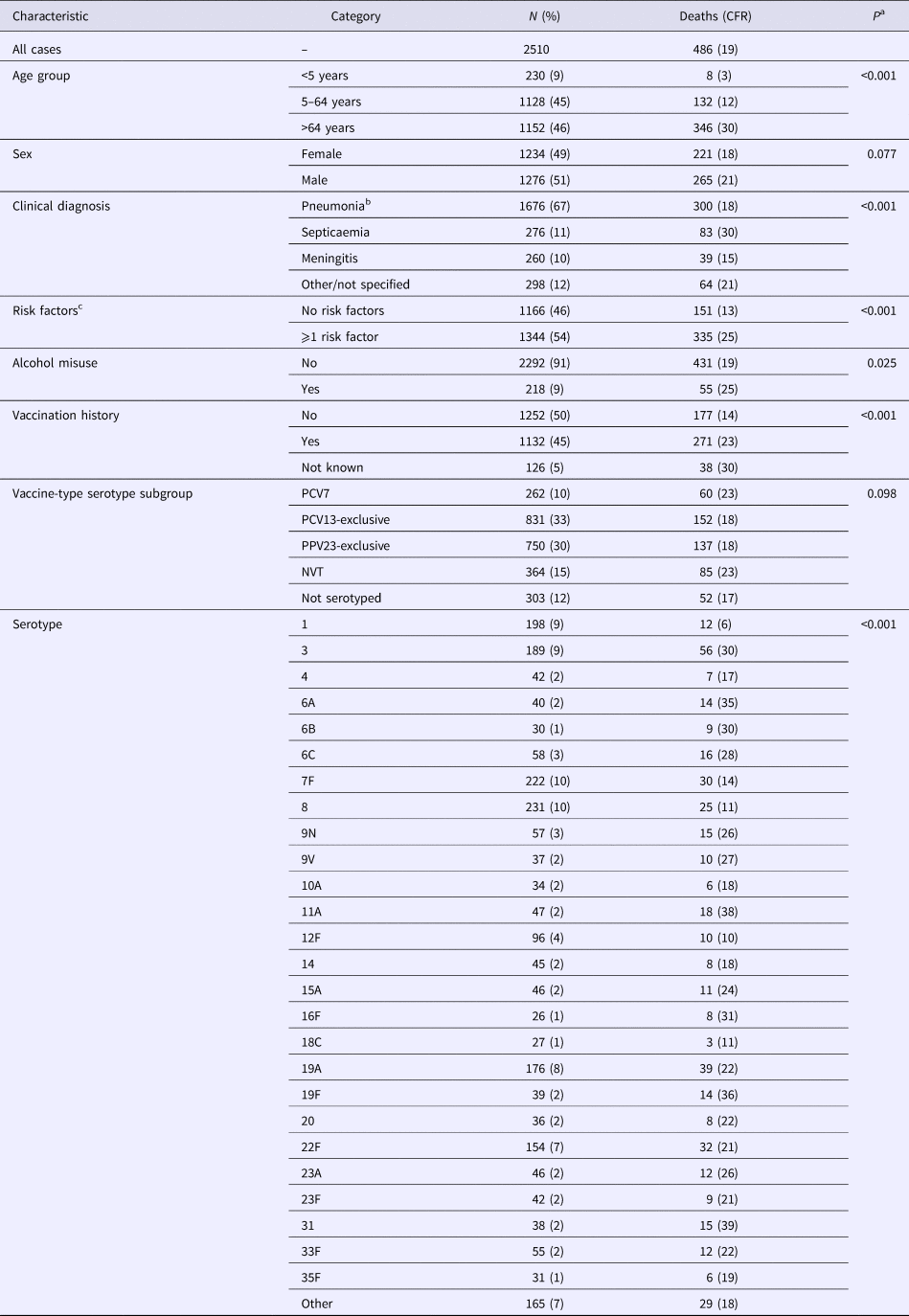
a Fisher's exact test.
b Bacteraemic pneumonia.
c Chronic heart disease, chronic lung disease, chronic liver disease, chronic renal disease, diabetes, immunosuppression, asplenia, cochlear implant, cerebrospinal fluid leak.
CFR, case fatality rate (%); PCV7, seven-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PPV23, 23-valent pneumococcal polysaccharide vaccine.
Of the 2510 cases included in the multivariable analysis, corresponding to an average annual incidence rate of 9.7 (range 6.5–12.1) per 100 000 population, 486 cases had a date of death within 30 days of their specimen date (average annual CFR 19%, range 16–24%). Serotype was available for 88% of cases; PCV13 serotypes accounting for 50%, PPV23-exclusive serotypes 34% and NVT serotypes 16%. Twenty-six serotypes each accounted for >1% of cases (⩾26 cases per serotype) (Table 1).
Multivariable analysis
The final multivariable model (2192 cases; 303 cases excluded with unknown serotype and 15 cases excluded as main diagnosis not recorded) for association with 30-day all-cause mortality contained epidemiological year, age, sex, clinical diagnosis, presence of clinical risk factors, alcohol misuse and pneumococcal serotype (Table 2). This model had an overall acceptable goodness of fit (Hosmer–Lemeshow χ 2 7.89, degrees of freedom (DF) 8, P = 0.444), improved fit compared to the starting model (LRT χ 2 = 330.52, DF 34, P < 0.001) and acceptable discrimination of cases (area under the Receiver Operating Characteristic curve 0.77).
Table 2. Associations between characteristics of invasive pneumococcal disease cases and 30-day all-cause fatality rate in North East England, April 2006–March 2016
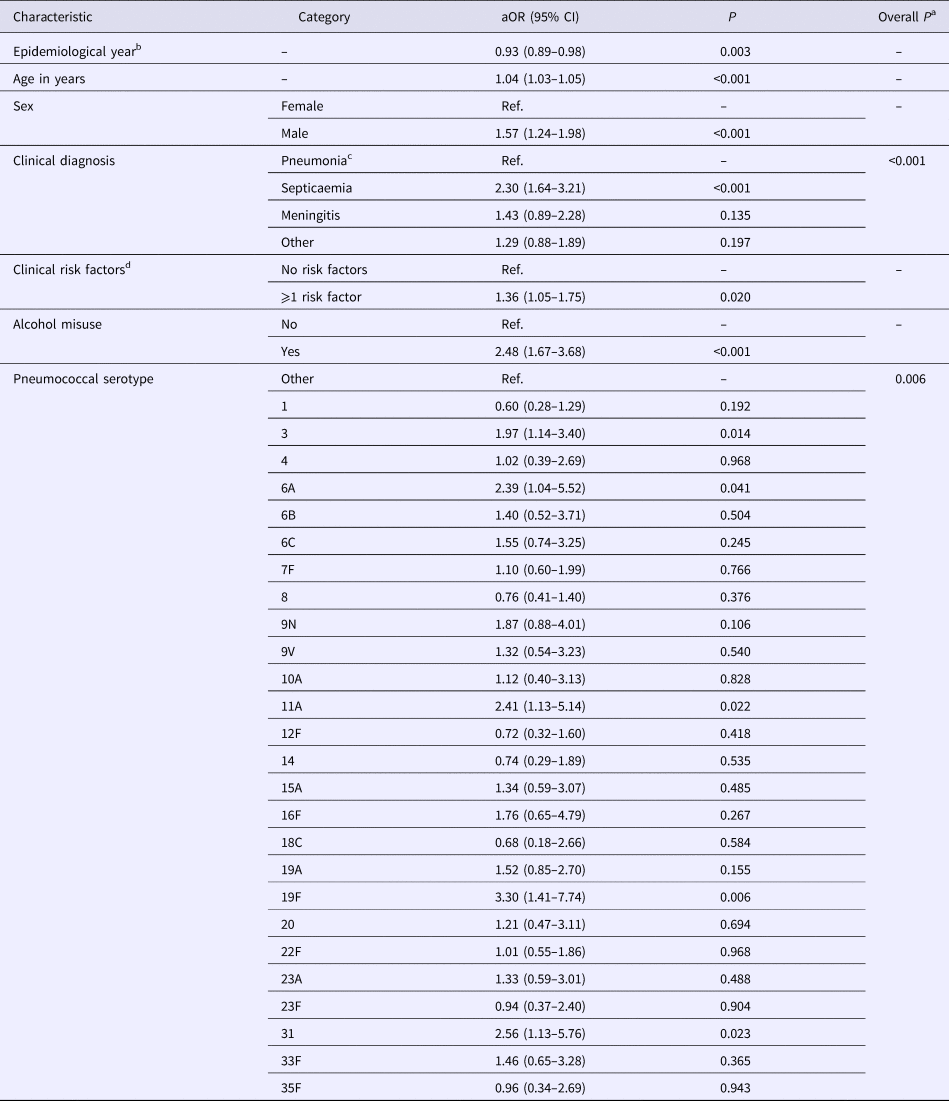
a Wald test.
b April–March.
c Bacteraemic pneumonia.
d Chronic heart disease, chronic lung disease, chronic liver disease, chronic renal disease, diabetes, immunosuppression, asplenia, cochlear implant, cerebrospinal fluid leak.
aOR, adjusted odds ratio; Ref, reference group.
After adjustment for other independent predictors, 30-day all-cause mortality demonstrated a small but significant decrease by epidemiological year (adjusted odds ratio (aOR) 0.93, 95% confidence interval (CI) 0.89–0.98, P = 0.003), corresponding to a decline in adjusted average CFR from 24% (20–27%) in the epidemiological year 2006/2007 to 16% (13–18%) in the epidemiological year 2015/2016 (Fig. 1). Although a multivariable fractional polynomial model indicated significant improvement of fit of the final model with a non-linear transformation of age (P = 0.014), this did not result in substantial changes to the aOR of covariates (median change 2.0%, range 0.27–14.39%) and all covariates retained statistical significance. Importantly, the aOR for epidemiological year differed by just 0.52% (aOR 0.93, 95% CI 0.89–0.98).
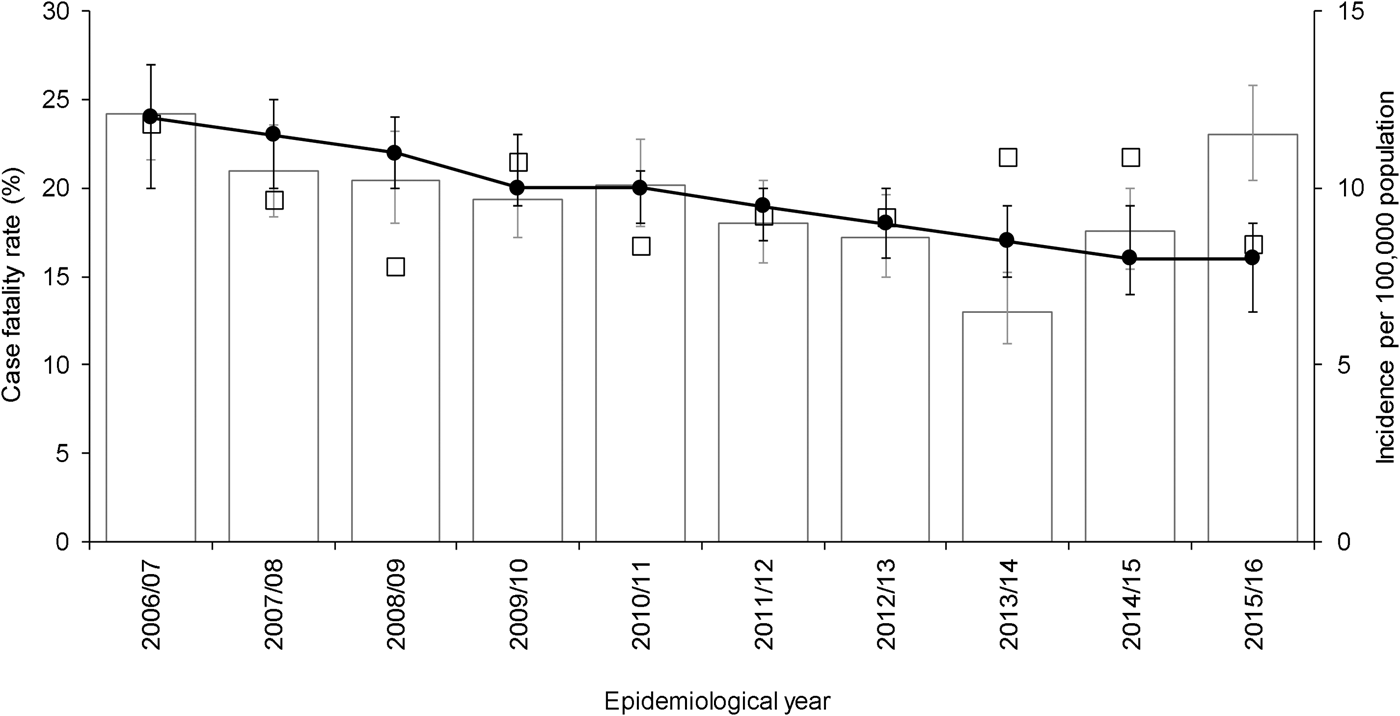
Fig. 1. Crude 30-day all-cause case fatality rate, adjusted average predicted probabilities and incidence of invasive pneumococcal disease by epidemiological year (April–March) in North East England, April 2006–March 2016. Bars show incidence. Line indicates average predicted probability of 30-day all-cause mortality for cases presented as case fatality rate (CFR), adjusted for age, sex, clinical presentation, risk factors and pneumococcal serotype. Epidemiological year is April–March. Open squares indicate crude CFR. Error bars indicate 95% confidence intervals.
A multivariable fractional polynomial model indicated no significant non-linearity in the association between 30-day all-cause mortality and epidemiological year (P > 0.05).
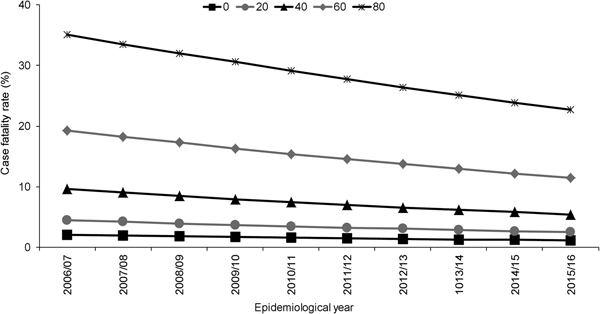
Independent to the association with epidemiological year, increasing age, male sex, septicaemia, presence of ⩾1 clinical risk factor, alcohol misuse and pneumococcal serotype were significant predictors of increased 30-day all-cause mortality. Of the individual serotypes included, serotype 1 had the lowest crude CFR (6%) and serotype 31 the highest (39%) (Table 1). Serotype 1 also had the lowest aOR (0.60) although this was not statistically significant (Table 2). Although no significant interaction was observed between the annual trend in 30-day all-cause mortality and age groups (<5, 5–64, ⩾65 years, P = 1.000) or age quintiles (P = 0.103), the pattern was expectedly more pronounced for older ages (Fig. 2).
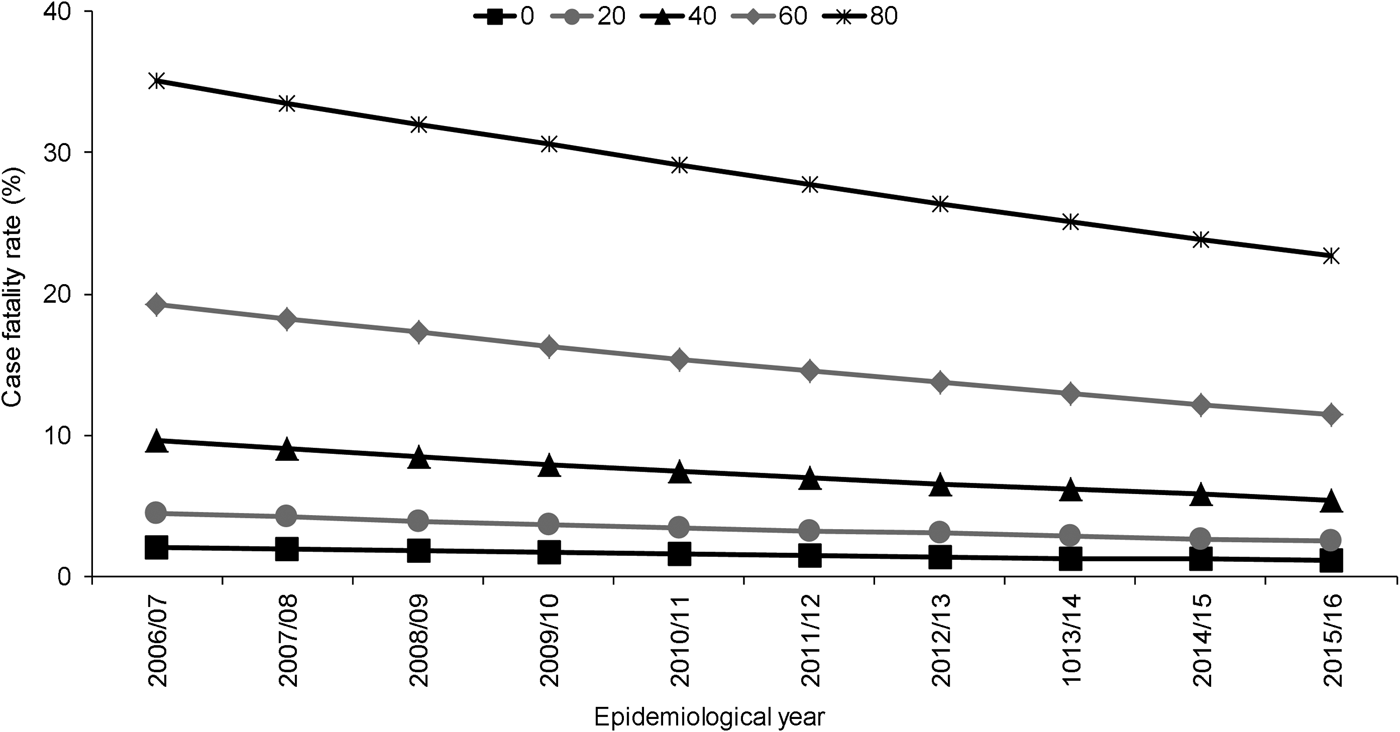
Fig. 2. Adjusted predicted probabilities of 30-day all-cause case fatality rate by epidemiological year (April–March) in North East England by age, April 2006–March 2016. Lines indicate predicted probability of 30-day all-cause mortality presented as case fatality rate (CFR) for individuals of specific ages (years) adjusted for sex, clinical presentation, risk factors and pneumococcal serotype using mean values for covariates. Epidemiological year is April–March.
Discussion
Despite recent increases in IPD in NEE in the epidemiological years 2014/2015 and 2015/2016, following a sustained period of reducing incidence [Reference Houseman4], our study provides reassuring evidence that this increased incidence does not seem to be associated with the emergence of serotypes associated with worse outcomes. Moreover, a decline in adjusted CFR has continued. Our study provides evidence of decreasing CFR following IPD and, to the best of our knowledge, has for the first time estimated the long-term trend in CFR following IPD after adjustment for known individual risk factors.
Given that the rise in IPD incidence in NEE has not disrupted a general decreasing trend in adjusted CFR, it is not surprising that only one (9N) of the six emergent serotypes seen in our population (8, 9N, 12F, 15A, 23A, 25F) [Reference Houseman4] has been previously associated with increased CFR in NEE [Reference Hughes18]. Of the four serotypes previously shown to be significantly associated with increased CFR following IPD in NEE [Reference Hughes18], 9N is the only serotype where the results of this current study are not consistent with those of the earlier study and the reason for this requires further exploration. The observation of rising incidence with declining CFR is consistent with the hypothesised relationship between increasing invasiveness and reduced virulence – those serotypes that have emerged as causative agents of IPD in NEE in recent years represent primary pathogens with an intrinsically low CFR compared to other more heavily encapsulated serotypes which function as opportunistic pathogens causing severe disease in those with underlying risk factors [Reference Weinberger19]. Certainly, the increasing prevalence of the low CFR serotype 8 [Reference Hughes18–Reference Grabenstein and Musey20] in NEE [Reference Houseman4] further supports this hypothesis.
The reasons for the existence of a significant relationship between epidemiological year and declining CFR, after adjusting for risk factors and infecting serotype (all of which have been observed and described elsewhere [Reference Hughes18, Reference Grabenstein and Musey20, Reference Navarro-Torné21, Reference Harboe22]), requires further exploration. Factors may include: improved diagnostic and laboratory practices (that may have led to disproportionate identification of uncomplicated cases [Reference Backhaus11], or earlier diagnoses with better prognosis); improved treatment for risk factors associated with CFR (thus reducing their relative impact on CFR following IPD); reduced prevalence of smoking [Reference Cruickshank, Jefferies and Clarke23]; and/or improved treatment of IPD. Certainly there is evidence for a declining prevalence of smoking in NEE (https://fingertips.phe.org.uk/profile/tobacco-control) but we were unable to adjust for smoking at an individual level due to limited data availability.
Our study indicates that a small but significant trend in declining 30-day all-cause CFR following IPD has been observed in NEE between 2006 and 2016. This suggests that selection pressure due to the use of effective vaccines has not led to the emergence of serotypes of S. pneumoniae that are associated with more severe outcomes. The existence of a declining CFR raises interesting questions related to trends in case ascertainment bias that require further investigation. Despite this encouraging finding, particularly following recent increases in IPD incidence, certain risk groups have an increased probability of dying following IPD and work should continue to reduce the burden of mortality and morbidity from IPD.
Author ORCIDs
G. J. Hughes, 0000-0002-3781-0117
Acknowledgements
We thank the Public Health England North East Health Protection Team for their participation in IPD enhanced surveillance, the Public Health England Respiratory and Vaccine Preventable Bacteria Reference Unit for providing serotype results, all North East National Health Service microbiology laboratories for reporting cases of IPD, teams from primary care and acute National Health Service trusts across NEE for providing enhanced surveillance and Nick Hinton for undertaking linkage to death data.
Financial support
This work was supported by a grant from the Health Protection Agency Strategic Research and Development Fund, April 2009–March 2012; an unrestricted educational grant from Sanofi Pasteur MSD (UK12C1036), April 2012–June 2014; and an unrestricted grant from Pfizer UK Ltd (WI194024), August 2015. The funders had no role in the study design, data collection and analysis or manuscript preparation.
Conflict of interest
None.