Introduction
The human immunodeficiency virus (HIV) pandemic has been described by DeCock [Reference De Cock, Jaffe and Curran1] as a ‘patchwork of different epidemics moving through different groups and communities at different times.’ (pp. 1026).
The initial cases of HIV/acquired immune deficiency syndrome (AIDS) were identified in homosexual men in the USA [Reference De Cock, Jaffe and Curran1]. Studies from the mid-1980s in Africa found that heterosexuals of both sexes in Zaire (now The Democratic Republic of Congo) [Reference Piot, Taelman, Bila Minlangu, Mbendi, Ndangi, Kalambayi, Bridts, Quinn, Feinsod and Wobin2] and Rwanda [Reference Van de Perre, Lepage, Kestelyn, Hekker, Rouvroy, Bogaerts, Kayihigi, Butzler and Clumeck3] were affected. Detailed overviews of the development of the HIV epidemic in Africa have been described elsewhere and reinforce the interplay between disease, culture and society [4, Reference Sankoh, Arthur, Nyide and Weston5].
In South Africa, the HIV epidemic amongst heterosexuals is thought to have been introduced via the sex trade associated with the major sea ports located in KwaZulu Natal on the east coast. This was followed by a subsequent spread firstly to the Witwatersrand region in Gauteng Province, the principal centre for migrant employment in the country and then to other rural regions [Reference Collinson, Wolff, Tollman and Kahn6]. A pattern of oscillatory migration between the urban areas where men spent the majority of their time and the rural communities where they returned to families exacerbated the spread of the infection. The vast majority of these men were contracted in to work in the mining sector [Reference Wilson7]. This pattern of migration resulted in a fracturing of societal and family structures and created the unique environment for the epidemic to spread once it was introduced [Reference Hargrove8].
Data collected from South African antenatal clinics indicates that the most rapid early development of the epidemic was in KwaZulu Natal province in the late 1980s, with a time lag before the same development was seen in other more isolated rural areas regions [Reference Williams and Campbell9]. These results also indicate that HIV prevalence in the general population was very low up to 1993 and increased substantially thereafter.
Lurie investigated the relationship between migration and the risk of infection in HIV discordant couples in rural KwaZulu Natal [Reference Lurie10]. He identified a pattern in which the infection was spread in the community both by the men returning to the rural area and the women who were their partners, through sexual relations other than their primary relationship. Similar patterns were reported by Collinson et al. based on studies carried out in the site of the present study [Reference Collinson, Wolff, Tollman and Kahn6].
A comparison of the age distribution of adult HIV prevalence for the Agincourt sub-district in 2011 [Reference Gómez-Olivé, Angotti, Houle, Klipstein-Grobusch, Kabudula, Menken, Williams, Tollman and Clark11] with that for the whole of South Africa in 2012 [Reference Shisana, Rehle, Simbayi, Zuma, Jooste, Zungu, Labadorios and Onoya12] page 38 is shown in Fig. 1. In all age groups, HIV prevalence was substantially higher in the Agincourt sub-district than that nationally. For men peak rates were seen in 35–39 years old both nationally and the Agincourt sub-district, whereas for women the highest rates were seen in 30–34 years old nationally and for 35–39 years old in Agincourt.
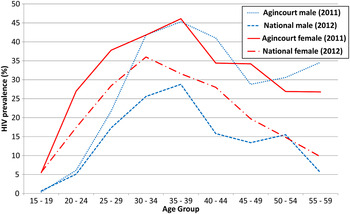
Fig. 1. Comparison of HIV prevalence for the Agincourt sub-district (2011) [Reference Gómez-Olivé, Angotti, Houle, Klipstein-Grobusch, Kabudula, Menken, Williams, Tollman and Clark11] and the whole of South Africa (2012) [Reference Shisana, Rehle, Simbayi, Zuma, Jooste, Zungu, Labadorios and Onoya12]. The overall HIV prevalence in the Agincourt sub-district for those aged over 15 in 2011 was 23.9% for females and 10.6% for males. For the whole of South Africa in 2012 the HIV prevalence in those aged over 15 was 17.8% for females and 11.4% for males.
HIV Mortality studies in the Agincourt sub-district
Studies in this area using data from 1992 to 2007 identified distinct localised clusters of all-cause and cause-specific mortality [Reference Sartorius, Kahn, Vounatsou, Collinson and Tollman13]. A recent study assessed changes in HIV/AIDS related mortality over the period of the roll-out of ART in this community [Reference Mee, Collinson, Madhavan, Root, Tollman, Byass and Kahn14]. This study found a 30% decrease in adult HIV-related mortality between 2007 and 2008, the period immediately before Antiretroviral therapy (ART) was easily accessible in this area, and 2009–2010. In most of the villages the HIV-related mortality decreased whilst for a small number of villages the rate either levelled or increased. A subsequent analysis identified several socio-demographic and geographic factors which were associated with an increased risk of HIV-related mortality [Reference Mee, Collinson, Madhavan, Kabudula, Gómez-Olivé, Kahn, Tollman, Hargreaves and Byass15]. These included; living more than 5 km from the health centre which was the main provider of ART, being a man, being of an older adult age (50–65 years old), being a recent in-migrant to the community, being a regularly returning labour migrant and having lower levels of household wealth and education. In addition, there was also evidence for a lower risk of mortality amongst those of Mozambican origin.
In the current study, we extend the previous research through an analysis of the spatial patterns of development of the epidemic and the nature of the shifting burden on different sub-groups within the population. We also estimate the extent of the excess mortality amongst adults due to HIV in the Agincourt sub-district compared with that expected in its absence.
Methods
The study was carried out in the Agincourt sub-district which is underpinned by a robust Health and Demographic Surveillance System (HDSS) and is located in the Bushbuckridge area of Ehlanzeni municipality of Mpumalanga Province in North-East South Africa. The sub-district has a predominantly rural character with nearby peri-urban settlements [Reference Mee, Collinson, Madhavan, Root, Tollman, Byass and Kahn14, Reference Kahn, Collinson, Gómez-Olivé, Kabudula CW, Mokoena, Shabangu, Tibane, Twine, Garenne and Clark16, Reference Kahn, Tollman, Collinson, Clark, Twine, Clark, Shabangu, Gómez-Olivé, Mokoena and Garenne17] and is close to the southern Mozambican border. There are strong familial and cultural links between the region and Mozambique and approximately one-third of the population are of Mozambican origin [Reference Polzer Ngwato18].
There is high unemployment locally which has resulted in persistent patterns of labour migration, with adults working in the major population centres of the country but retaining strong links with their home community, returning regularly to the area.
Provision of testing and treatment for HIV/AIDS in the Agincourt sub-district
Voluntary Counselling and Testing for HIV has been available in the study site since 2002 [Reference Pronyk, Kim, Makhubele, Hargreaves, Mohlala and Hausler19]. Between 2004 and 2005 ART became available in two district hospitals serving the study population, albeit some distance away. In 2007, the Bhubezi community health centre, a public–private initiative, was established in one of the study villages. This provided general clinical care with a focus on provision of testing and treatment for those with HIV/AIDS. From 2008 ART became available in other clinics and health centres within or close to the study area. The numbers enrolled on ART in these public sector clinics was initially very limited.
Data used in this study and analytical approach
The data used for this analysis were drawn from the Agincourt Health and Demographic Surveillance Study (HDSS) in which information on all births, deaths, migrations and other socio-demographic characteristics of residents is updated through annual visits to all households in the area [Reference Kahn, Collinson, Gómez-Olivé, Kabudula CW, Mokoena, Shabangu, Tibane, Twine, Garenne and Clark16]. Where a death is reported a detailed verbal autopsy (VA) questionnaire is completed through an interview with the closest care-giver of the deceased [Reference Khan, Tollman, Garenne and Gear20, Reference Kahn, Tollman, Garenne and Gear21]. VAs have been carried out on all deaths since the inception of the site in 1992. Causes of death were assigned using the InterVA-4 model version 4.02 [Reference Byass, Fottrell, Dao, Berhane, Corrah, Kahn, Muhe and Do22, Reference Byass, Kahn, Fottrell, Collinson and Tollman23], which is aligned with the standardised 2012 WHO VA instrument [24]. These variables were extracted from the Agincourt VA questionnaire [Reference Mee, Collinson, Madhavan, Root, Tollman, Byass and Kahn14]. InterVA-4 has been shown to produce cause of death assignments well aligned to the physicians’ assessments of VA questionnaires [Reference Byass, Kahn, Fottrell, Mee, Collinson and Tollman25, Reference Byass, Calvert, Miiro-Nakiyingi, Lutalo, Michael, Crampin, Gregson, Takaruza, Robertson and Herbst26]. Due to the high level of HIV co-infection amongst those whose cause of death was assigned as pulmonary tuberculosis (TB) [Reference Byass, Calvert, Miiro-Nakiyingi, Lutalo, Michael, Crampin, Gregson, Takaruza, Robertson and Herbst26] and the difficulty in distinguishing between these in the VA these two causes were combined in a single category (HIV/TB).
For each individual, overall person time was subdivided into 1 year blocks and values for time-changing covariates assigned for each year. The study population comprised all individuals aged 15 years and above at any time during the period of analysis and resident in the Agincourt HDSS study site, on a dynamic cohort basis. To control for the changing age-structure the INDEPTH 2013 standard population [Reference Sankoh, Sharrow, Herbst, Whiteson Kabudula, Alam, Kant, Ravn, Bhuiya, Thi Vui and Darikwa27] was used to calculate age and sex standardised cause-specific mortality rates for each year of the analysis. In order to reduce the effect of single year random variations mortality rates were aggregated in 2 year intervals and moving average values were calculated using a ± 4 year time window. Data analysis was carried out using Stata™ version 10 [28].
Age standardised HIV/TB mortality rates were plotted for the period 1992–2013. These were stratified by: village of residence, age/sex and country of origin/sex. Village names were assigned as letters to maintain anonymity. An individual's country of origin was assigned as either Mozambique or South Africa based on their father's place of birth.
Estimation of the excess mortality due to HIV between 1992 and 2013
A recent study of five sub-Saharan African longitudinal HIV cohorts estimated the excess mortality rate amongst people living with HIV/AIDS (PLWHA) [Reference Slaymaker, Todd, Marston, Calvert, Michael, Nakiyingi-Miiro, Crampin, Lutalo, Herbst and Zaba29] in the periods before, during and after the introduction of ART. One of these sites, uMkhanyakude in KwaZulu Natal South Africa has similar levels of HIV prevalence to those in the Agincourt sub-district [Reference Gómez-Olivé, Angotti, Houle, Klipstein-Grobusch, Kabudula, Menken, Williams, Tollman and Clark11, Reference Streatfield, Khan, Bhuiya, Hanifi, Alam, Millogo, Sie, Zabré, Rossier and Soura30] and is also geographically closest to the site. For this reason the values from that site were used in this analysis. HIV prevalence values for Mpumalanga Province in which the Agincourt sub-district is located were extracted from the South African National HIV prevalence survey [Reference Shisana, Rehle, Simbayi, Zuma, Jooste, Zungu, Labadorios and Onoya12] for the years 2002, 2005, 2008 and 2012. Using the assumption that the first cases of HIV occurred in the site in 1990 we used a linear extrapolation between the known values to estimate the values for the intervening years. Values for Mpumalanga Province were used due to the geographical proximity and the fact that the prevalence for those aged over 14 years of 19.7% in 2012 was very close to that for Agincourt in 2011 of 19.4% [Reference Gómez-Olivé, Angotti, Houle, Klipstein-Grobusch, Kabudula, Menken, Williams, Tollman and Clark11]. The estimated HIV prevalence for each year was multiplied by the actual person years of residence in that year in order to estimate the number of HIV-positive individuals of each sex in the study population. The estimated numbers who were HIV positive were multiplied by the sex-specific HIV attributable mortality rates for uMkhanyakude for the relevant ART period [Reference Slaymaker, Todd, Marston, Calvert, Michael, Nakiyingi-Miiro, Crampin, Lutalo, Herbst and Zaba29] in order to estimate the number of excess deaths for men and women. This figure was compared with the actual number of deaths recorded in the Agincourt HDSS in order to estimate the percentage of deaths directly or indirectly attributable to HIV. The calculation is shown in online Supplementary Table S1 of the supplementary materials associated with this paper.
Results
The 20 plots in Fig. 2 show the HIV/TB epidemic development in each village that was included in the Agincourt HDSS at baseline in 1992. The majority of villages follow the trend seen for the entire population (dashed red line) with peak HIV mortality between 2005 and 2007. Villages O and R had somewhat higher HIV/TB mortality over this period compared with the other villages, whilst that for village B was lower. There is some evidence for a levelling or small increase in mortality for village S from 2007 to 2013. Due to the relatively small numbers of deaths and consequently wide confidence intervals, none of these differences are statistically significant.
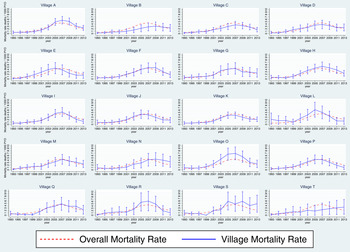
Fig. 2. HIV/TB epidemic profiles of mortality for each of the 20 original Agincourt HDSS villages compared with that for the whole study population. The overall mortality rate for all villages is represented as a red dashed line and that for each individual village as a solid blue line.
The plots in Fig. 3 indicate that younger adult women (aged 15–39) have had a higher risk of dying of HIV/TB at least since 1999. This pattern is reversed in the older age groups (40–59 & 60 years and older) with men showing significantly higher mortality rates than women.

Fig. 3. HIV/TB epidemic profiles of mortality (1992–2013) subdivided by age and gender in the Agincourt sub-district, South Africa. The rates for females are represented as a red-dashed line and that for males as a solid blue line.
There is some evidence that both South African and Mozambican men have had higher mortality rates than women throughout the epidemic although the differences were generally not significant (Fig. 4). For South Africans, the mortality rates for men and women appear to converge between 2005 and 2007. For both sexes the mortality rates were somewhat higher for Mozambicans than South Africans at the height of the epidemic.
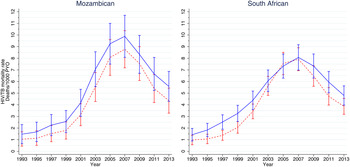
Fig. 4. HIV/TB epidemic profiles of mortality for the Agincourt sub-district subdivided by country of origin and gender. The rates for females are represented as a red dashed line and that for males as a solid blue line.
The estimation of excess mortality indicates that over 60% of the deaths occurring between 1992 and 2013 in this community were attributable to HIV. The trends over time are shown in Fig. 5 of particular note is the steady decline in the percentage of HIV attributable deaths from 2001 onwards.
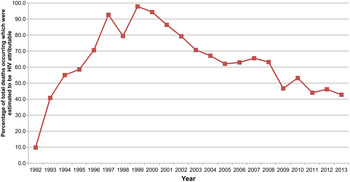
Fig. 5. Plot showing the estimated numbers of HIV attributable deaths occurring between 1992 and 2013 in the Agincourt sub-district (see online Supplementary Table S1 in the supplementary materials for details of how the estimated were derived).
Discussion
As the longitudinal dataset includes all deaths reported in the study area from 1993 to 2013, it allows a unique opportunity to study various dimensions of the local HIV epidemic from close to its origin. This investigation builds on previous studies by investigating; how the development differed by sex and ethnic origin, the spatial development of the epidemic and by estimating the excess mortality due to HIV.
Our findings suggest that the HIV epidemic in the area was generalised in character from the beginning with early deaths seen in all villages rather than being a point source epidemic, which was introduced into one village and subsequently spread through contacts within the community. It seems likely that the origins of the HIV epidemic can be traced to migrant men becoming HIV infected whilst working in higher prevalence urban areas and then returning to their home communities which were distributed throughout the study area [Reference Collinson, Wolff, Tollman and Kahn6, Reference Lurie10]. There is some evidence that the subsequent epidemic followed different trajectories in different village communities although these differences were not statistically significant.
Among individuals aged over 40, men had higher HIV/TB mortality rates than women, whilst the HIV prevalence for the two sexes was very similar. One explanation for this is that men were less likely to get tested for HIV and subsequently initiate and remain on ART treatment. There is evidence for this pattern of healthcare seeking behaviour in the 2012 South African HIV prevalence survey [Reference Shisana, Rehle, Simbayi, Zuma, Jooste, Zungu, Labadorios and Onoya12]. This found that women living with HIV were significantly more likely than men to be on anti-retroviral therapy and also more likely to have ever been tested, to have been tested recently and to know where HIV testing was available locally. This suggests that the lower mortality rates seen for women over 40 compared with men may be due in part to their more effective healthcare seeking behaviour. A different pattern was seen for those aged 15–39 where HIV/TB mortality was higher for women than men at the peak of the epidemic. This probably reflects higher HIV prevalence among women in these younger age groups (Fig. 1) which outweighs their better health seeking behaviour. One important factor contributing to this is likely to be the age disparities in sexual relationships with younger women tending to form relationships with older men [Reference Ott, Bärnighausen, Tanser, Lurie and Newell31, Reference Katz and Low-Beer32]. This trend towards an increase in mortality amongst younger women was first identified in a 1995 study of mortality trends in the Agincourt sub-district [Reference Tollman, Kahn, Garenne and Gear33]. In this community, adult men have been shown to have a higher risk of death due to external causes such as accident or suicide than women [Reference Sartorius, Kahn, Collinson, Sartorius and Tollman34]. This competing risk of death due to causes other than HIV may explain some of the differences in mortality rates between sexes shown in this study.
Peak HIV/TB mortality rates in 2007 for Mozambicans were higher than those for South Africans whilst by 2013 the two groups had very similar rates. This confirms evidence from previous quantitative [Reference Mee, Collinson, Madhavan, Kabudula, Gómez-Olivé, Kahn, Tollman, Hargreaves and Byass15] and qualitative [Reference Polzer Ngwato18] studies that cultural differences between the two groups are decreasing over time as Mozambicans are assimilated into the host South African communities. This pattern is consistent with previous research carried out between 2003 and 2009 comparing women of Mozambican and South Africans origin. This study identified a convergence of fertility patterns and socio-economic parameters over this period [Reference Williams, Ibisomi, Sartorius, Kahn, Collinson, Tollman and Garenne35].
We see evidence for the huge burden of HIV in rural communities such as these from the estimate that over 60% of deaths between 1992 and 2013 can be attributed to HIV. Limiting the analysis to the period from 2002 onwards when the HIV prevalence for Mpumalanga province was available and hence the estimates used in the model were likely to be more robust, we see that over 50% of the deaths were attributable to HIV. The increase in non-HIV attributable deaths over the same period provides further evidence of the growing burden of non-communicable disease in this community [Reference Gomez-Olive, Thorogood, Clark, Kahn and Tollman36, Reference Clark, Gómez-Olivé, Houle, Thorogood, Klipstein-Grobusch, Angotti, Kabudula, Williams, Menken and Tollman37].
This analysis of a localised HIV epidemic from its beginning shows the significant progress in reducing the burden of HIV associated mortality in this community, but also emphasises the considerable progress that still needs to be made. The UNAIDS-Lancet commission on Defeating AIDS – Advancing Global Health recently published a major report on the current state of the epidemic and the steps that will be needed to tackle it [Reference Piot, Abdool Karim, Hecht, Legido-Quigley, Buse, Stover, Resch, Ryckman, Møgedal, Dybul and Goosby38]. These included an increased emphasis on HIV prevention, an expansion of access to treatment and efforts to address structural factors in society which put people at an increased risk. The report also identified the need for better data to enable a more detailed context-dependent understanding of the characteristics of the epidemic and the impact of interventions aimed at lowering the barriers to accessing testing and treatment for HIV. In the context of this study for example, we identified that the levels of HIV-related mortality were significantly higher in men than women in the older age groups. By investigating the upstream determinants of mortality such as the level of engagement with HIV treatment programmes and long-term retention in care, specific strategies could be developed to address the barriers to testing and treatment experienced by older men in this community. This emphasises the importance of the continuation of longitudinal surveillance studies such as this which collect data on morbidity and mortality in order to help to plug these data gaps.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/gheg.2016.3
Acknowledgements
We acknowledge the Agincourt community for their long-term support for the program of work from which these data are drawn and the invaluable contribution of the Agincourt HDSS field workers responsible for the primary data collection.
Financial Support
The Agincourt HDSS was funded by the Wellcome Trust, UK (grant no. 058893/Z/99/A, 069683/Z/02/Z and 069683/Z/08/Z), the National Institute on Aging of the NIH (grants 1R24AG032112–01 and 5R24AG032112–03), the William and Flora Hewlett Foundation (grant no. 2008–1840) and the Andrew W Mellon Foundation, USA; the University of the Witwatersrand, Medical Research Council and the Anglo American Chairman's Fund, South Africa.
This work was carried out in collaboration with the Umeå Centre for Global Health Research, supported by Forte, the Swedish Research Council for Health, Working Life and Welfare (grant no. 2006–1512), and the European Union International Research Staff Exchange Scheme (grant no. 295168).
Declaration of Interest
None.
Ethical Approval
Ethical clearance for the data collection for this study was given by the University of the Witwatersrand Human Research Ethics Committee (Medical). Clearance Certificates M960720 and M110138 ‘Investigating and Responding to Changes in the Health and Population Dynamics of Rural South Africans’. Verbal consent was obtained from the respondent prior to every follow-up survey.







