Introduction
Kefir has its origin in the Caucasus, Tibetan or Mongolian mountains, where before 2000 years BC the grains were already being traditionally passed from generation to generation among the Caucasus tribes, being considered a source of family wealth. The name kefir originates from the Slavic Keif, meaning ‘well-being’ or ‘living well’, due to the overall sense of health and well-being generated in those who consume it( Reference Farnworth 1 ). Kefir differs from other fermented products because it is produced from kefir grains that comprise a specific and complex mixture of lactic acid- and acetic acid-producing bacteria, and lactose-fermenting and non-fermenting yeast, which live in a symbiotic association( Reference Lopitz-Otsoa, Rementeria and Elguezabal 2 ).
Kefir grains, when inoculated into a culture medium such as milk, produce acidified fermented milk that is slightly carbonated and contains small amounts of alcohol. During fermentation, lactic acid, bioactive peptides, exopolysaccharides, antibiotics and numerous bacteriocins are produced( Reference Farnworth 1 , Reference Pogačić, Šinko and Zamberlin 3 ). According to the Codex Alimentarius (Codex Stan 243-2003)( 4 ), a typical kefir (fermented milk obtained from kefir grains) should contain at least 2·7 % of protein, 0·6 % of lactic acid, and less than 10 % of fat. The percentage of alcohol is not established. The total number of micro-organisms in the fermented milk produced should be at least 107 colony-forming units (CFU)/ml and the yeast number not less than 104 CFU/ml( 4 ).
The micro-organisms present in kefir possess probiotic potential. Numerous bacterial species isolated from kefir demonstrate high resistance to the low pH and bile salts in the gastrointestinal tract, and are able to adhere to the intestinal mucus( Reference Golowczyc, Gugliada and Hollmann 5 ). Additionally, the microbiota present in kefir can produce antagonistic substances, such as organic acids and bacteriocins( Reference Silva, Rodrigues and Filho 6 ), and interfere with the adherence of pathogenic bacteria in the intestinal mucosa( Reference Xie, Zhou and Li 7 ), potentially contributing to the improvement of gut health.
Kefir has raised interest in the scientific community due to its suggested beneficial properties, including improved digestion and tolerance to lactose( Reference Hertzler and Clancy 8 ), antibacterial effect( Reference Rodrigues, Caputo and Carvalho 9 ), hypocholesterolaemic effect( Reference Taylor and Williams 10 ), control of plasma glucose( Reference Hadisaputro, Djokomoeljanto and Judiono 11 ), anti-hypertensive effect( Reference Maeda, Zhu and Omura 12 ), anti-inflammatory effect( Reference Rodrigues, Caputo and Carvalho 9 , Reference Lee, Ahn and Kwon 13 ), antioxidant activity( Reference Guzel-Seydim, Seydim and Greene 14 ), anti-carcinogenic activity( Reference Gao, Gu and Ruan 15 ) and anti-allergenic activity( Reference Lee, Ahn and Kwon 13 ). Therefore, the present review focuses on the nutritional and microbiological composition of kefir and presents relevant findings associated with the beneficial effects of kefir on human and animal health.
Characteristics of kefir grains
Kefir grains have a similar shape to the cauliflower. They are elastic, irregular, gelatinous, with an ivory or white colour, and variable size, from 0·3 to 3·5 cm in diameter( Reference Garrote, Abraham and de Antoni 16 , Reference Gaware, Kotade and Dolas 17 ) (Fig. 1). In general, kefir grain consists of 4·4 % fat, 12·1 % ash, 45·7 % mucopolysaccharide, 34·3 % total protein (27 % insoluble, 1·6 % soluble and 5·6 % free amino acids), vitamins B and K, tryptophan, Ca, P and Mg( Reference Marshall and Cole 18 ).

Fig. 1 Appearance of kefir grains.
The presence of d-glucose and d-galactose in a 1:1 ratio in the complex structure of polysaccharides (kefiran) is responsible for the connection between the micro-organisms in kefir grains( Reference Lin, Chen and Liu 19 ). Kefiran features include viscosity, water solubility and resistance to bowel enzymic hydrolysis. The production of kefiran is mainly related to the presence of Lactobacillus kefiranofaciens and Lactobacillus kefiri in the grains( Reference Lopitz-Otsoa, Rementeria and Elguezabal 2 , Reference Otles and Cagindi 20 ).
In kefir grains, the peripheral portion is composed almost exclusively of bacteria, predominantly Bacillus, whereas the inner portion of the grain contains yeasts, and the interface of the inner and outer portions has a mixed composition, where bacteria with long polysaccharide filaments, yeasts and fungi are found( Reference Lopitz-Otsoa, Rementeria and Elguezabal 2 , Reference Lin, Chen and Liu 19 ).
The grains can be stored in different ways. When stored at 4°C, they are active for only 8 to 10 d. Lyophilisation or drying at room temperature for 36 to 48 h allows maintenance of the activity for 12 to 18 months( Reference Garrote, Abraham and de Antoni 16 ). Wszolek et al. ( Reference Wszolek, Kupiec-Teahan and Skov Guldager 21 ) proposed a conventional method of drying at 33°C or vacuum drying to preserve the grains. However, Garrote et al. ( Reference Garrote, Abraham and de Antoni 22 ) observed that freezing at –20°C was the best method for grain preservation. Kefir grains remain stable for many years without losing their activity, if stored under favourable conditions. The process of reconstitution consists of performing successive incubations in milk. The grains slowly re-establish their structure and, subsequently, new kefir grains are formed( Reference Sarkar 23 ).
Production of kefir
Kefir can be produced from whole, semi-skimmed or skimmed pasteurised cow, goat, sheep, camel or buffalo milk( Reference Santos 24 ). Kefir from cows’ milk is the most common. The kefir grains can be added to the fermentation substrate as a starter culture( Reference Santos 24 ).
Although there is an ideal relationship between the grains and the fermentation substrate (1:30 to 1:50 (w/v) in the case of animal milk), in practice, the measures are made empirically( Reference Farnworth 1 ). Fermentation typically occurs at temperatures ranging from 8 to 25°C, in a partially closed container, at a variable time from 10 to 40 h. However, the most common incubation time is 24 h( Reference Güven, Güven and Gülmez 25 – Reference Urdaneta, Barrenetxe and Aranguren 27 ).
After fermentation, the grains are separated from the fermented milk by filtration using a sieve( Reference Lopitz-Otsoa, Rementeria and Elguezabal 2 ). When milk is used as a substrate, the kefir is similar to yogurt. The higher the fat content in the milk, the thicker and creamier the kefir ( Reference Santos 24 ). Kefir grains may increase in size by up to 2 % of the original to form a new biomass, which allows continuous production, since the grains can be further added to a fermentation substrate( Reference Farnworth 1 , Reference Garrote, Abraham and de Antoni 16 , Reference Gaware, Kotade and Dolas 17 ). Pure starter and lyophilised culture can be used, eliminating the step of recovering the kefir grains. Kefir can be consumed immediately after grain separation or can be refrigerated for later consumption. During the cooling step, alcoholic fermentation leads to the accumulation of CO2, ethanol and vitamin B complex( Reference Farnworth 1 , Reference Santos 24 ). This maturation step reduces the lactose content, making the product desirable for consumption by individuals with lactose intolerance and diabetes( Reference Farnworth 1 ) (Fig. 2).
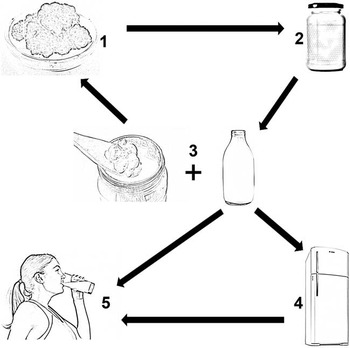
Fig. 2 Domestic production of kefir. (1) Separation of kefir grains. (2) Addition of milk to the kefir grains in a half-open container at room temperature to ferment for 10 to 24 h. (3) Filtration and separation of kefir grains. Possible addition of the kefir grains to fresh milk to start a new fermentation. The kefir is adequate for consumption. (4) The kefir can be refrigerated (4°C). (5) The kefir is safe and ready to drink.
Nowadays, micro-organisms isolated from kefir grains or starter cultures containing freeze-dried lactic acid bacteria (LAB) and kefir yeasts are being used in kefir production. However, the composition of the final fermented milk presents a lower number and variety of micro-organisms than the fermented milk produced from kefir grains( Reference Arslan 28 ).
Nutritional composition of kefir
The nutritional composition of kefir varies widely and is influenced by milk composition, the origin and composition of the grains used, the time/temperature of fermentation and storage conditions. However, the nutritional composition of kefir is still not well described in the literature.
Regarding the chemical composition, moisture is the predominant constituent (90 %), followed by sugars (6 %), fat (3·5 %), protein (3 %) and ash (0·7 %)( Reference Sarkar 23 ). During fermentation, proteins become easily digestible due to the action of acid coagulation and proteolysis. Kefir shows a similar profile of amino acids to the milk used as the fermentation substrate( Reference Ferreira 29 ). The levels of ammonia, serine, lysine, alanine, threonine( Reference Guzel-Seydim, Seydim and Greene 14 ), tryptophan, valine, lysine, methionine, phenylalanine and isoleucine are higher in kefir compared with unfermented milk( Reference Liut Kevičius and Šarkinas 30 ). According to Liutkevičius & Šarkinas( Reference Liutkevičius and Šarkinas 31 ), the essential amino acid contents in kefir are in descending order: lysine (376 mg/100 g); isoleucine (262 mg/100 g); phenylalanine (231 mg/100 g); valine (220 mg/100 g); threonine (183 mg/100 g); methionine (137 mg/100 g); and tryptophan (70 mg/100 g).
The lactose from milk is degraded to acid during the fermentation process, which causes pH reduction and increase in consistency. Approximately 30 % of milk lactose is hydrolysed by the β-galactosidase enzyme, turning lactose into glucose and galactose. Furthermore, bacteria present in kefir convert glucose into lactic acid( Reference Ferreira 29 ). In this context, kefir is a good option for lactose-intolerant individuals.
The lipid content (monoacylglycerols, diacylglycerols and TAG, NEFA and steroids) in kefir can vary depending on the type of milk used in the fermentation. In the fermented milk, the presence of NEFA contributes to the improvement of digestibility( Reference Otles and Cagindi 20 ).
Kefir contains a rich vitamin composition, when it is ready for consumption. The vitamin content depends on the quality of the milk used, micro-organisms present in the kefir grains, and the way of preparation. Kefir presents vitamins B1, B2, B5, C( Reference Sarkar 23 ), A and K, and carotene in its composition. According to Liut Kevičius & Šarkinas( Reference Liut Kevičius and Šarkinas 30 ), the concentration of pyridoxine, vitamin B12, folic acid, biotin, thiamin and riboflavin increase during the fermentation process.
Among the minerals, kefir is a good source of Mg, Ca and P( Reference Otles and Cagindi 20 ). Additionally, minerals such as Zn, Cu, Mn, Fe, Co and Mo are found in milk kefir.
Lactic acid, CO2 and ethanol are the main products that originate from the lactic fermentation process. Kefir also contains formic, propionic and succinic acids, aldehydes, traces of acetone and isoamyl alcohol, and a variety of folates( Reference Güven, Güven and Gülmez 25 ). The pH of kefir varies between 4·2 and 4·6, ethanol content between 0·5 and 2·0 % (v/v), lactic acid between 0·8 and 1·0 % (w/v) and CO2 between 0·08 and 0·2 % (v/v)( Reference Saloff Coste 32 ). Biogenic amines such as putrescine, cadaverine, spermidine and tyramine are also found in kefir samples as a consequence of the LAB activity( Reference Altay, Karbancioglu-Guler and Daskaya-Dikmen 33 ). The high levels of biogenic amines are related to the depreciation of the sensorial properties of fermented milk, and are considered to be an important indicator of quality and acceptability. The high concentration of bioactive amines in fermented products, especially putrescine, cadaverine, agmatine and N-methylputrescine, as well as monoamines such as penicillamine and histamine are positively correlated with inharmonious bitter taste( Reference Takahashi and Kohno 34 ). Özdestan & Uren( Reference Özdestan and Üren 35 ) reported total biogenic amines contents in kefir samples between 2·4 and 35·2 mg/l, with tyramine being the most abundant bioactive amine. These values, however, are far below the recommended limits.
Finally, several compounds that are generated during fermentation exert a direct influence on the aroma and taste of kefir, such as lactic acid, acetic acid, pyruvic acid, hippuric acid, propionic acid, butyric acid, diacetyl and acetaldehyde( Reference Ahmed, Wang and Ahmad 36 ).
Microbiological composition of kefir
The microbiota present in kefir and its grains include numerous bacterial species from lactic acid and acetic acid groups, yeasts and filamentous fungi, which develop complex symbiotic associations( Reference Pogačić, Šinko and Zamberlin 3 ). In this relationship, yeasts produce vitamins, amino acids and other essential growth factors that are important for bacteria. Likewise, the metabolic products of bacteria are used as an energy source for the yeasts. This symbiosis allows the maintenance of stability, so that throughout the fermentation cycle, the microbiological profile of kefir grains and kefir remains unaltered, despite variations in the quality of the milk, microbial contamination, presence of antibiotics and other inhibitory substances( Reference Santos 24 ).
The identification of microbiota present in kefir and its grains is important since it is directly related to the quality of the probiotic product( Reference Garbers, Britz and Witthuhn 37 ). Different methodologies have been applied to study the microbiota of kefir; however, the classical approach of culturing micro-organisms in nutrient media (universal and selective) and identification of isolated cultures is still being performed( Reference Chen, Wang and Chen 38 ). Nowadays, the understanding of microbial ecology of foods has dramatically changed. The use of a combined approach using culture-dependent and culture-independent methods, such as functional genomics, transcriptomics, proteomics and metabolomics, are encouraged to understand the behaviour of micro-organisms in foods( Reference Ercolini 39 ). Using culture-independent methods, including metagenomics, has allowed characterisation of a number of previously unknown micro-organisms in kefir ( Reference Gao, Gu and He 40 ). In particular, analysis of the 16S rRNA gene libraries and/or molecular techniques such as denaturing gradient gel electrophoresis are very useful to evaluate and understand the complex microbial populations and diversity of strains from the probiotic kefir ( Reference Nielsen, Gürakan and Ünlü 41 ).
The microbial diversity of kefir described in the literature varies greatly. In our review, we present a complete description of the bacteria and yeasts that have been identified in kefir to date (Table 1). The number of different microbial species in kefir is estimated to be more than 300. The microbial composition of kefir also varies according to microbiological culture medium, origin of kefir grains, different techniques employed during processing, different room temperatures, type and composition of milk used, storage conditions of kefir and kefir grains. Additionally, the amount of grain added to the milk, agitation and incubation temperature can influence the extent of acidification and consequently the microbiological composition of the final fermented milk. Witthuhn et al. ( Reference Witthuhn, Cilliers and Britz 42 ) observed that the population of bacteria in kefir may vary from 6·4×104 to 8·5×108 CFU/g and yeasts from 1·5×105 to 3·7×108 CFU/g. After 24 h of fermentation, kefir presented 108 CFU/ml of Lactobacillus, 105 CFU/ml of Lactococcus, 106 CFU/ml of yeasts and 106 CFU/ml of acetic acid bacteria( Reference Irigoyen, Arana and Castiella 43 ).
Table 1 Species found in the microbiota of kefir and its grains

According to Lopitz-Otsoa et al. ( Reference Lopitz-Otsoa, Rementeria and Elguezabal 2 ), the microbial composition of kefir grains comprised 65 to 80 % of Lactobacillus and Lactococcus and the remaining portion was completed by yeasts. Hallé et al. ( Reference Hallé, Leroi and Dousset 44 ) found that 80 % of Lactobacillus belonged to Lactobacillus kefiri and the remaining 20 % belonged to Lactobacillus paracasei subsp. paracasei, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus plantarum and Lactobacillus kefiranofaciens.
The diversity of yeasts present in kefir can also be assessed using culture-dependent and culture-independent methods. Yeast species such as Saccharomyces cerevisiae, Saccharomyces unisporus, Candida kefyr, Kluyveromyces marxianus subsp. marxianus, Torulaspora delbrueckii, Pichia fermentans, Kazachastania aerobia, Lachanceae meyersii, Yarrowia lipolytica and Kazachstania unispora are present in kefir and kefir grains in greater numbers( Reference Leite, Mayo and Rachid 45 , Reference Wang, Chen and Liu 46 ).
Kefir consumption
In Russia, USA, Japan and Central and Northern Europe, kefir has been used in the control of many diseases due to its nutritional and therapeutic aspects. Recently, a new formulation of kefir with the addition of enzymes such as lipase or α-amylase to prevent and control obesity was patented in Japan. In the former Soviet Union countries, the consumption of kefir has been informally recommended for healthy individuals to reduce the risk of chronic diseases and also for patients with gastrointestinal and metabolic disorders, hypertension, IHD, weight control and allergies( Reference Figler, Mosik and Schaffer 47 ).
The industrial production of kefir is large in Germany, Austria, France, Luxembourg, Norway, Switzerland, Czech Republic, Slovakia, Poland and Israel. In Brazil, the consumption of kefir occurs domestically with spontaneous fermentation of kefir grains in milk, without the control of the time or temperature of fermentation. The consumption and industrial production of kefir on a larger scale are lacking so far.
Health effects of kefir
Kefir has a wide spectrum of important health benefits, including physiological, prophylactic and therapeutic properties. These effects are a result of a wide variety of bioactive compounds produced during the fermentation process and the highly diverse microbiota, which act either independently or synergistically to influence these health benefits( Reference de Oliveira Leite, Miguel and Peixoto 48 ). Therefore, the main studies reporting the beneficial effects of kefir on animal models and human subjects in the last 10 years, besides those described throughout this review, are presented in Tables 2 and 3. A schematic diagram of the potential beneficial effects of kefir on human physiology and health is shown in Fig. 3.
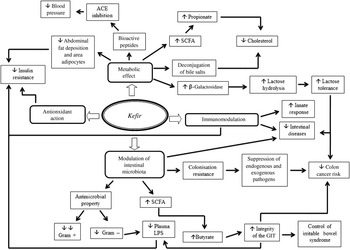
Fig. 3 Schematic diagram of the beneficial physiological effects of kefir on human health. ACE, angiotensin-converting enzyme; LPS, lipopolysaccharide; GIT, gastrointestinal tract.
Table 2 Health benefits of milk kefir in animal studies

CFU, colony-forming unit.
Table 3 Health benefits of milk kefir in human studies

Effect of kefir on lactose intolerance
Milk and dairy products contain high concentrations of lactose. Intestinal absorption of lactose requires hydrolysis of this disaccharide and its subsequent absorption in the small-intestinal mucosa. However, a significant proportion of the world population demonstrates limitations in the digestion of lactose due to insufficient activity of intestinal β-galactosidase( Reference de Vrese and Marteau 49 ). This enzyme, naturally present in kefir grains, reduces lactose content of the kefir during fermentation, which in turn makes the final product suitable for individuals with lactose intolerance( Reference Ahmed, Wang and Ahmad 36 ). Moreover, fermented products such as kefir are characterised by a delayed gastric emptying, which helps in lactose digestion. Hertzler & Clancy( Reference Hertzler and Clancy 8 ) found that the consumption of kefir, which is similar to yogurt, was able to improve lactose digestion and tolerance in healthy adult subjects clinically diagnosed with lactose intolerance. In this study, yogurt and kefir were similarly able to reduce the severity of flatulence related to milk by 54 % to 71 %. According to Alm( Reference Alm 50 ), after the fermentation period, kefir has a reduction of 30 % in the content of lactose, compared with the unfermented milk, providing better comfort for individuals with lactose intolerance. In addition, enzymes released from the lysed micro-organisms may aid in lactose digestion in the gut in a similar manner to most probiotic preparations containing LAB. It is important to note that there are only a few studies on kefir concerning lactose intolerance, and more work is needed to better understand the effects of kefir consumption and its possible effectiveness in reducing the unpleasant symptoms of lactose intolerance in humans. The amount and regularity of consumption of kefir to perform these desirable effects should also be studied.
Antimicrobial properties of kefir
Studies of the early twentieth century observed that the positive effect on life expectancy of regular consumption of yogurt containing lactic acid-producing micro-organisms was due to the existing competition between LAB and harmful pathogens. Since then, antifungal and antibacterial activities of probiotics like kefir have been extensively studied( Reference Lopitz-Otsoa, Rementeria and Elguezabal 2 ).
Antibacterial properties of kefir are related to a combination of several factors, including competition for available nutrients and the inherent action of organic acids, H2O2, acetaldehyde, CO2 and bacteriocins produced during the fermentation process( Reference Powell 51 ). These substances also exhibit some effects similar to those of nutraceuticals, preventing gastrointestinal disorders and vaginal infections( Reference Ahmed, Wang and Ahmad 36 ).
Kefir exerts bactericidal effects on Gram-negative bacteria; however, it is more potent against Gram-positive bacteria( Reference Czamanski, Greco and Wiest 52 ). This antagonistic action has been observed against bacteria, such as Salmonella ( Reference Schneedorf and Anfiteatro 53 ), Shigella, Staphylococcus ( Reference Rodrigues, Caputo and Carvalho 9 , Reference Schneedorf and Anfiteatro 53 ), Helicobacter pylori ( Reference Oh, Osato and Han 54 ), Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, Bacillus subtilis, Micrococcus luteus ( Reference Kwon, Park and Cho 55 ), Listeria monocytogenes, Streptococcus pyogenes and also against the yeast Candida albicans ( Reference Rodrigues, Caputo and Carvalho 9 ).
Silva et al. ( Reference Silva, Rodrigues and Filho 6 ) reported antimicrobial activity of kefir against Candida albicans, Escherichia coli, Staphylococcus aureus, Salmonella typhi and Shigella sonnei. Ulusoy et al. ( Reference Ulusoy, Colak and Hampikyan 56 ) observed that kefir produced from lyophilised commercial grain (PROBAT KC3; Danisco) presented antibacterial effect against Staphylococcus aureus, Bacillus cereus, Salmonella enteritidis, Listeria monocytogenes and Escherichia coli. The results were comparable with the antibacterial action of ampicillin and gentamicin.
Kefir grains have shown to have higher antibacterial activity than kefir. This is observed especially against Gram-positive cocci, including staphylococci and Gram-positive bacilli( Reference Farnworth 1 ). The antifungal and antibacterial activities may explain the wide use of kefir in the prevention of infectious diseases and tumour development( Reference Liu, Wang and Lin 57 ).
According to Brialy et al. ( Reference Brialy, Rivalland and Coiffard 58 ), fresh kefir presented an intrinsic inhibitory potential against Staphylococcus aureus, Kluyveromyces lactis and Escherichia coli. However, this effect was not verified against Saccharomyces cerevisiae and Candida albicans. Kefir has been shown to lose its intrinsic inhibitory effect after lyophilisation and re-constitution in distilled water or milk.
In an in vitro study, Ismaiel et al. ( Reference Ismaiel, Ghaly and El-Naggar 59 ) tested the antimicrobial activity of kefir grains and kefir suspension against several species of bacteria and fungi and observed higher inhibitory action against Streptococcus faecalis and Fusarium graminearum. The concentration of kefir from 7 to 10 % (w/w) was able to completely inhibit the sporulation of Aspergillus flavus, and consequently the production of aflatoxin B1, which demonstrates the antifungal properties of kefir against filamentous fungi. The organic acids produced during fermentation of kefir can change the molecule aflatoxin B1, by converting it into less toxic forms such as aflatoxicol aflatoxin B and B2a( Reference Westby, Reilly and Bainbridge 60 ) In this context, kefir appears as a safe alternative for food preservation, providing protection against poisoning from aflatoxin B1.
Additionally, kefir was able to increase the population of LAB and reduce the levels of Enterobacteriaceae and Clostridium in the intestinal mucosa of mice( Reference Marquina, Santos and Corpas 61 ). Oral administration of milk kefir or soya milk kefir in mice over a period of 28 d was able to significantly increase Lactobacillus and Bifidobacterium while reducing Clostridium perfringens in animal faeces( Reference Liu, Wang and Chen 62 ).
Thus, the antimicrobial activity of kefir is differentiated when the fermented milk is used in the reconstituted or liquid form, and also when kefir grains are used. However, it is noted that both kefir grains and fermented milk constitute interesting alternatives that can be used for the prevention of some infections, especially those involving the gastrointestinal tract. We emphasise that it is necessary to perform in vivo studies to investigate the antimicrobial properties of kefir, especially with regard to the reduced infection rates and severity of symptoms with kefir consumption in animal and human studies, since the results of in vitro studies conducted until now show promising results.
Hypocholesterolaemic effect of kefir
The consumption of probiotic dairy products has been proposed as a strategy to reduce levels of circulating cholesterol. Guo et al. ( Reference Guo, Liu and Zhang 63 ) in a meta-analysis with thirteen trials, including 485 participants with high, borderline high and normal cholesterol levels, observed that the consumption of probiotic dairy products was able to lower serum cholesterol (mean net change of 6·40 mg/dl; 0·17 mmol/l), LDL-cholesterol (mean net change of 4·90 mg/dl; 0·13 mmol/l) and TAG (mean net change of 3·95 mg/dl; 0·04 mmol/l) concentrations. Some mechanisms are proposed to justify these findings:
-
(1) The LAB inhibit the absorption of exogenous cholesterol in the intestine due to binding and incorporation of cholesterol by the bacterial cells. The high count of LAB present in kefir may directly or indirectly reduce cholesterol in the medium by up to 33 %( Reference Hosono and Tanako 64 ). Vujičić et al. ( Reference Vujičić, Vulić and Könyves 65 ) verified that after 24 h of fermentation, kefir cultures were able to absorb from 28 to 65 % of the cholesterol present in the culture medium.
-
(2) Probiotic bacteria increase the production of SCFA. Among the different SCFA produced, propionate reduces the production of cholesterol by inhibiting hydroxymethylglutaryl CoA (HMG-CoA) reductase activity. Additionally, plasma cholesterol is redistributed to the liver, where the synthesis and secretion of bile acids are increased, since the activity of the 7α-hydrolase enzyme is stimulated. Moreover, propionate inhibits the intestinal expression of genes involved in the biosynthesis of cholesterol( Reference Arora, Sharma and Frost 66 ).
-
(3) Another possible pathway involves the deconjugation of bile acids, which may be increased in the large intestine, caused by the bile salt hydrolase (BSH) enzyme. The BSH enzyme catalyses the hydrolysis of glycine and/or taurine conjugated to the bile salts in residual amino acids and free bile salts, increasing excretion. With the increasing excretion of bile salts, fewer of them are carried back to the liver by the enterohepatic circulation, which increases the demand for cholesterol for de novo synthesis of bile salts in the liver. Thus, the liver increases the hepatic uptake of LDL from the circulation( Reference Lecerf and de Lorgeril 67 ) which leads to the reduction of serum LDL-cholesterol concentrations.
Some animal studies have demonstrated the hypocholesterolaemic effect of kefir ( Reference Maeda, Zhu and Omura 12 , Reference Xiao, Kondo and Takahashi 68 ). Hamsters fed a hypercholesterolaemic diet supplemented with freeze-dried kefir (milk or soya milk) showed a significant reduction in TAG concentration and in the atherogenic index( Reference Liu, Wang and Chen 69 ). In this study, the effects were partially related to increased faecal excretion of neutral sterols and bile acids. Also, Lactobacillus plantarum MA2 isolated from kefir grains originated from Tibet was effective in reducing plasma and liver cholesterol and TAG concentrations. This micro-organism was also able to increase faecal excretion of cholesterol and TAG in mice fed a high-fat diet( Reference Wang, Xu and Xi 70 ).
Animals that consumed hyperlipidaemic diets associated with kefiran showed a reduction in serum total cholesterol, LDL-cholesterol and TAG concentrations, as well as a reduction in liver cholesterol and TAG concentrations compared with controls( Reference Maeda, Zhu and Omura 12 ). Uchida et al. ( Reference Uchida, Ishii and Inoue 71 ) evaluated the anti-atherogenic effect of kefiran in rabbits fed a high-cholesterol diet and observed lower atherosclerotic lesion in the abdominal aorta and lower concentrations of hepatic cholesterol and lipid peroxidation in the animals fed kefiran in comparison with the control group.
The consumption of kefir (0·5 litres/d) by hypercholesterolaemic adult men for 4 weeks did not affect the circulating concentrations of total cholesterol, HDL-cholesterol, LDL-cholesterol or TAG; however, it increased the SCFA concentrations in their faeces( Reference St-Onge, Farnworth and Savard 72 ). Ostadrahimi et al. ( Reference Ostadrahimi, Taghizadeh and Mobasseri 73 ) conducted a double-blind randomised placebo-controlled clinical trial with diabetic patients, who consumed 600 ml of kefir daily for 8 weeks. Kefir consumption was not able to influence serum TAG, total cholesterol, LDL-cholesterol and HDL-cholesterol levels compared with the control, showing that kefir was unable to reduce plasma lipids in diabetic patients.
The benefits of kefir in lowering cholesterol have shown conflicting results. Such inconsistent results may be due to different experimental protocols used, origin of the grains, and fermentation conditions of kefir, and consequently the variety of kefir composition. This scenario may be related to lack of standardisation in nutritional and microbiological composition of kefir used in scientific research.
Control of plasma glucose by kefir
The regular consumption of probiotics has the ability to improve blood sugar levels. This effect has been attributed mainly to the probiotic ability to positively modulate the composition of the intestinal microbiota and hence reduce intestinal permeability, oxidative stress and inflammation( Reference Gomes, Bueno and de Souza 74 ).
Similar effects can be observed with regular consumption of kefir. Hadisaputro et al. ( Reference Hadisaputro, Djokomoeljanto and Judiono 11 ) evaluated the effect of kefir consumption for 30 d in controlling glycaemia in Wistar rats induced to diabetes mellitus by administration of streptozotocin. Kefir supplementation was able to reduce plasma glucose compared with the control group.
In a clinical trial, diabetic adults that consumed 600 ml/d kefir, for 8 weeks, showed a significant decrease in fasting glucose levels and glycosylated Hb compared with baseline. Additionally, these same parameters were found to be significantly reduced in individuals who consumed kefir compared with control subjects who consumed a conventional fermented milk( Reference Ostadrahimi, Taghizadeh and Mobasseri 73 ).
The regular intake of probiotics can reduce the amount of Gram-negative bacteria in the intestinal lumen, and therefore lower the amount of lipopolysaccharide (LPS). In addition, probiotics can improve intestinal barrier function leading to the reduction of intestinal permeability. The absorption of lower amounts of LPS may therefore diminish the low-grade chronic inflammatory process characteristic of diabetes. Moreover, lower LPS may restore the function of insulin receptors leading to a better control of blood glucose. We can thus conclude that kefir might be used in the prevention of diabetes; however, more studies are needed to demonstrate such effects.
Anti-hypertensive effect of kefir
Some evidence indicates that probiotic bacteria or their fermented products play an important role in controlling blood pressure. The anti-hypertensive effects have been observed in experimental and clinical studies( Reference Parvez, Malik and Kang 75 ), although the data are limited and controversial.
Quirós et al. ( Reference Quirós, Hernández-Ledesma and Ramos 76 ) found that kefir is able to inhibit the activity of angiotensin-converting enzyme (ACE) through the action of bioactive peptides generated from casein during the milk fermentation process. According to Maeda et al. ( Reference Maeda, Zhu and Omura 12 ), the antihypertensive activity observed in their study was due to the ability of kefiran to inhibit ACE activity.
The ACE-inhibitory peptides inhibit the production of the vasoconstrictor angiotensin I, and consequently the production of aldosterone, a hormone that stimulates the increase of serum Na concentration, causing an increase in blood pressure. Additionally, ACE-inhibitory peptides also inhibit the breakdown of bradykinin, a hormone that has vasodilating action, contributing to the decrease in blood pressure( Reference Hernández-Ledesma, Contreras and Recio 77 ).
Experimental and especially clinical studies that have evaluated the antihypertensive effect of milk kefir are rare in the literature to date. Furthermore, the milk kefir peptides that exhibit the ability to inhibit ACE action have not yet been identified.
Anti-inflammatory properties of kefir
The inflammatory state is associated with the development of some chronic diseases such as obesity, diabetes and cancer( Reference Arthur and Jobin 78 ). Therefore, the number of studies that have evaluated the immunomodulatory properties of probiotics is increasing.
The immunomodulatory properties of kefir may result from direct action of the microbiota or may be indirect, through different bioactive compounds produced during the fermentation process( Reference Zhou, Liu and Jiang 79 ). The bioactive peptides, produced during milk fermentation by the microbiota present in kefir, are able to activate macrophages, increase phagocytosis, suppress the Th2 immune response, increase the production of NO and cytokines, and stimulate the secretion of IgG and IgA by B lymphocytes in the intestinal lumen( Reference Adiloǧlu, Gönülateş and Işler 80 ). According to Vinderola et al. ( Reference Vinderola, Duarte and Thangavel 81 ), these bioactive compounds are able to promote the cell-mediated immune response against infections and intracellular pathogens( Reference Liu, Wang and Lin 57 ).
The anti-inflammatory potential of kefir was evaluated in an animal model of asthma, sensitised with ovalbumin. The administration of kefir (50 mg/kg) was found to significantly inhibit the total number of inflammatory cells and eosinophils in the bronchoalveolar fluids. In addition, kefir administration decreased IL-4, IL-13 and IgE to a normal level( Reference Lee, Ahn and Kwon 13 ). Thus, kefir has therapeutic potential for the prevention of allergic bronchial asthma.
Rodrigues et al. ( Reference Rodrigues, Caputo and Carvalho 9 ) evaluated the anti-inflammatory action of kefir in rats using a protocol of oedema and granuloma induction. In this study, water kefir, milk kefir and kefiran extract inhibited the inflammatory process by 41, 44 and 34 %, respectively. The treatments also significantly reduced oedema in the animals. The results demonstrate the presence of anti-inflammatory compounds in the symbiotic cultures of kefir.
The immunomodulatory effect of kefir can be attributed to the ability of this probiotic to decrease or restore intestinal permeability. Thus, the contact between the host and the antigens present in the intestinal lumen is decreased, which in turn can reduce the inflammatory response.
In this situation, kefir may be able to reduce intestinal permeability against food-borne antigens. Liu et al. ( Reference Liu, Wang and Chen 62 ) observed that animals treated with ovalbumin and consumed milk and soya milk kefir, during 28 d, exhibit lower concentrations of IgE and IgG than the control animals. These results suggest the potential of kefir in the prevention of food allergy and in the improvement of mucosal resistance against pathogen infection.
The effect of kefir is not restricted to the modulation of the immune system in the gastrointestinal tract but goes far beyond it. Such effect is a consequence of the micro-organisms and bioactive compounds present in kefir, which positively modulate the composition of the intestinal microbiota and consequently the immune system of the host.
Antioxidative activity of kefir
Harmful biological effects of reactive oxygen species in vivo are controlled by a broad spectrum of antioxidant defence mechanisms, including dietary compounds and enzymes with antioxidant activity.
According to Güven et al. ( Reference Güven, Güven and Gülmez 25 ), in a toxicity test with carbon tetrachloride (CCl4) in rodents, kefir exerted a higher antioxidant effect than vitamin E. Additionally, Ozcan et al. ( Reference Ozcan, Kaya and Atakisi 82 ) evaluated the effect of kefir supplementation in rodents induced to oxidative stress by the use of Pb. After 6 weeks of treatment, the consumption of kefir increased glutathione peroxidase and reduced malondialdehyde to levels comparable with those of the non-induced group. The results support the hypothesis that kefir is a potential tool in the control of oxidative stress.
Liu et al. ( Reference Liu, Lin and Chen 83 ) evaluated the antioxidant activity of kefir prepared from goat and cow milk. The authors reported the great ability of kefir to bind the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and superoxide radicals, besides the inhibition of linoleic acid peroxidation. In this situation, the antioxidative activity of kefir can reduce DNA damage, which explains its anticarcinogenic potential( Reference Grishina, Kulikova and Alieva 84 ).
It is known that the increased concentration of free radicals has a strong relationship with an increased risk of chronic diseases. Therefore, kefir consumption should be encouraged since it is a natural source of antioxidant compounds and also stimulates the activity of enzymes of the antioxidant system.
Anticarcinogenic activity of kefir
The regular consumption of kefir is able to positively modulate the composition of the intestinal microbiota and immune system of its host. Therefore, it is believed that this fermented milk may play an important role in the modulation of carcinogenesis.
Hosono et al. ( Reference Hosono, Tanabe and Otani 85 ) observed that all bacterial strains isolated from kefir had a remarkable ability of binding to mutagens (>98·5 %), which could be further eliminated with the faeces, protecting the colonocytes from damage. Also, Khoury et al. ( Reference Khoury, El-Hayek and Tarras 86 ) showed the ability of kefir to inhibit proliferation and induce apoptosis in HT 29 and Caco 2 colorectal cancer cells. Here, kefir was able to induce cell cycle arrest at the G1 phase, decrease mRNA expression of transforming growth factor-α (TGF-α) and transforming growth factor-β1 (TGF-β1) in HT 29 cells, and to up-regulate protein expression of Bax:Bcl-2 ratio and p53-independent-p2, indicating its pro-apoptotic effect in vitro. Therefore, the regular consumption of kefir can decrease the risk for colon cancer development; however, more studies are needed to understand the mechanisms of action involved in this process.
Also, in a murine breast cancer model, de Moreno de Leblanc et al. ( Reference de Moreno de LeBlanc, Matar and Farnworth 87 ) showed that the antitumour effect of kefir was related to the immune response in mammary gland of mice. Additionally, oral administration of milk and soya milk kefir in mice inoculated with sarcoma 180 ascites tumour resulted in inhibition of 64·8 and 70·9 % of tumour growth, respectively, compared with administration of unfermented milk. Furthermore, kefir was able to induce cell lysis by apoptosis and increase levels of IgA in the intestinal mucosa of animals after 30 d of consumption. These data suggest that kefir is a promising probiotic in cancer prevention( Reference Liu, Wang and Lin 57 ).
The reduction in cancer risk can also be attributed to the presence of some polysaccharides and bioactive compounds, such as specific proteins and peptides, present in kefir. In this way, water-soluble polysaccharides of kefir grains showed a protective effect against pulmonary metastasis, whereas the water-insoluble polysaccharide fraction inhibited melanoma metastasis in mice( Reference Lopitz-Otsoa, Rementeria and Elguezabal 2 ). Additionally, the bioactive compounds of kefir can prevent cancer initiation or suppress the initiated tumour growth by hindering certain enzymes, avoiding the conversion of procarcinogens to carcinogens( Reference Ahmed, Wang and Ahmad 36 ).
The antimutagenic activities of milk, yogurt and kefir were compared using the Ames test. Kefir showed a significant reduction in the mutagenicity induced by methyl methanesulfonate, sodium azide, and aflatoxin B1, while yogurt and milk reduced mutagenicity to a lesser degree. In kefir, higher levels of conjugated linoleic acid isomers and butyric, palmitic, palmitoleic and oleic acids were found in relation to milk and yogurt, factors that may have contributed to the reported outcomes( Reference Guzel-Seydim, Seydim and Greene 88 ).
Moreover, kefir has shown protective effects against radiation-induced gastrointestinal damage in mice. Diluted kefir solutions were able to protect the crypts from radiation and promote crypt regeneration( Reference Teruya, Myojin-Maekawa and Shimamoto 89 ). Matsuu et al. ( Reference Matsuu, Shichijo and Okaichi 90 ) also verified that kefir protected colonic crypt cells against radiation-induced apoptosis and reduced active caspase-3 expression. Thus, the use of kefir may be an alternative to help cancer patients who undergo radiotherapy.
The possible anticancer effect of milk kefir can be considered systemic, since its regular consumption has a potential in cancer prevention to influence both the gastrointestinal tract and other organs, such as breasts and lungs. This beneficial effect can be a result of the improvement of gut microbiota and immune system associated with the increased consumption of bioactive compounds produced by the kefir microbiota.
Healing action of kefir
Recent studies have explored the beneficial effects of probiotics far beyond the intestine. Some of these novel benefits include healthier skin, improvement of eczema, atopic dermatitis and burns, healing of scars, and rejuvenation( Reference Lew and Liong 91 ).
Rodrigues et al. ( Reference Rodrigues, Caputo and Carvalho 9 ) tested the scar ability of a 70 % kefir and kefiran gel in skin wounds infected with Staphylococcus aureus from Wistar rats. The treatment with kefir and kefiran for 7 d showed a protective effect on connective tissue, greatly improving tissue healing compared with treatment with 5 mg/kg neomycin–clostebol emulsion.
The healing properties of kefir were tested in an animal model with experimental burn and contamination with Pseudomonas aeruginosa. Kefir grains and gels prepared with kefir culture after 24, 48 and 96 h of incubation were evaluated. After 2 weeks of treatment, the wound area and the percentage of inflammation were reduced in animals treated with kefir grains and gel compared with those treated with silver sulfadiazine cream, used for the topical treatment of burns of second and third degrees. In addition, the percentage of epithelialisation and healing in animals treated with kefir was also improved. The authors concluded that treatment with kefir gel was effective in improving outcomes from a severe burn compared with conventional treatment( Reference Huseini, Rahimzadeh and Fazeli 92 ).
The ability of kefir to heal wounds can result from its antimicrobial and anti-inflammatory activities, which may act synergistically contributing to the healing( Reference Huseini, Rahimzadeh and Fazeli 92 ). Thus, the beneficial health effects provided by kefir go far beyond the gastrointestinal tract, contributing to wound healing.
Conclusion
Kefir contains a large variety of beneficial micro-organisms and bioactive compounds, being considered a product with a great potential as a functional food. Kefir could be an interesting alternative as a probiotic drink, since it is safe, can be produced at home, has a low production cost, and can be easily incorporated in the diet. The numerous physiological effects described in the literature and highlighted in the present article support the health-promoting benefits of kefir. However, many questions still need to be answered. The methodological standardisation of studies constitutes an important step to better understand the physiological benefits of kefir. First, it is worth mentioning that the detailed knowledge of kefir composition is still scarce, and needs to be characterised for the understanding of the in vivo physiological effects and for finding new possibilities for kefir application. Second, more animal and human studies demonstrating clear cause and effect of kefir consumption and the reduction of disease risk must be performed. Unfortunately, numerous human studies with kefir and other probiotics have often been poorly designed, frequently driven by costs rather than scientific need. The sample size and period of time of experiment usually are not coherent with the objectives of the study and the analyses performed to verify changes in the metabolic parameters. Therefore, different study designs of experimental and clinical trials with the use of kefir cause difficulties in drawing clear conclusions. There is also a need for good clinical studies targeting specific mechanisms of action to better evaluate and understand the physiological effects of kefir as part of a diet. Third, different manufacturing conditions of kefir may alter the original characteristics of micro-organisms, which therefore may influence their effects on health. The methods of producing kefir, time and temperature of fermentation, type of milk used, different origin of grains, ratio of grains:milk (w/v) and cooling time of the product after fermentation may influence the chemical and microbiological composition of the fermented milk. In this context, it is necessary to better understand the mechanisms of action of kefir in oxidative stress, immune-modulatory action, anti-inflammatory properties, modulation of gut microbiota and maintenance of gut integrity, which can have a beneficial effect on attenuation or delay of the progression of chronic diseases, and thus positively affect human health.
Acknowledgements
We would like to thank the Foundation of Research Support of the Minas Gerais State – Brazil (FAPEMIG), the National Council of Technological and Scientific Development (CNPq – Brazil), and Coordination for the Improvement of Higher Education Personnel (CAPES) for financial support during the writing of this paper.
D. D. R. conducted the review of the literature and drafted the manuscript. M. M. S. D., S. A. R. and L. L. C. performed the literature search and contributed to the writing of the manuscript. L. M. G. and M. C. G. P. provided supervision and critical revision of the manuscript. All authors contributed to and approved the final version of the manuscript.
The authors declare no conflicts of interest.









