β2-1 Fructans are polymers of fructose and occur naturally in many grains, vegetables and fruits( Reference Singh and Singh 1 , Reference Coussement 2 ). As human digestive enzymes are unable to cleave the β2-1 bond, these polymers pass into the colon where they are metabolised by gut bacteria( Reference Bouhnik, Raskine and Simoneau 3 , Reference Gibson and Roberfroid 4 ). β2-1 Fructans are widely used in processed foods because of their effects on product rheology, texture and baking characteristics( Reference Singh and Singh 1 ). β2-1 Fructans are also claimed to improve health because of their actions as prebiotics( Reference Gibson and Roberfroid 4 , Reference Schaafsma and Slavin 5 ). The potential health-promoting effects of prebiotics are thought to result from the selective growth stimulation of colonic bifidobacteria and lactobacilli( Reference Gibson and Roberfroid 4 ). Possible benefits attributed to stimulating growth of these bacteria in the colon include an enhanced ability of the host to exclude pathogens from the gut, acidification of the colon due to lactic acid production and stimulation of the host immune system( Reference Looijer-van Langen and Dieleman 6 , Reference Lomax and Calder 7 ). These broadly based claims imply that healthy subjects will benefit from increased β2-1 fructan intakes( Reference Brooks and Kalmokoff 8 ).
In rodents, β2-1 fructans elicit a variety of physiological effects including increased butyrate excretion( Reference Le Blay, Michel and Blottiere 9 ), lowered concentrations of serum lipids( Reference Kok, Taper and Delzenne 10 ), changes in gut nitrogen cycling( Reference Younes, Garleb and Behr 11 ) and increased caecal tissue accretion( Reference Campbell, Fahey and Wolf 12 ). Their rapid fermentation in the caecum also affects gut barrier function, resulting in increased translocation of gut bacteria, mucin sloughing and irritation of the lower gut epithelial surface( Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink 13 , Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink 14 ). Increased mucin sloughing has been observed in one human β2-1 fructan study( Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink 15 ), but not in a second( Reference Scholtens, Alles and Willemsen 16 ). In humans, both positive and neutral effects have been reported on both serum lipid concentrations( Reference Davidson, Maki and Synecki 17 , Reference Wong, Kendall and de Souza 18 ) and bowel movement regularity( Reference Scholtens, Alles and Willemsen 16 , Reference Slavin and Feirtag 19 , Reference Dahl, Whiting and Isaac 20 ). β2-1 Fructan supplementation has also been associated with a wide range of minor gastrointestinal (GI) complaints such as increased flatulence, bloating and diarrhoea( Reference Scholtens, Alles and Willemsen 16 , Reference Lomax, Cheung and Tuohy 21 ).
The implications of a stimulatory immune effect of β2-1 fructans in humans are unclear( Reference Lomax and Calder 7 ). Much of the supporting data have been obtained using rodent models( Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink 14 , Reference Bovee-Oudenhoven, Ten Bruggencate and Lettink-Wissink 22 , Reference Ryz, Meddings and Taylor 23 ), and only a few studies have been conducted in healthy human subjects( Reference Lomax, Cheung and Tuohy 21 , Reference Seidel, Boehm and Vogelsang 24 ). For example, increased faecal IgA concentrations have been observed in feeding studies involving mice or human infants( Reference Nakamura, Nosaka and Suzuki 25 , Reference Scholtens, Alliet and Raes 26 ), but not in healthy adults( Reference Ramnani, Costabile and Bustillo 27 ). Increases in certain immune cell populations and cytokine levels have been observed in the Peyer’s patches of rodents fed β2-1 fructans( Reference Ryz, Meddings and Taylor 23 , Reference Roller, Rechkemmer and Watzl 28 ), whereas in humans increases in circulating B cells in combination with decreases in both intercellular adhesion molecule-1 and percentages of natural killer (NK) cells were observed( Reference Seidel, Boehm and Vogelsang 24 ). In contrast, a more recent trial on healthy adults found that β2-1 fructan supplementation had no effects on circulating Ig concentrations, immune cell subsets or on neutrophil, monocyte and NK cell activities, indicating minimal impact of β2-1 fructans on the immune system in the absence of an immune challenge( Reference Lomax, Cheung and Tuohy 21 ).
β2-1 Fructans may have an effect of modulating inflammation associated with various pathological conditions( Reference Looijer-van Langen and Dieleman 6 ). For example, in women with type 2 diabetes, circulating concentrations of the pro-inflammatory cytokine TNF-α and of lipopolysaccharide (LPS) decreased following 8 weeks of β2-1 fructan supplementation( Reference Dehghan, Gargari and Jafar-Abadi 29 ), whereas in Crohn’s disease patients feeding β2-1 fructans increased the numbers of lamina propria dendritic cells (DC) producing IL-10 and expressing toll-like receptor (TLR) 2 and 4 and reduced disease activity as determined by the Harvey Bradshaw index, although some symptoms increased( Reference Lindsay, Whelan and Stagg 30 ).
The most consistent response to β2-1 fructan supplementation has been increases in faecal bifidobacteria content( Reference Lomax, Cheung and Tuohy 21 , Reference Bouhnik, Raskine and Simoneau 31 ). However, this change in the gut microbial community has yet to be linked to a repeatable health outcome in humans that would support a defined health benefit. In this study, we report the results from a randomised, double blind, cross-over clinical trial investigating the physiological, psychological and immunological impacts of β2-1 fructan supplementation in thirty healthy adults. The impact of β2-1 fructan supplementation on change within the faecal microbial communities will be reported in a separate publication.
Methods
Subjects
Subjects were recruited by a newspaper advertisement. Individuals interested in participating attended a clinical orientation and provided their written informed consent to take part in the study (Fig. 1). Subjects first completed a clinical orientation where their health status was assessed and evaluated against inclusion/exclusion criteria. Inclusion criteria were as follows: males and females, aged 18–50 years, in good general health and a BMI of 18–30 kg/m2. Exclusion criteria were as follows: subjects taking medication or any health supplement, regular ingestion of high inulin-containing food products, having known GI diseases, food allergies or intolerance to any ingredients in the supplements. In all, thirty subjects (seventeen women and thirteen men) were enrolled to the study and completed both phases. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the research ethics boards at Health Canada, McMaster University, University of Ontario Institute of Technology and by the Canadian SHIELD Ethics Review Board. The study was registered as ‘Effects of fructan prebiotics on the intestinal microbiota’ at www.ClinicalTrials.gov (clinical trial identifier NCT01277445). Clinical visits, enrolment, examination of the participants and generation of random treatment allocations were all carried out at Nutrasource Diagnostics Inc., Guelph, Ontario, Canada. The clinical trial was initiated on 15 November 2010 and was completed on 5 April 2011.

Fig. 1 Consolidated Standard of Reporting Trials diagram: progress of subjects through the trial.
Study design
The present study followed a double-blind, placebo-controlled, randomised, cross-over design having two 28-d supplementation periods separated by a minimum 14-d washout period. Individually packaged supplements (5 g) consisted of β2-1 fructan (Orafti® Synergy1 (BENEO), 50:50 mixture of inulin and short-chain oligosaccharides; 15 g/d total, 3×5 g ingested with each meal) or placebo (maltodextrin; 15 g/d total, 3×5 g ingested with each meal). Investigators and subjects were blinded to the identity of the supplement or placebo, and subjects were randomised based on the order in which they were to receive either supplement (A or B). The randomisation code (Seed no. 26285) was generated using an online programme (Tufts University, www.tufts.edu/~gdallal/random_block_size.htm). Subjects ingested either pre-packaged supplement in its entirety by dissolving the contents contained in each envelope in coffee, tea, a beverage of choice or in their food at each meal. Subjects returned any unopened supplement envelopes. Compensation occurred on day 28 of each phase.
Subjects were provided with a booklet outlining how information pertaining to the study would be collected as well as a diary to record bowel functions (the frequency of daily bowel movements) and to record self-reported adverse GI events (gas, flatulence, diarrhoea, bloating, stomach ache, heartburn and abdominal cramping) or other daily events (variations in normal activity, stress, changes in exercise patterns, medications, headaches and non-GI events such as menstrual cramps, head cold, etc.). Materials and instructions for the collection and storage of 1-d faecal samples were also provided. Subjects were instructed to fast for 12 h before each clinical visit, and to return unopened envelopes as well as their frozen faecal samples at the start and end of each supplement arm. Subjects completed the health-related quality-of-life short form 36 survey (HRQoL SF-36, www.sf-36.org), the General Health Questionnaire-28 (GHQ-28)( Reference Goldberg 32 ) and the Gastrointestinal Symptom Rating Scale (GSRS)( Reference Revicki, Wood and Wiklund 33 – Reference Kulich, Madisch and Pacini 35 ).
Subjects made six visits over 3 months, including at the initiation (day 1) and at the end of each phase (day 28), as well as one bi-weekly, non-clinical visit within each phase to receive a 2-week supply of envelopes and a faecal collection kit. One day before each clinical visit, subjects stored their faecal samples in their own freezer. On arrival at the clinic, faeces were stored at −20°C, and a 20-ml fasting blood sample was collected. Subjects then submitted their diaries and unconsumed supplements, and at the end of each phase they completed the HRQoL, GHQ-28 and GSRS surveys. Compliance was assessed on the basis of the returned unopened supplement envelopes.
Estimates of dietary β2-1 fructan intake
Dietary β2-1 fructan intakes were estimated for each subject based on a semi-quantitative FFQ, completed at the end of each phase, which included the most common β2-1 fructan-containing foods( Reference Moshfegh, Friday and Goldman 36 ). Average portion sizes were obtained from the Canadian Community Health Survey (http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/cchs_focus-volet_escc-eng.php), and the midpoint of reported ranges was used to calculate consumption in g/d dietary β2-1 fructan( Reference Moshfegh, Friday and Goldman 36 ).
Impact of β2-1 fructans on faecal bifidobacteria populations
DNA was isolated from individual faecal samples as previously described( Reference Brooks, McAllister and Sandoz 37 ). Total bacteria and bifidobacteria contents were determined by quantitative-PCR using the Agilent Brilliant Ultra-fast SYBR green master mix kit (Agilent Technologies) and an Applied Biosystems ViiA real-time instrument (Applied Biosystems). Quantification of faecal eubacteria was carried out using the primer set HDA1/HDA2 (V2-3 region of the 16S rRNA gene) as previously described( Reference Abnous, Brooks and Kwan 38 ). For bifidobacteria, the primer set Bif582 (5'-GGTGTGAAAGYCCATCGC) and Bif815 (5'-CACATCCAGCRTCCACCG) targeting the 16S rRNA gene was used at a concentration of 5 µm and an annealing temperature of 58°C. For each sample, three technical replicates were run with copy numbers determined by comparison against a standard curve prepared from cloned 16S rRNA genes (Escherichia coli or Bifidobacterium animalis). Bifidobacteria abundance was expressed as a percentage of the total bacterial community (bifidobacteria 16S rRNA copy number/HDA1/HDA2 copy number) within each sample.
Faecal analyses
Faecal SCFA and branched-chain fatty acid (BCFA) concentrations were determined as previously described( Reference Scheppach, Fabian and Kasper 39 ). Faecal β2-1 fructan content (g/100 g faecal dry weight) was measured using the 0–12 % fructan assay procedure recommended in the Fructan Assay Kit (Megazyme).
Blood analysis
Blood analyses were carried out by LifeLabs Medical Services (Toronto, Ontario, Canada). Measurements included serum biochemistry (fasting glucose, creatinine, aspartate aminotransferase, γ-glutamyl-transferase, C-reactive protein (CRP), total globulin, albumin and total blood protein), lipid profiles (total cholesterol, LDL-cholesterol, HDL-cholesterol and TAG) and standard haematological profiles. Blood urea nitrogen was determined in serum as previously described( Reference Kalmokoff, Zwicker and O’Hara 40 ). Serum LPS concentrations were determined using the Limulus Amebocyte Lysate Assay QCL-100 (Lonza) according to the manufacturer’s procedure.
Cell population analysis
Immune cell populations were determined by flow cytometry and immunofluorescence (online Supplementary Table S1). Flow cytometry was performed on a BD FACSCalibur™ flow cytometer (BD Biosciences) equipped with a blue laser (488 nm) and a red diode laser (635 nm). Data were analysed using BD FACSDIVA™ software (BD Biosciences). Blood samples were first treated with the Immunoprep™ Reagent System (Beckman Coulter) to remove erythrocytes before staining.
In a subset of six randomly selected subjects, whole blood was stimulated with 1 μg/ml LPS (Sigma-Aldrich) and incubated at 37°C, 5 % CO2 to investigate functional TLR responses in DC and macrophages by measuring intracellular cytokine production. Following 1 h incubation with LPS, 1 mg/ml Brefeldin A (Sigma-Aldrich) was added to each sample to block cytokine secretion, and samples were further incubated at 37°C, 5 % CO2 for 2 h. Cells were collected by centrifugation and permeabilised to allow direct intracellular staining with anti-cytokine antibodies to measure production of intracellular IL-12p70 and TNF-α by flow cytometry. Antibodies used for intracellular cytokine determinations are also listed in the online Supplementary Table S1.
Cytokine profiles
Circulating cytokines were quantified by ELISA. The following cytokines were measured in serum previously frozen at −80°C: granulocyte-colony stimulating factor, granulocyte-macrophage colony stimulating factor (GM-CSF), interferon-γ (IFN-γ), IL receptor antagonist, IL-1β, IL-4, IL-6, IFN-γ-induced protein 10, soluble CD40 ligand/TNFSF5 (R&D Systems), IL-10, IL-12p70, TNF-α (BioLegend) and IL-8 (Invitrogen). Concentrations of active and total transforming growth factor- β were measured in plasma (R&D Systems). All cytokines were quantified by ELISA according to the manufacturer’s procedures, and ELISA plates were read at 450 nm using a Synergy HTTR microplate reader (BioTek).
Analysis of toll-like receptor agonist-induced responses of peripheral blood cells
An ex vivo protocol was utilised to investigate TLR responsiveness to antigens. Whole blood was collected in heparin-containing tubes at the end of each supplement phase, and was stimulated with either the TLR2 agonist Pam3Cys (P3C) (Novabiochem) or the TLR4 agonist LPS to analyse cytokine production profiles and assess alterations in TLR agonist sensitivity. Whole blood was stimulated in 5-ml polypropylene round-bottom tubes with either 1 μg/ml LPS or with 10 μg/ml P3C reconstituted in Roswell Park Memorial Institute (RPMI) 1640 medium. The tubes were subsequently incubated at 37°C in a 5 % CO2-humidified incubator for 20 h with an unstimulated negative control for each individual. Supernatants were collected from these samples and stored at −80°C until analysis. Cytokine profiles were investigated using the BD Cytometric Bead Array Human flex set (BD Biosciences) containing IFN-α, IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF-α. Flow cytometric acquisition was performed on a BD LSRFortessa™ flow cytometer (BD Biosciences) equipped with multiple lasers (blue 488, red 640 and violet 405).
Ig profiles
Serum Ig levels were quantified with the human Ig isotyping magnetic bead panel (MILLIPLEX Map Kit; Millipore) using a Luminex 200 analyzer. Faecal IgA, IgG and IgM were quantified by ELISA (Bethyl Laboratories Inc.). Samples were prepared in phosphate-buffered saline (PBS) containing 10 mg/ml bovine serum albumin and 1 % Protease Inhibitor Cocktail (Sigma-Aldrich). Thawed faeces samples were mixed with PBS solution at a ratio of 1:5 (w/v), incubated for 10 min at room temperature, homogenised using a Brinkmann Polytron PT3000 homogenizer (Kinematica) and centrifuged (4000 g for 30 min at 20°C). Supernatants were collected and stored at −80°C. ELISA plates were read at a wavelength of 450 nm (Infinite® 200 PRO series; Tecan).
Statistical analyses
In most instances, data analyses were performed using repeated-measures ANOVA with time (day 0 and day 28) and supplement (placebo and fructan) as the repeated variables (Statistica version 12; StatSoft). In the case of peripheral blood immune cell phenotypes and SCFA (absence of day 0 values), data were also analysed by repeated-measures ANOVA but with supplement as the only repeated measure. When significant (P<0·05), differences among groups were tested using a Tukey’s honest significant difference (HSD) test. For some measures, there was a high proportion of individuals with values below the limit of quantification (LoQ). In these cases, the value was set to LoQ/2( Reference Armbruster, Tillman and Hubbs 41 ) and the groups were analysed using the non-parametric Friedman’s test (one-way repeated-measures ANOVA by ranks) followed by a Wilcoxon’s matched-pairs signed-rank test to determine differences and corrected for false discovery rate. Values are reported as means with their standard errors. For ANOVA where P>0·05, results are reported as non-significant. Dependent variables were assessed for normality by four methods: visual inspection of a means v. variance plot, inspection of the distribution of residuals, Levene’s test and significance of the λ parameter after Box–Cox transformation. If required, data were normalised by Box–Cox transformation before parametric tests.
Survey-based results (estimates of dietary β2-1 fructan intakes, self-reported adverse events, HRQoL, SF-36, GHQ and GSRS) were also analysed using a Friedman’s test with post hoc testing carried out using a Wilcoxon’s matched-pairs signed-rank test corrected for false discovery rate. Associations between bifidobacteria, physiological and immune parameters (continuous variables) were assessed by Pearson’s (linear) correlation analysis. When multiple pair-wise comparisons or correlations were performed, the P values were corrected for false discovery rate using a web-based correction calculator (http://www.sdmproject.com); P<0·05 was considered to be significant.
Results
Subject characteristics
The baseline characteristics of the subjects are presented in Table 1 and online Supplementary Table S2. In total, thirty adults (thirteen male, seventeen female) completed both supplement phases. All thirty subjects had good health status before starting the trial. Compliance (number of supplements used/total given) in the placebo phase was 97·6 (sem 0·5) % and in the β2-1 fructan phase 99·9 (sem 6·9) %. No change in cohort BMI occurred over the course of the trial. No significant differences were found in estimates of dietary β2-1 fructan daily intakes in subjects across the cohort (1·09 g/d in placebo v. 0·96 g/d for β2-1 fructan phase; P=0·15; two-tailed t test; online Supplementary Table S3).
Table 1 Participant characteristicsFootnote * (Mean values, standard deviations and 95 % confidence intervals)
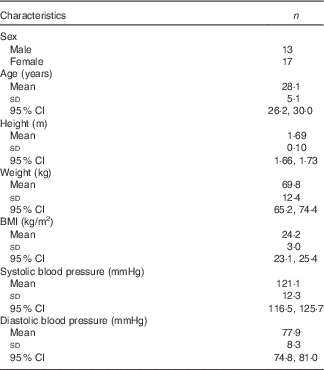
* Data are shown for subjects who completed the study (n 30).
Well-being and gut symptomology
GI symptoms and well-being were assessed using three different surveys completed during the clinical orientation (baseline) and at the end of each supplement phase. The GHQ-28 was used to assess psychiatric stress. No significant effect was associated with either supplement (P=0·84; Fig. 2(a)) nor were there any significant differences in the different domains within the survey (Fig. 2(b)). Significant differences were found with the GSRS, used to assess the effect of treatment on gastrointestinal symptoms. β2-1 Fructan consumption increased GI symptoms compared with the placebo and baseline (P=0·04; Fig. 3(A)). Analysis of the distinct domains of the GSRS (Fig. 3(B)) indicated that β2-1 fructan significantly increased indigestion (P<0·001) and there was a trend towards increased abdominal pain (P=0·05) compared with the baseline and placebo. No significant changes were noted in quality of life as assessed using the HRQoL SF-36 questionnaire (P=0·63 for the physical component and P=0·42 for the mental component; online Supplementary Fig. S1). β2-1 Fructan consumption was associated with higher frequency of self-reported adverse GI events such as gas, bloating and cramping (P<0·001), specifically a higher frequency of GI issues (P=0·01) and headaches (P=0·043; Table 2). No effect on the frequency of daily bowel movements was observed (placebo 1·61 (sem 0·76) v. β2-1 fructan 1·67 (sem 0·70), P=0·31).

Fig. 2 (a) Assessment of emotional distress in subjects at baseline (![]() ) and following supplementation with placebo (
) and following supplementation with placebo (![]() ) or β2-1 fructan (
) or β2-1 fructan (![]() ) based on results derived from the General Health Questionnaire-28 (GHQ-28) questionnaire. Values are means (n 30), and standard errors represented by vertical bars. The total possible scores for the GHQ-28 ranges from 0 to 84, with higher scores indicating greater distress. No significant differences were found between baseline and either treatment (P=0·84). (b) Comparison of individual domains within the GHQ-28 questionnaire, including somatic symptoms, anxiety and social dysfunction: baseline (
) based on results derived from the General Health Questionnaire-28 (GHQ-28) questionnaire. Values are means (n 30), and standard errors represented by vertical bars. The total possible scores for the GHQ-28 ranges from 0 to 84, with higher scores indicating greater distress. No significant differences were found between baseline and either treatment (P=0·84). (b) Comparison of individual domains within the GHQ-28 questionnaire, including somatic symptoms, anxiety and social dysfunction: baseline (![]() ), placebo (
), placebo (![]() ) and β2-1 fructan (
) and β2-1 fructan (![]() ). No significant differences were found in any of the four domains.
). No significant differences were found in any of the four domains.

Fig. 3 (A) Assessment of gastrointestinal symptoms in subjects at baseline (![]() ) and following supplementation with placebo (
) and following supplementation with placebo (![]() ) or β2-1 fructan (
) or β2-1 fructan (![]() ) based on results derived from the Gastrointestinal Symptom Rating Scale (GSRS) questionnaire. Values are means (n 30), and standard errors represented by vertical bars. Higher scores indicate greater symptom severity. a,b Mean values with unlike letters were significantly different between baseline and supplements (P=0·04), as determined by a Wilcoxon’s matched-pairs signed-rank test. (B) Comparison of individual domains within the GSRS questionnaire, including abdominal pain, reflux, diarrhoea, indigestion and constipation: baseline (
) based on results derived from the Gastrointestinal Symptom Rating Scale (GSRS) questionnaire. Values are means (n 30), and standard errors represented by vertical bars. Higher scores indicate greater symptom severity. a,b Mean values with unlike letters were significantly different between baseline and supplements (P=0·04), as determined by a Wilcoxon’s matched-pairs signed-rank test. (B) Comparison of individual domains within the GSRS questionnaire, including abdominal pain, reflux, diarrhoea, indigestion and constipation: baseline (![]() ), placebo (
), placebo (![]() ) and β2-1 fructan (
) and β2-1 fructan (![]() ). β2-1 Fructans significantly increased indigestion (P<0·001) and there was a trend towards increased abdominal pain (P=0·05).
). β2-1 Fructans significantly increased indigestion (P<0·001) and there was a trend towards increased abdominal pain (P=0·05).
Table 2 Self-reported adverse gastrointestinal (GI) symptoms and other adverse events in subjects receiving placebo or β2-1 fructan (n 30)
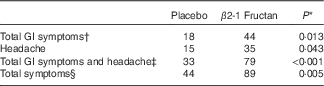
* Values differ significantly as determined by the Wilcoxon’s matched-pairs signed-rank test.
† Total GI symptoms include abdominal cramping and pain, bloating, constipation, diarrhoea, flatulence, gas, heartburn, indigestion and reflux.
‡ Includes total GI symptoms and headache.
§ All symptoms reported including GI symptoms, non-GI symptoms (allergies, sore throat, congestion, menstrual cramps) and headache.
Faecal bifidobacteria populations, SCFA concentrations and residual β2-1 fructan
β2-1 Fructan supplementation increased the content of faecal bifidobacteria 16S rRNA genes by almost 3-fold (1·93 (sem 0·35) v. 5·71 (sem 1·00) %; n 30; P<0·001; Fig. 4; online Supplementary Table S4). No correlation between the increased faecal bifidobacteria content and any of the measured parameters was found. β2-1 Fructan supplementation significantly increased total faecal SCFA concentrations (89·54 (sem 5·8) v. 81·62 (sem 6·2) µmol/g wet weight for placebo; P<0·001), increasing the proportion of propionate and butyrate at the expense of acetate and reducing the proportions of BCFA with the exception of heptanoic acid (Fig. 5; online Supplementary Table S5). Average residual β2-1 fructan content of faeces was higher following the placebo phase (0·10 (sem 0·42) v. 0·04 (sem 0·04)g/100 g dry weight for the prebiotic phase), although this difference was not significant (P=0·19; one tailed t test).

Fig. 4 Change in the abundance of faecal bifidobacteria over the duration of each supplement phase: placebo (![]() ), β2-1 fructan (
), β2-1 fructan (![]() ). Box and whisker plot indicating the mean values and standard errors and the means±1·96×standard errors for each determination. Faecal bifidobacteria content was significantly different (P<0·001). a,b Mean values with unlike letters were significantly different at P<0·05 level as determined by Tukey’s HSD. Corresponding data are available in online Supplementary Table S4.
). Box and whisker plot indicating the mean values and standard errors and the means±1·96×standard errors for each determination. Faecal bifidobacteria content was significantly different (P<0·001). a,b Mean values with unlike letters were significantly different at P<0·05 level as determined by Tukey’s HSD. Corresponding data are available in online Supplementary Table S4.

Fig. 5 Change in faecal SCFA and branched-chain fatty acid (BCFA) proportions at the end of the placebo (![]() ) and β2-1 fructan (
) and β2-1 fructan (![]() ) supplement phase. Values are means (n 30), and standard errors represented by vertical bars. a Values are expressed as a percentage of the total SCFA/BCFA content; those demonstrating a significant increase or decrease in relative proportion. Individual SCFA/BCFA proportions under both phases are available in online Supplementary Table S5.
) supplement phase. Values are means (n 30), and standard errors represented by vertical bars. a Values are expressed as a percentage of the total SCFA/BCFA content; those demonstrating a significant increase or decrease in relative proportion. Individual SCFA/BCFA proportions under both phases are available in online Supplementary Table S5.
Blood biochemistry
β2-1 Fructan supplementation had no effect on any blood biochemistry analytes or haematological profiles (online Supplementary Table S6). As an indirect measure of bacterial translocation, serum LPS concentrations were determined (Table 3). Serum LPS concentrations were significantly higher at the end of the β2-1 fructan phase compared with the placebo phase and day 0 of the β2-1 fructan phase (P=0·03).
Table 3 Circulating lipopolysaccharide (LPS), cytokine and chemokine concentrations in subjects receiving placebo or β2-1 fructan supplements (Mean values with their standard errors, n 30)

EU, endotoxin units; sCD40L, soluble CD40 ligand; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; IFN, interferon; IL-1Ra, receptor antagonist; <LoQ, below limit of quantitation; IP-10, IFN-γ-induced protein 10; TGF, transforming growth factor.
* P values determined by repeated-measures ANOVA. a,b,c Mean values within a row with unlike superscript letters were significantly different as determined by Tukey’s HSD
† P values determined by the Friedman’s test. a,b,c Mean values within a row with unlike superscript letters were significantly different as determined by Wilcoxon’s matched-pairs signed-rank test.
Circulating immune cell populations and cytokine profiles – serum and faecal Ig concentrations
There were no differences in T or B lymphocyte populations between phases (Table 4). However, percentages of CD282+/TLR2+ myeloid dendritic cells (mDC) (P=0·008) were higher following the β2-1 fructan phase. A trend towards higher percentages of CD284+/TLR4+ granulocytes (P=0·05) and CD284+/TLR4+ mDC (P=0·06) was also observed. Certain serum cytokines associated with key T cell subsets and with pro-inflammatory and regulatory immune activities showed significant changes after β2-1 fructan supplementation (Table 3). β2-1 Fructan consumption lowered concentrations of regulatory cytokine IL-10 (P<0·001) but increased concentrations of T helper 2 cytokine IL-4 (P<0·001) and pro-inflammatory cytokine GM-CSF (P=0·045).
Table 4 Peripheral blood immune cell phenotypes at the end of placebo or β2-1 fructan phases (Mean values with their standard errors, n 30)

pDC, plasmacytoid dendritic cells; mDC, myeloid dendritic cells.
* Determined by leucocytes differential count on a Beckman Coulter instrument.
The effects of treatment on serum and faecal Ig concentrations are shown in Table 5. Although significant differences were found in serum and faecal Ig concentrations over the course of the trial, these differences did not directly correlate with either treatment phase. The results for serum Ig concentrations were confirmed by retesting samples from six randomly selected subjects using ELISA.
Table 5 Serum and faecal Ig concentrations in subjects receiving placebo or β2-1 fructan supplements (Mean values with their standard errors, n 30)

a,b,c Mean values within a row with unlike superscript letters were significantly different P<0·05 level as determined by Tukey’s HSD.
* n 29.
Responses of ex vivo toll-like receptor agonist stimulation
P3C-induced IL-10 production was higher following the β2-1 fructan phase (P=0·016) but LPS-induced IL-10 production was unaffected by either supplement (Table 6). LPS (TLR4 agonist) induced higher concentrations of TNF-α than did stimulation with P3C (TLR2 agonist), although there was no significant effect associated with treatment phase. IFN-α remained below the level of detection after TLR agonist stimulation, whereas IL-12p70 was detected in only one subject after P3C stimulation in whole blood culture collected at the end of the β2-1 fructan phase. To further examine cytokine production in response to TLR activation at the cell-specific level, intracellular cytokine accumulation was measured in mDC and monocytes using flow cytometry following LPS stimulation of whole blood. Intracellular production of TNF-α and IL-12p70 in mDC and monocytes was measured in a subgroup of six subjects at phase end points. Although stimulation with LPS-induced TNF-α production in mDC and monocytes, no differences were found between treatments (P=0·94). Intracellular IL-12p70 concentrations following stimulation were minimal (data not shown).
Table 6 Ex vivo cytokine production following stimulation of toll-like receptors in whole blood collected from subjects at the end of placebo or β2-1 fructan phases (lipopolysaccharide (LPS) stimulation (n 30), Pam3Cys (P3C) stimulation (n 24)) (Mean values with their standard errors)
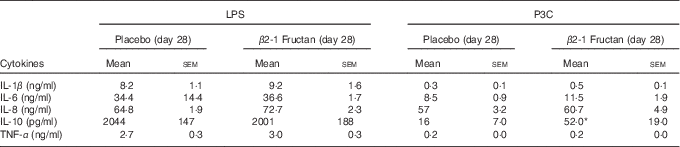
* Significantly different from the corresponding placebo group (P=0·016) as determined by a Wilcoxon’s matched-pairs signed-rank test.
Discussion
β2-1 Fructans are widely claimed to be health promoting, although there is little evidence to support this, particularly regarding their use in healthy subjects. In order to identify potential health benefits, we investigated their impact on a wide range of factors relating to the psychological, physiological and immunological status of healthy adults. We selected a commercially available product (Orafti® Synergy1) at an intake level recommended by the manufacturer. Supplementation at 15 g/d resulted in intakes well above estimates of daily dietary intakes for North Americans (1–4 g/d)( Reference Coussement 2 ). Nevertheless, the material was completely fermented, suggesting that the colonic fermentation capacity was not exceeded. β2-1 Fructan supplementation significantly increased the content of faecal bifidobacteria across the cohort, in agreement with previous findings( Reference Lomax, Cheung and Tuohy 21 , Reference Lindsay, Whelan and Stagg 30 ). Under the current definition for prebiotics, any potential health claim would require that this change in the gut microbiota be causally linked with a measurable and consistent response that could reasonably be considered to represent an improvement in health. An association would be considered supportive evidence( Reference Brooks and Kalmokoff 8 ).
The intakes of the present study did, however, suggest that 15 g/d exceeds the average human tolerance for this material, as indigestion was significantly higher and there was a trend towards increases in other minor GI complaints. Although the results from the GSRS survey are retrospective, they were consistent with the increased incidence of GI complaints based on the analysis of the daily diaries. Similar complaints have been reported in other trials involving healthy subjects with β2-1 fructan supplementation at 20 g/d for 21 d( Reference Slavin and Feirtag 19 ), 8 g/d for 4 weeks( Reference Lomax, Cheung and Tuohy 21 ) and 5–20 g/d for 2 weeks( Reference Bruhwyler, Carreer and Demanet 42 ). In contrast to previous studies involving β2-1 fructan supplementation (25–30 g/d for 2 weeks( Reference Scholtens, Alles and Willemsen 16 ), 13 g/d for 3 weeks( Reference Dahl, Whiting and Isaac 20 ) and 8 g/d for 4 weeks( Reference Lomax, Cheung and Tuohy 21 )), we found no difference in regularity under either supplement. An unusual finding was the association of β2-1 fructan with an increased incidence of headaches. However, gut health and overall host well-being are connected through the gut–brain axis( Reference Collins and Bercik 43 ). The impact of supplementation on perceptions of well-being and overall health represents an important but often ignored dimension. Changes in host well-being are used to clinically assess treatment efficacy in various disease states, and the relationship between GI health and sense of well-being is well documented( Reference De Palma, Collins and Bercik 44 ). Note that, despite the minor complaints associated with β2-1 fructans, we found no significant changes in the subject’s general sense of well-being or perceptions of overall health between the β2-1 fructan or placebo treatment phases.
β2-1 Fructan supplementation moderately, although significantly, altered total faecal SCFA and BCFA concentrations. We found that the proportions of propionate and butyrate tended to increase at the expense of acetate, whereas others have reported increases in acetate at the expense of butyrate( Reference Scholtens, Alles and Willemsen 16 ) or no changes at all( Reference Slavin and Feirtag 19 ). Increased SCFA concentrations occur in response to increased dietary fibre, and this effect is not specific to β2-1 fructans. The proportions of most BCFA also moderately decreased in response to β2-1 fructan supplementation. BCFA are formed during the fermentation of branched-chain amino acids and represent a marker for protein fermentation in the gut( Reference Cummings and Macfarlane 45 ). The reduction in gut protein fermentation is consistent with a previous study( Reference Geboes, De Hertogh and De Preter 46 ), and likely results from a shift in the balance of available gut peptidyl nitrogen sources towards microbial growth at the expense of energy production (i.e. protein fermentation) due to very rapid fermentation in the proximal colon. This effect is also not likely specific to β2-1 fructans, and can be expected to occur with other soluble rapidly fermented polymers. Finally, as in previous studies involving healthy adults( Reference Pedersen, Sandstrom and Van Amelsvoort 47 ), we found that β2-1 fructan supplementation had no effect on blood lipid or cholesterol concentrations.
Changes in Ig concentrations and isotype profiles can serve as a marker for alterations to host adaptive immune activity( Reference Cassidy, Nordby and Dodge 48 , Reference Albers, Antoine and Bourdet-Sicard 49 ). For example, in formula-fed infants, β2-1 fructans appear to increase secretory IgA concentrations( Reference Bakker-Zierikzee, Tol and Kroes 50 ). In common with a previous study in healthy adults( Reference Lomax, Cheung and Tuohy 21 ), we found no association between β2-1 fructan treatment and Ig concentrations or isotype profiles, although we did observe considerable variation in both serum and faecal Ig concentrations over the duration of the trial. However, serum Ig concentrations are subject not only to short-term periodic fluctuations( Reference Veys, Wieme and Van Egmond 51 ) but also vary with sex, ethnicity, age and changes in season( Reference Cassidy, Nordby and Dodge 48 , Reference Nordby and Cassidy 52 , Reference Maddison, Stewart and Farshy 53 ). β2-1 Fructan-associated increases in serum or faecal IgA have been observed in infants following vaccination( Reference Scholtens, Alliet and Raes 26 , Reference Firmansyah, Pramita and Carrie Fassler 54 , Reference Saavedra and Tschernia 55 ), and increases in serum antibody titers against a seasonal flu vaccine were observed in healthy middle-aged adults( Reference Lomax, Cheung and Noakes 56 ). As the present study was carried out in adults under resting conditions, it remains possible that differences might become apparent following an immune challenge. Without stimulating the subject’s immune system by vaccination to induce an adaptive immune response, we were limited to measuring changes in total Ig concentrations. However, it is clear that the observed changes in Ig concentrations of healthy adults were driven by more complex factors than diet alone.
β2-1 Fructan supplementation has been reported to affect other immune parameters in individuals potentially having compromised health. For example, increases in peripheral T cell subsets have been observed in elderly adults supplemented with β2-1 fructans( Reference Guigoz, Rochat and Perruisseau-Carrier 57 ) and in Crohn’s disease patients, where increases in the percentages of TLR2+ and TLR4+ DC producing IL-10 were observed( Reference Lindsay, Whelan and Stagg 30 ). In contrast, others have reported no changes in circulating immune cell populations in healthy adults supplemented with β2-1 fructans( Reference Lomax, Cheung and Tuohy 21 ). Our findings regarding immune cell populations were consistent with the latter study, despite supplementing nearly twice the amount of β2-1 fructan (8 v. 15 g/d). However, in common with the study carried out with Crohn’s patients, we did observe increases in subpopulations of immune cells expressing TLR 2 and 4. Innate immune cells serve as sentinels and are highly sensitive to the presence of microbial components, which are sensed through pattern recognition receptors such as TLR2 and TLR4( Reference Medzhitov, Preston-Hurlburt and Janeway 58 , Reference Yang, Mark and Gray 59 ). Specifically, CD282+/TLR2+ mDC subpopulations were significantly increased, and an upward trend in both CD284+/TLR4+ granulocytes and CD284+/TLR4+ mDC was also observed at the end of the β2-1 fructan phase. Changes in circulating cytokine profiles provide insight into the type of immune activity, and we observed that β2-1 fructan supplementation was associated with lower concentrations of the regulatory cytokine IL-10, but increased concentrations of pro-inflammatory cytokine GM-CSF and of the T helper 2 cytokine IL-4. Changes in circulating cytokine profiles, in combination with increases in percentages of immune cells expressing TLR, are suggestive of encounters of the immune system with pro-inflammatory stimuli. The third approach we used to assess the response of circulating innate immune cells to microbial-derived stimuli was TLR stimulation assays( Reference Ida, Shrestha and Desai 60 , Reference Della Bella, Giannelli and Taddeo 61 ), which provided both a sensitive measure of changes in host innate immunity( Reference Blimkie, Fortuno and Yan 62 ) and insight into the ability of the host to respond to infection( Reference Turvey and Hawn 63 , Reference Barreiro, Ben-Ali and Quach 64 ). A significantly higher concentration of IL-10 induced by the TLR2 agonist P3C was associated with the β2-1 fructan phase. Again, an increased sensitivity towards microbial-derived TLR agonists was consistent with the observed increase in TLR2+ subpopulations.
Although other properties of β2-1 fructans, such as direct TLR stimulation of intestinal epithelial cells, could possibly contribute to these immunological effects( Reference Vogt, Ramasamy and Meyer 65 ), our data suggest otherwise. First, serum LPS concentrations were significantly higher following the β2-1 fructan phase. Circulating LPS is commonly used as an indirect index of gut permeability( Reference Bala, Marcos and Gattu 66 , Reference Bischoff, Barbara and Buurman 67 ). Second, increased translocation of gut bacteria has been previously observed in studies in rats fed diets containing β2-1 fructans – this appears to result from irritation and damage to the gut due to the very rapid fermentation of these substrates in the caecum( Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink 13 , Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink 14 ). Increased TLR expression and heightened sensitivity to TLR agonists coupled with changes in circulating cytokine concentrations in combination with higher serum LPS concentrations all point towards an increase in microbial translocation during the β2-1 fructan supplementation phase. Despite our evidence for increased translocation, we found no significant supplement phase-related change in serum CRP, indicating that alterations to gut barrier function were of non-acute nature.
In conclusion, supplementing healthy adults with β2-1 fructan had no effect on perceptions of health or well-being. However, in common with previous studies, minor self-reported GI events were identified, as well as an association with an increased incidence of headaches. More importantly, β2-1 fructans were found to affect host immune activity, altering circulating regulatory and pro-inflammatory cytokine concentrations, increasing the proportions of immune cells expressing TLR2 and 4 and influencing TLR2 agonist responsiveness in an ex vivo whole blood stimulation assay. These changes were consistent with modulation of the innate immune system resulting from increased contact with microbial-derived stimuli, as also mirrored by higher concentrations of serum LPS. Often, the purported immune-stimulating properties of β2-1 fructans are claimed as beneficial. However, chronic stimulation of the immune system by intestinal luminal contents has also been linked to several intestinal and extra-intestinal disorders including metabolic disease( Reference Bischoff, Barbara and Buurman 67 ). Moreover, although the immunological effects observed in our study can only be described as moderate, this might not be the case in individuals ingesting these supplements over a longer period or in those having pre-existing conditions( Reference Gibson and Shepherd 68 ). Although supplementation with β2-1 fructans did increase the content of faecal bifidobacteria, we found no direct relationship between this change and any of the parameters measured across the study, nor could we link this change with a host response, which could reasonably be considered to represent an improvement in health. Our study involved only thirty healthy adults, and, although similar to the number of subjects used in previous controlled cross-over studies (eleven to fifteen subjects)( Reference Scholtens, Alles and Willemsen 16 , Reference Slavin and Feirtag 19 , Reference Dahl, Whiting and Isaac 20 ) and parallel studies (eighteen to twenty-two subjects)( Reference Lomax, Cheung and Tuohy 21 , Reference Seidel, Boehm and Vogelsang 24 ), there remains the possibility that additional effects associated with β2-1 fructan prebiotics may be identified in future studies examining larger cohorts.
Acknowledgements
The authors thank Emily Chomyshyn (Health Canada) and Fernando Matias (Health Canada) for their excellent technical assistance throughout the study.
This study was a project undertaken by the gut health group of the Advanced Food and Materials Network, and was supported with funding from Agriculture and Agri-Food Canada (RPBI no. 1501, M. K., G. D. I., L. J. Y.), Health Canada (S. P. J. B.), General Mills (L. B. S., M. K.), Alberta Innovates Bio Solutions (G. D. I.), the Advanced Food and Materials Network (L. B. S., M. K.) and National Science and Engineering Research Council (J. M. G.-J.).
Researchers of this group were involved in the original conception of the project (J. M. G.-J., D. D. R., P. B., G. D. I., L. J. Y., L. B. S., S. P. J. B., M. K.). D. D. R. and J. G. organised and supervised the clinical trial. C. A. and P. B. analysed and interpreted the survey-based data. S. T. C. and J. G. conducted the immunological analyses. S. T. C. and S. P. J. B. undertook and provided the statistical analyses. S. T. C., J. M. G.-J. and M. K. prepared the first draft of the manuscript. All additional authors (S. P. J. B., D. D. R., P. B., C. A., G. D. I., J. G., L. J. Y. and L. B. S.) contributed to the final content. M. K., L. B. S., J. M. G.-J. and G. D. I. receive research support from General Mills Inc. and Alberta Innovates Bio Solutions.
All other authors have no conflicts of interest to declare.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516000908














