An estimated 4.4 million CT scans were performed in 2011-2012 in Canada. 1 Although CT scans have been reported to compose only 17% of all diagnostic imaging tests employing ionizing radiation, they account for 49% of the collective radiation dose administered by all radiographic and nuclear medicine procedures.Reference Mettler, Thomadsen and Bhargavan 2 Recent literature has highlighted the need for CT radiation dose reduction given the potential risk of secondary carcinogenesis.Reference Brenner and Hall 3 , Reference Pearce, Salotti and Little 4 A number of dose-reduction strategies have already been adopted into CT scanner design, including tube current modulation and automatic exposure control.Reference McCollough, Primak, Braun, Kofler, Yu and Christner 5 More recent clinical implementation of iterative reconstruction has provided additional optimization of patient dose relative to conventional filtered back-projection (FBP) techniques.Reference Willemink, Leiner and de Jong 6
Opportunities for dose reduction are limited in FBP given the trade-off between image noise and sharpness and the resultant loss of image quality at lower tube current settings.Reference Baumueller, Winklehner and Karlo 7 Iterative reconstruction introduces a correction loop with image regularization into the reconstruction process, which reduces noise with maintained or improved image resolution by offering some degree of decoupling of noise and spatial resolution.Reference Korn, Fenchel and Bender 8 Technological advances have enabled workflow-efficient iterative reconstruction on CT workstations and all major CT vendors have developed software for clinical use.Reference Willemink, de Jong and Leiner 9 One of the newest commercially available algorithms, sinogram affirmed iterative reconstruction (SAFIRE) (Siemens Healthcare, Forchheim, Germany), performs iterations in the raw data (sinogram) and image domains to optimize image noise and sharpness.Reference Baumueller, Winklehner and Karlo 7 , Reference Willemink, de Jong and Leiner 9 Recent studies have reported that SAFIRE offers substantial radiation dose savings in imaging of the chest, abdomen, and head.Reference Baumueller, Winklehner and Karlo 7 , Reference Kalra, Woisetschläger and Dahlström 10 - Reference Korn, Bender and Fenchel 12
The purpose of this study was to further evaluate the effects of SAFIRE on radiation dose reduction and quantitative and qualitative measures of image quality for noncontrast adult head CT for the scanning parameters employed at our institution and to validate the technique for local clinical use.
Methods
Patients
Institutional review board approval was obtained for this retrospective study. Between June 1 and June 15, 2012, 168 consecutive adult patients underwent noncontrast head CT scans for any indication with SAFIRE reconstruction immediately after a software upgrade. Following appropriate sample size/power calculations, a test group of the first 50 consecutive patients in this group was selected for the quantitative CT dataset analysis. The control group for the quantitative CT dataset analysis comprised 50 patients from a consecutive series who underwent noncontrast CT head examinations for any indication with FBP reconstruction between May 16 and 28, 2012, before the software upgrade on the same CT scanner. Subjects within the control group were age-matched within 5 years of the test group to limit confounding secondary to age-related white matter changes. For the qualitative CT dataset analysis, the test population comprised 14 of the 168 patients imaged between June 1 and 15, 2012, who had previously undergone noncontrast CT head examinations for any indication between May 19, 2011, and May 20, 2012, with FBP reconstruction before the software upgrade on the same CT scanner. Five patients were included in both the quantitative and qualitative arms of the study.
CT Data Acquisition and Reconstruction
Imaging was performed on a Siemens SOMATOM Definition Flash CT scanner (Siemens Healthcare). Acquisition and reconstruction parameters are listed in Table 1.
Table 1 CT data acquisition and reconstruction parameters

FBP=filtered back-projection; FOV=field of view; SAFIRE=sinogram-affirmed iterative reconstruction.
Quantitative CT Dataset Analysis
One reader (MRB, second-year radiology resident) blinded to patient identity, scan date, and reconstruction algorithm, performed two independent sets of circular region of interest (ROI) attenuation measurements in Hounsfield units (HU) on head CT images for the 50 patients in the control group (FBP) and the 50 patients in the test group (SAFIRE). Region-of-interest (ROI) attenuation measurements were performed in the lentiform nucleus for gray matter (GM) and in the internal capsule for white matter (WM). Both mean and standard deviation (SD) values were recorded for each ROI measurement. These values were subsequently used to calculate signal-to-noise and contrast-to-noise ratios (SNR, CNR). The SNR represents the quality of the signal intensity within a given tissue, whereas the CNR reflects tissue differentiation based on photon attenuation with respect to background noise.Reference Rapalino, Kamalian and Kamalian 13 The SNR of a given tissue is defined as its mean attenuation (HU) divided by the SD.Reference Mullins, Lev and Bove 14 The CNR of adjacent tissues, in this case GM and WM, is defined as the difference in their mean tissue attenuations divided by the square root of the sum of their variances.Reference Mullins, Lev and Bove 14
Qualitative CT Dataset Analysis
Qualitative analysis of images obtained with FBP and SAFIRE was performed on the 14 patients who had head CT scans with both FBP and SAFIRE reconstruction. Two readers (MRB, second-year radiology resident; JSS, subspecialty-trained neuroradiologist) were trained in the qualitative grading system before reviewing the study datasets. Readers graded study datasets according to randomized lists on a PACS workstation (Barco, Kortrijk, Belgium). The “demographic toggle” function was employed to ensure appropriate blinding to patient identity, scan date, and reconstruction algorithm. Qualitative variables and their grading system are listed in Table 2.Reference Rapalino, Kamalian and Kamalian 13 For visual assessment of image noise, ratings ≥2 represented diagnostic quality studies. Posterior fossa artifact comprised beam hardening and partial volume averaging. GM-WM differentiation was assessed at the level of the centrum semiovale. Small structure visibility included evaluation of Virchow-Robin spaces at the level of the basal ganglia and intracranial vasculature. Image sharpness was rated according to the visibility of the margins of the subarachnoid space.
Table 2 Qualitative variables and grading system
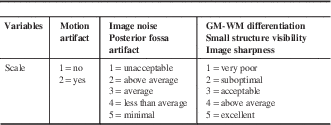
GM=gray matter; WM=white matter.
Radiation Dose
CT dose index volume (CTDIvol) and dose-length product (DLP) were recorded from scanner-generated CT dose reports for all patients. An effective dose per unit dose-length product of 0.0021 mSv/(mGy·cm) for the head was used to calculate effective dose for a 70-kg adult patient.Reference McCollough, Primak, Braun, Kofler, Yu and Christner 5
Statistical Analysis
Statistical analysis was performed with the PASW Statistics 18.0 software package (IBM Corporation, Somers, NY). Numerical data are presented as the mean (SD, range). Intraobserver variation for the repeated attenuation measurements was assessed with the coefficient of repeatability.Reference Bland and Altman 15 Interobserver agreement for the qualitative variables was assessed with a quadratic-weighted kappa value.Reference Landis and Koch 16 The independent samples t-test and the related samples Wilcoxon signed-rank test were employed for significance testing of quantitative and qualitative data, respectively. Significance was taken at p<0.05.
Preliminary measurements of the quantitative variables assessed in this study (SNR, CNR, CTDIvol, DLP, and effective dose) were performed to estimate study power.Reference Dupont and Plummer 17 The sample size of 50 provided greater than 95% power with α=0.05 representing the Type I error probability.
Results
The quantitative study population consisted of 100 adult patients; 50 underwent noncontrast CT head examinations with FBP (mean [SD] age, 57.1 [20.0] years; range, 22-92 years; 31 women, 19 men) and 50 underwent noncontrast head CT examinations with SAFIRE (mean [SD] age, 56.4 [19.9] years; range, 21-91 years; 27 women, 23 men). There were no significant differences in age (p=0.853) or gender (p=0.418) between the FBP or SAFIRE groups.
The qualitative study population consisted of 14 adult patients (four women, 10 men) who underwent noncontrast CT head examinations with FBP and SAFIRE. The mean age at the time of the FBP examination was 63.2 (22.1, 24-92) years. The mean time was between the FBP and SAFIRE examinations was 202 (147, 18-381) days. One patient developed bilateral subdural hygromas in the interval between studies.
Quantitative CT Dataset Analysis
The mean ROI area for attenuation measurements was 22.8 (0.8, 18.3-26.3) mm2. Each ROI contained approximately 110 voxels. Mean GM attenuation values for the FBP and SAFIRE populations were 38.1 (1.1, 34.1-40.0) HU and 39.3 (1.0, 36.3-41.8) HU, respectively. For WM, the attenuation values were 28.3 (1.1, 25.2-30.6) HU for FBP and 27.5 (1.1, 24.6-29.9) HU for SAFIRE. The coefficient of repeatability for the two sets of attenuation measurements was 1.8 (95% confidence interval: 1.5-2.0). Figure 1 demonstrates relatively tight clustering of the GM and WM attenuation values about the line of equality.

Figure 1 Intraobserver variation for GM and WM attenuation values. Measurement 1 versus measurement 2 with the line of equality.
Figures 2a and 2b displays boxplots of the GM SNR and WM SNR stratified by reconstruction technique. The mean GM SNR significantly increased from 10.2 (1.1, 7.4-13.1) for FBP to 15.8 (2.2, 12.7-22.4) for SAFIRE (p<0.0001). Similarly, the mean WM SNR increased from 7.4 (0.9, 5.4-9.1) for FBP to 10.0 (1.3, 7.3-14.4) for SAFIRE (p<.0001). Figure 3 displays a boxplot of the GM-WM CNR stratified by reconstruction technique. There was also a significant improvement in GM-WM CNR with SAFIRE, which increased from 1.8 (0.3, 1.1-2.4) to 3.1 (0.4, 2.1-4.2) (p<0.0001).

Figure 2 Boxplots of GM SNR (a) and WM SNR (b) with FBP and SAFIRE. The horizontal line within the box is the median value; the box defines the 25th to 75th quartile with whiskers to the minimum and maximum values.
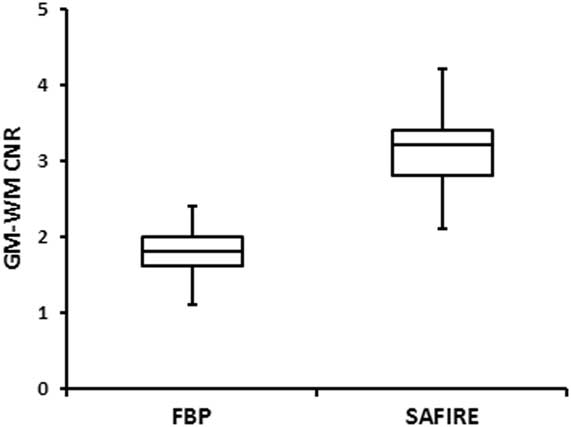
Figure 3 Boxplot of GM-WM CNR with FBP and SAFIRE. The horizontal line within the box is the median value; the box defines the 25th to 75th quartile with whiskers to the minimum and maximum values.
Qualitative CT Dataset Analysis
There was perfect agreement between the readers for the presence of motion artifact, and two of the 14 patients were excluded from analysis secondary to motion artifact on the FBP scans. Readers either agreed or were within 1 unit of each other for 114/120 (95%) of the rankings in the remaining 12 patients. Measures of interobserver agreement were: image noise, 0.82 (very good); posterior fossa artifact, 0.50 (moderate); GM-WM differentiation, 0.60 (moderate); small structure visibility, 0.71 (good); and image sharpness, 0.65 (good). Mean reader qualitative rankings are listed in Table 3. Image noise and posterior fossa artifact were significantly reduced with SAFIRE (p=0.002). Similarly, GM-WM differentiation, small structure visibility, and image sharpness were significantly improved with SAFIRE (p<0.005). Examples of FBP and SAFIRE non-contrast head CT images are displayed in Figures 4a and 4b, respectively.

Figure 4 A 41-year-old man with a seizure disorder. (a) Axial noncontrast head CT image (width, 80 HU; level, 40 HU) reconstructed with FBP from a raw CT dataset acquired with a tube current of 300 mAs. (b) Axial noncontrast head CT image (width, 80 HU; level, 40 HU) reconstructed with SAFIRE from a raw CT dataset acquired with a tube current of 247 mAs. The scans were performed 1 month apart.
Table 3 Mean qualitative rankings (n=12)

FBP=filtered back-projection; GM=gray matter; SAFIRE=sinogram-affirmed iterative reconstruction; SD=standard deviation; WM=white matter.
Radiation Dose
The mean CTDIvol and DLP values for FBP were 55.9 (1.0, 55.6-59.4) mGy and 951.4 (58.6, 849-1144) mGy·cm. These values were significantly reduced to 47.4 (2.4, 42.3-51.9) mGy and 802.9 (76.9, 654-959) mGy·cm for SAFIRE (P<0.0001). Using the effective dose per unit dose-length product, the mean effective doses for FBP and SAFIRE were calculated as 2.0 (0.1, 1.8-2.4) mSv and 1.7 (0.2, 1.4-2.0) mSv, respectively, corresponding to a dose reduction of 15% with SAFIRE (p<0.0001).
Discussion
Several other recent studies have also assessed the efficacy of iterative reconstruction for head CT and have shown that image quality can be preserved and even improved with concomitant radiation dose reduction.Reference Korn, Fenchel and Bender 8 , Reference Korn, Bender and Fenchel 12 , Reference Rapalino, Kamalian and Kamalian 13 , Reference Kilic, Erbas, Guryildirim, Arac, Ilgit and Coskun 18 , Reference Vorona, Zuccoli, Sutcavage, Clayton, Ceschin and Panigrahy 19 Iterative reconstruction has been reported to reduce patient dose for head CT by 15-30% for iterative reconstruction in image space (Siemens Healthcare),Reference Korn, Fenchel and Bender 8 24-31% for adaptive statistical iterative reconstruction (ASiR) (GE Healthcare, Milwaukee, WI),Reference Rapalino, Kamalian and Kamalian 13 , Reference Kilic, Erbas, Guryildirim, Arac, Ilgit and Coskun 18 , Reference Vorona, Zuccoli, Sutcavage, Clayton, Ceschin and Panigrahy 19 and 20% for SAFIRE.Reference Korn, Bender and Fenchel 12 Reported mean effective doses for head CT in adults range from 2.2-2.7 mSv for FBP to 1.5-2.0 mSv for different iterative methods.Reference Korn, Fenchel and Bender 8 , Reference Korn, Bender and Fenchel 12 , Reference Rapalino, Kamalian and Kamalian 13 , Reference Kilic, Erbas, Guryildirim, Arac, Ilgit and Coskun 18 Our results of 2.0 mSv for FBP and 1.7 mSv for SAFIRE are in good agreement with these values.
We identified significant improvements in SNR and CNR for the acquisition parameters in our study. Korn et al.Reference Korn, Bender and Fenchel 12 report 28%, 31%, and 25% increases in GM SNR, WM SNR, and GM-WM CNR with SAFIRE at reduced-dose head CT (255 mAs) in comparison to routine-dose scans (320 mAs). Higher values of 55%, 35%, and 72% in our study likely relate to a combination of a lower tube current for our routine-dose scans (300 mAs) and a relatively higher quality reference mAs setting for our reduced-dose SAFIRE scans (320 mAs). An earlier report on image quality at different ASiR levels for routine- and reduced-dose head CT scans found that reduced-dose head CT scans had significantly higher SNR at ASiR levels ≥60% and CNR at ASiR levels ≥40%.Reference Rapalino, Kamalian and Kamalian 13 Although differences exist between the ASiR and SAFIRE algorithms, our results at a medium strength SAFIRE setting of 3 are comparable.
Subjective measures of CT image quality are similarly dependent on radiation dose and the method of image reconstruction. Furthermore, the generalizability of our results to the literature is limited by the lack of standardized ratings scales and variables.Reference Korn, Fenchel and Bender 8 , Reference Korn, Bender and Fenchel 12 , Reference Rapalino, Kamalian and Kamalian 13 , Reference Kilic, Erbas, Guryildirim, Arac, Ilgit and Coskun 18 , Reference Vorona, Zuccoli, Sutcavage, Clayton, Ceschin and Panigrahy 19 We employed the 5-point rating scales and variables of Rapalino et al.,Reference Rapalino, Kamalian and Kamalian 13 who similarly identified significant reductions in image noise and artifacts with improved GM-WM differentiation at ASiR levels ≥60% in reduced-dose head CT scans with respect to routine-dose head CT without ASiR. Although we report a reduction in posterior fossa artifact for SAFIRE, this finding may be in part be attributable to the narrower slice thickness of 3 mm, which would be expected to reduce partial volume averaging through the skull base. Korn et al.Reference Korn, Bender and Fenchel 12 also report significantly reduced image noise, GM-WM differentiation, and distinctness of the posterior fossa contents with SAFIRE in reduced-dose head CT scans. Although our results of improved small structure visibility with SAFIRE have not previously been reported, this finding may relate to the use of a lower tube current for the routine-dose scan (300 mAs).
The difference in slice thickness between the FBP (5 mm) and SAFIRE (3 mm) groups may have contributed to an underestimation of the potential CT dose reduction offered by SAFIRE for the SNR values reported in this study. Radiation dose is proportional to the mAs or the number of photons used to acquire a CT dataset. These quantities have traditionally been expressed in relation to SNR, pixel volume, and slice thickness whereby the number of photons used to create a CT dataset is proportional to the square of the SNR divided by the product of the pixel volume and the slice thickness.Reference Bushberg, Siebert, Leidholdt and Boone 20 For example, increasing the slice thickness from 3 to 5 mm for a given reconstruction algorithm with a fixed pixel volume provides a theoretical dose reduction of up to 40% if the mAs required to maintain a fixed SNR is appropriately reduced at the time of scanning. The SNR values measured from the SAFIRE datasets in this study may be achievable at 1 mSv with a slice thickness of 5 mm, or 60% of the reported 1.7 mSv dose with a slice thickness of 3 mm. In comparison to the 2.0 mSv FBP dose, this represents a dose savings of up to 50%. The inherent reduction in image noise in iterative reconstruction also offers an additional opportunity for radiation dose optimization. Using the relationship described previously at a fixed pixel volume, estimated mean effective doses of 0.9 mSv for 3-mm SAFIRE datasets and 0.6 mSv for 5-mm SAFIRE datasets may be obtained for the mean SNR values reported for the 5-mm FBP datasets in this study. These values are 45% and 30%, respectively, of the reported 2.0 mSv FBP dose. However, these estimates require experimental verification for modern CT scanners, and the diagnostic quality of such low-dose datasets would also need to be confirmed.
Although we only evaluated noncontrast adult head CT scans, SAFIRE may be considered for additional head and neck CT applications. Children would benefit from reduced doses given heightened concerns of radiosensitivity and lifetime cumulative dose in this population.Reference Vorona, Zuccoli, Sutcavage, Clayton, Ceschin and Panigrahy 19 A role for iterative reconstruction in contrast-enhanced head CT has previously been described,Reference Korn, Fenchel and Bender 8 and further reductions in radiation dose may be also achievable in CT angiography and dedicated bone imaging where GM-WM CNR is less important.Reference Shankar and Pretty 21 SAFIRE could be considered for CT brain perfusion imaging, but has the potential to delay the initiation of tissue plasminogen activator therapy in the setting of acute ischemic stroke because of slightly longer reconstruction times.Reference Korn, Bender and Fenchel 12
There are limitations to this study. First, a fixed tube current of 300 mAs for the FBP scan protocol in comparison to a quality reference mAs setting of 320 mAs for SAFIRE likely resulted in an underestimation of the dose reduction offered by SAFIRE for head CT because previous studies have employed fixed tube currents of 320-340 mAs for FBP.Reference Korn, Fenchel and Bender 8 , Reference Korn, Bender and Fenchel 12 , Reference Kilic, Erbas, Guryildirim, Arac, Ilgit and Coskun 18 Second, the 3-mm slice thickness for the SAFIRE datasets may also have contributed to an underestimation of the achievable dose reduction with respect to 5-mm FBP datasets as described previously. The reduced posterior fossa artifact for the SAFIRE datasets may be attributable in part to the narrower slice thickness, given the expected reduction in partial volume averaging through the skull base. Third, routine-dose datasets (300 mAs) could not be reconstructed with SAFIRE to assess image quality at a fixed tube current because of the retrospective study design. Despite this limitation, measures of image quality with SAFIRE would likely be improved at higher radiation doses. Similarly, reduced-dose datasets were not reconstructed with FBP, but would likely have been noisier than the routine-dose scans with FBP reconstruction and reduced-dose scans with SAFIRE. Fourth, the characteristic appearance of SAFIRE images may have limited reader blinding for the qualitative CT dataset analysis,Reference Rapalino, Kamalian and Kamalian 13 which would also benefit from a larger sample size. Finally, the diagnostic accuracy of SAFIRE in the clinical setting was not assessed because this study focused on the characterization of image quality.
In conclusion, SAFIRE for noncontrast adult head CT reduces patient radiation dose by 15% for the scanner settings employed at our institution. Additional dose reduction is likely achievable given the significant improvements in SNR, CNR, and multiple qualitative measures of image quality with reduced-dose SAFIRE. Further research is also required to validate the technique for multiple head and neck applications. Future advances may also facilitate adoption of this technique into more computationally demanding applications such as CT brain perfusion imaging for acute ischemic stroke.









