Falls are a growing concern in seniors (≥65 years).Reference Stinchcombe, Kuran and Powell1 Cognitive declineReference Stinchcombe, Kuran and Powell1 and vestibular impairment (VI) increase with age and are correlated with an increased risk of falling.Reference Agrawal, Carey, Della Santina, Schubert and Minor2 Vestibular rehabilitation (VR) is used to treat VI.Reference Herdman3
We tested the feasibility of a VR program in Memory Clinic patients with previous falls. The aim of this study was to assess the feasibility of recruitment, ability to complete screening tests/questionnaires, feasibility of the program and compliance to a VR program in patients with mild cognitive decline and VI.
Patients who had attended the Memory Clinic and were ≥65 years of age, English speaking, had a diagnosis of MCI/AD/VaD/mixed dementia,Reference Albert, DeKosky and Dickson4–Reference Rockwood, Macknight and Wentzel7 fell within the past year and able to provide consent were contacted. Exclusion criteria consisted of additional neurological disease (i.e. seizures or stroke), severe psychiatric disease, current inner-ear infection, severe neck arthritis, Montreal cognitive assessment (MoCA) score < 15 or > 26 (v7.1)Reference Nasreddine, Phillips and Bedirian8 or other aetiology of dementia (i.e. Parkinson’s disease). Eligible patients were called (1 message/patient) and invited to a screen for VI. The MoCA, dizziness-handicap inventory (DHI) and activities-specific balance confidence scale (ABC) were administered for cognitive state, levels of perceived dizziness and confidence in performing daily activities without losing balance, respectively. VI was assessed using the Dix–Hallpike test,Reference Dix and Hallpike9 both the bedside- and video head impulse test (HIT and vHIT),Reference MacDougall, Weber, McGarvie, Halmagyi and Curthoys10 the Headshake test (HST)Reference Hain, Fetter and Zee11 and the modified clinical test of sensory interaction on balance (mCTSIB).Reference Cohen, Blatchly and Gombash12
Definite vestibular impairment (DVI) was diagnosed if patient failed Dix–Hallpike, HIT/vHIT or HST. Failure of mCTSIB alone was diagnostic of probable VI (PVI), unless failed additional test, in which the patient would be categorized as having a DVI. Patients were diagnosed with unilateral vestibular hypofunction (UVH) if there was an abnormal test result on one side or bilateral vestibular hypofunction (BVH) if both sides were affected.
Those with VI went on to baseline assessment including the dynamic gait index (DGI) for gait, geriatric depression scale (GDS) for depression and both the WHOQOL-BREF and QOL-AD for quality of life. Patients were then randomized to a VR or control-arm (CON) via a coin toss.
Patients in the VR arm were assigned 12 weeks of exercises, whereas patients in CON continued with standard care (no exercise program). Twelve weeks later, patients returned and retested for changes from baseline (ABC, DHI, DGI, WHOQOL-BREF and QOL-AD). In addition, patients were given the Problematic Experiences of Therapy Scale (PETS). The PETS was used to gauge barriers to VR adherence.Reference Kirby, Donovan-Hall and Yardley13 At 24 weeks, patients were called to ask if any falls occurred.
Participants in the VR arm performed vestibular exercises 3 times daily, 3–10 minutes/session (see Supplementary Materials). Exercises progressed every 2 weeks. Patients were called biweekly to monitor compliance.
Feasibility metrics included: prevalence of DVI, recruitment of patients screened to intervention, compliance to exercises, attrition during the intervention period and ability of participants to complete the questionnaires. Feasibility criteria were set at: a recruitment rate of 45%,Reference Cooper, Ketley and Livingston14 compliance rate as ability to carry out 60% of the exercises,Reference Ries, Hutson, Maralit and Brown15 attrition rate of 30% or lessReference Ries, Hutson, Maralit and Brown15 and 60% of participants to complete a given questionnaire in its entirety.Reference Hoe, Katona, Roch and Livingston16
Flow of patients is shown in Figure 1. There were 424 patients over the age of 65 with MCI/early dementia; 209/424 (49.3%) had experienced a prior fall, 114/424 (26.9%) had no falls and in 101/424 (23.8%) fall history unknown (Supplementary Table 1). Of 209, 48 participants experienced a fall within the past year and met inclusion criteria (Supplementary Table 2). Of 48, 34 (70.8%) patients were contacted by phone; 14/34 (41.2%) agreed to participate in the study. Of 14, 12 (85.5%) participants attended screening. Screening results are given in Table 1. Mean age of those screened was 77.3 ± 6.7 years old and 8/12 (66%) were men. Of 12, 8 (75.0%) of screened participants were diagnosed with a DVI. Of those with DVI, 4/8 (50.0%) had UVH and 4/8 (50.0%) had BVH. Of 12, 1 participant had PVI due to failure of the mCTSIB alone and 3/12 (25.0%) had no vestibular impairment (NVI). Abnormal vestibular test results are given in Table 2, and mCTSIB results are given in Supplementary Table 1.
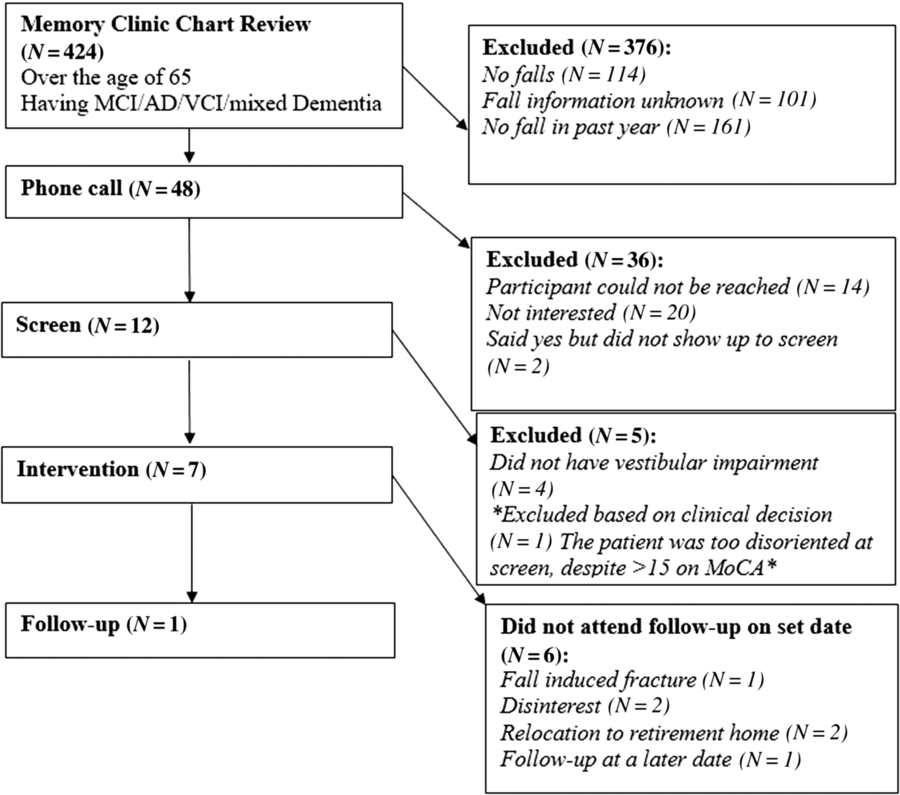
Figure 1: Patient flow throughout course of study.
Table 1: Screening questionnaire results at a Memory Clinic in those with and without vestibular impairment (N = 12)
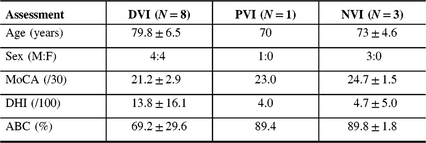
ABC = activities-specific balance confidence scale; DHI = dizziness handicap inventory; DVI = definite vestibular impairment; MoCA = Montreal cognitive assessment; NVI = no vestibular impairment; PVI = probable vestibular impairment.
Table 2: Abnormal tests of vestibular functioning at a Memory Clinic (N = 8)
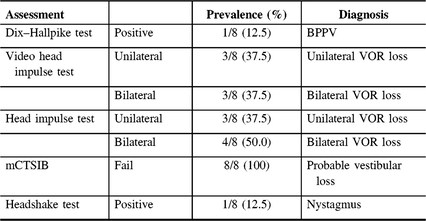
BPPV = benign paroxysmal positional vertigo; VOR = vestibulo-ocular reflex.
The mean MoCA score was 21.2 ± 2.9 for the DVI group, 23.0 for the one participant with PVI and 24.7 ± 1.5 for those with NVI. One participant with DVI that had been randomized to the intervention arm did not complete the MoCA due to time constraint.
Those with DVI reported lower confidence scores on the ABC (69.2 ± 29.6), compared to the participant with PVI (89.4) and to those with NVI (89.8 ± 1.8). One quarter (2/8) of participants had an ABC score lower than 67%, indicating fall risk.
There was greater reported dizziness on the DHI in those with DVI (13.8 ± 16.1) compared with the participant with PVI who scored a 4.0 and those with NVI (4.7 ± 5.0), respectively. Overall, for the participant with PVI and those with NVI, there was a mild perceived handicap due to dizziness (score 0–30). Of those with DVI, 2/8 (25%) had a moderate perceived handicap due to dizziness (score 31–60) and 6/8 (75%) had a mild handicap due to dizziness (0–30).
Of eight, seven (87.5%) participants with DVI continued onto the baseline assessment (87.5% recruitment rate). Those with PVI and NVI did not proceed to the next portion of the study. One participant did not continue to baseline because of decreased capacity to perform the exercises. All assessments and questionnaires in the baseline assessment were completed in their entirety (Table 3).
Table 3: Results from baseline assessment for those with definite vestibular impairment (N = 7)
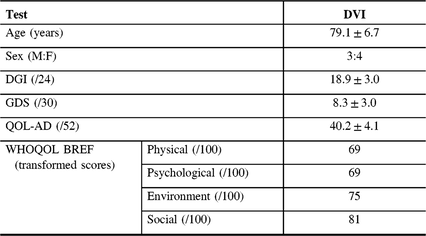
QOL-AD = Alzheimer’s disease quality of life; DGI = dynamic gait index; GDS = geriatric depression scale; WHOQOL-BREF = world health organization quality of life BREF.
Mean DGI (gait) score was 18.9 ± 3.0. The DGI score was <19 in 5/7 (71.4%) participants, indicating that most participants were at risk for falls. Mean GDS (depression) score was 8.3 ± 3.0, indicating no depressive symptoms. Only 2/7 (28.6%) of participants had a score of 10–19, indicating mild depressive symptoms. Mean QOL-AD score was 40.3 ± 4.1 for those with VI. Mean transformed WHO-QOL scores were 69 in the physical domain, 69 in the psychological domain, 75 in the environmental domain and 81 in the social domain, indicating good QOL.
Seven participants with DVI were randomized to the VR [5/7 (71.4%)] or CON [2/7 (28.6%)] arm. During the 12-week intervention period, 3/7 participants dropped out; one participant (VR) dropped out due to a fall with hip fracture in week 1 (Supplementary Table 5). Two participants (1 VR and 1 CON) dropped out at the 6-week mark because of disinterest. The remaining four participants continued their respective arms for 12 weeks (3 VR and 1 CON). One participant (VR) fell at week 7. Of those in the VR arm, bi-weekly phone calls indicated no reported difficulties carrying out the exercises.
Four participants originally agreed to attend the 12-week follow-up; however, only two attended: one participant from the arm control attended follow-up on the set date, and one participant from the intervention arm attended follow-up at a later date due to a flu. The remaining two participants could not attend follow-up due to relocation into a retirement home. Overall, there was a 71.4% attrition rate and retention in rehabilitation was not feasible. Questionnaire/assessment completeness was 100% at follow-up (Table 4).
Table 4: Follow-up assessment results for participant VS_01 (Intervention) and VS_04 (control)
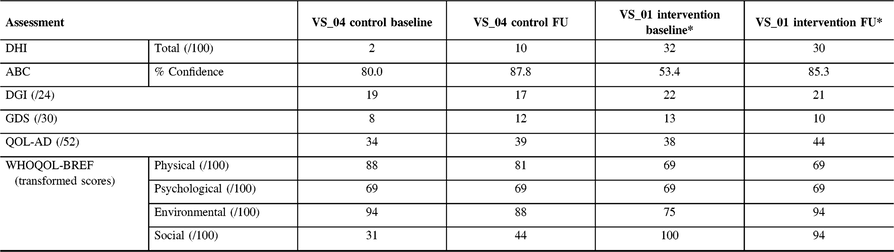
ABC = activities-specific balance confidence scale; QOL-AD = quality of life Alzheimer’s disease; DGI = dynamic gait index; DHI = dizziness handicap inventory; GDS = geriatric depression scale; WHOQOL = world health organization quality of life.
* The Intervention participant was unable to meet at the 12-week mark and was assessed 15 weeks post-baseline.
The participant from CON (VS_04) was able to complete all questionnaires upon return. This participant showed an increase in dizziness (DHI-10 from 2), impaired gait (DGI-17 from 19) and more depressive symptoms (GDS-12 from 8) compared to baseline. Interestingly, she showed a higher level of perceived confidence (ABC-87.8% from 80.0%) compared to baseline assessment and showed no significant change in QOL. One intervention participant (VS_01) attended follow-up 15 weeks after baseline. Overall, levels of dizziness decreased (DHI-30 from 32) but were still high. There was a large increase in confidence (ABC-85.3% from 53.4%) in ability to complete activities without loss of balance, which was clinically significant (>10% change).Reference Whitney, Wrisley, Marchetti and Furman17 DGI was 21 (from 22) and was not considered at risk for falls. Likewise, there was an increase in some QOL measures on the QOL-AD (44 from 38), WHOQOL-BREF (Physical-69 was same, Psychological-69 was same, Environmental-94 from 75, Social-94 from 100) and a slight decrease in levels of depression (GDS-10 from 13).
The control patient improved on the mCTSIB from baseline. At baseline, this participant was unable to complete condition three or four but upon return, they were able to complete condition three, but not four, suggesting a vestibular contribution to imbalance. Participant VS_01 was able to complete all three conditions, which was unchanged from baseline. VS_01 reported on the PETS that the obstacles to therapy were that exercises occasionally made symptoms worse and that it was difficult to remember to perform exercises. Compliance could not be recorded as VS_01 had forgotten to fill out the log, despite the phone calls. At 24 weeks, both the control (VS_04) and VR participant (VS_01) were contacted by telephone and neither had experienced a fall since the 12-week follow-up.
The self-directed VR protocol used in this study was not feasible for participants with both CI and VI. Few patients attended the screen; however, once participants were aware of a VI, they were easily recruited. Adherence to self-monitored VR could not be assessed and better methods of monitoring must be implemented into future trials. In addition, while not all participants completed the entire 12 weeks of the program, a majority remained in the study until the 6-week mark, suggesting that 6-week programs may be more feasible to complete. All assessments and questionnaires were completed at baseline and follow-up.
Overall, our results suggest that VI is common in those with CI and falls but that a modified VR program needs to be developed for participants with both CI and VI. A VR program that includes regular in-person monitoring or a group exercise program may be more beneficial for this patient population. This would allow for better compliance and assessment of technique in performing the exercises. Other contributors to falls including medication use and systemic illnesses need to be considered. Falls are multifactorial in nature, and therefore, it is critical to address all contributors to fall risk.
Acknowledgements
We would like to thank all patients and caregivers who participated in this study. We thank our colleagues from the Toronto General ENT clinic. We thank Dr. Naglie and Dr. Mansfield, who provided insight and expertise that greatly assisted the research.
Funding
This work has been supported by the following graduate awards and travel grants: Krembil Studentship in Dementia Research, Toronto General/Western Hospital Foundation Grant, Unilever Fellowship in Neurosciences, Alzheimer’s Association Travel Fellowship and SGS Travel Grant.
Conflict of Interests
The authors declare that they have no competing interests.
Statement of Authorship
BV designed the study and collected data, in addition to preparation of the manuscript. SS, CW, and WD assisted with the vestibular assessments and interpretation of the study. JR and MCT are PI, who contributed staff and guidance in the study. NM, KM, and CA assisted with questionnaires at the baseline assessment. MM and EC assisted with recruitment of patients. All authors have read and approve the manuscript.
Ethics Approval and Consent to Participate
The Research Ethics Board of the University Health Network approved the study. Written consent was obtained from all subjects, as outlined by the University Health Network research protocol.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2019.309







