Introduction
We report about the H2O sorption properties of a hygroscopic chloride recently identified in deposits on the Martian surface (Osterloo et al. Reference Osterloo, Hamilton, Bandfield, Glotch, Baldridge, Christensen, Tornabene and Anderson2008). Because Wierzchos et al. (Reference Wierzchos, Ascaso and McKay2006) and Davila et al. (Reference Davila, Gomez-Silva, de los Rios, Ascaso, Olivares, McKay and Wierzchos2008, Reference Davila, Hawes, Ascaso and Wierzchos2013) showed that deliquescence of halite (sodium chloride) in the hyper-arid Atacama Desert provides a habitable environment, the Martian chloride deposits also might have an astrobiological potential (Davila et al. Reference Davila, Duport, Melchiorri, Jänchen, Valea, de los Rios, Fairén, Möhlmann, McKay, Ascaso and Wierzchos2010). Möhlmann (Reference Möhlmann2011) proposed temporary liquid cryobrines on Mars available as water source for biological processes. This gives a good reason to investigate experimentally in more detail the sorption behaviour of such NaCl brines. Further, we have included in our study the hydration and dehydration behaviour of a biofilm because many of the microorganisms on Earth are hosted by hydrophilic biopolymers (Flemming & Wingender Reference Flemming and Wingender2010). The highly desiccation resistant filamentous cyanobacterium Nostoc commune has served as a model organism for our test because the biofilm in conjunction with the cryobrine may have significant implications for the characterization of such kind of habitats on Mars. Although N. commune is not tolerant to high salt concentrations (Sakamoto et al. Reference Sakamoto, Yoshida, Arima, Hatanaka, Takani and Tamaru2009), we chose this organism due to its high production of viscous extracellular polysaccharides (EPS). With this trait, the cyanobacterium is able to take up large amounts of brine, shows a higher hydration status at lesser values of humidity and thus acts as water sink (Tamaru et al. Reference Tamaru, Takani, Yoshida and Sakamoto2005).
N. commune has a cosmopolitan distribution and is hypothesized to be present already on the Early Earth in the Paleoproterozoic era more than 2 billion years ago (Amard & Bertrand-Sarfati Reference Amard and Bertrand-Sarfati1997; Potts Reference Potts, Whitton, Potts, Potts, Whitton and Potts2002; Sergeev et al. Reference Sergeev, Gerasimenko and Zavarzin2002). The cyanobacterium is adapted to a lifestyle as colonizer of nutrient-deficient open spaces and plays an important role as a carbon- and nitrogen-assimilating pioneer organism (Dodds et al. Reference Dodds, Gudder and Mollenhauer1995). The conditions in such environments are often extreme with regular periods of desiccation, high ultraviolet (UV) irradiation and temperature differences. To persist these extremes, N. commune forms large colonies by excreting EPS which are responsible for its remarkable desiccation and freezing tolerance (Tamaru et al. Reference Tamaru, Takani, Yoshida and Sakamoto2005). A tolerance to UV radiation is further achieved by synthesis of protective pigments and their excretion into the EPS (Ehling-Schulz et al. Reference Ehling-Schulz, Bilger and Scherer1997).
Communities of cyanobacteria and heterotrophic bacteria are well known to be successful communities in many extreme environments throughout the history of earth (Paerl et al. Reference Paerl, Pinckney and Steppe2000). Heterotrophs are known to be associated to filamentous, EPS-producing cyanobacteria either by inhabiting the polymeric matrix between the filaments or by attachment on the cell surface (Paerl Reference Paerl, Carr, Whitton, Paerl, Carr and Whitton1982; Albertano & Urzì Reference Albertano and Urzì1999). Still there is a lack of knowledge about the heterotrophic microbiota of terrestrial N. commune biofilms.
If a biofilm formed by a primary producer would be provided with water by deliquescence of halite under Martian conditions, it could serve as a habitat for other bacteria as well. Thus we put an emphasis on the identification of all prokaryotes in the biofilm samples and the viability of associated microorganisms. This was done by a test to ascertain a possible influence of desiccation, water content and salinity on recultivation. In addition to halite and biofilm we included montmorillonite in our study, as it has been identified in the Martian soil (Poulet et al. Reference Poulet, Bibring, Mustard, Gendrine, Mangold, Langevin, Arvidson, Gondet and Gomez2005; Vaniman et al. Reference Vaniman2014) and may form associates with bacteria being beneficial for their metabolism and survival (reviewed by Marshall Reference Marshall1975 and England et al. Reference England, Lee and Trevors1993) as well as being a source of water supply.
Palacio et al. (Reference Palacio, Azorin, Montserrat-Marti and Ferrio2014) discusses even the crystallization water of other minerals like gypsum as a relevant water source for plants in an extremely dry environment. Because huge deposits of sulphate hydrates are well known to occur on the Martian surface (Bibring et al. Reference Bibring2005; Gendrin et al. Reference Gendrin2005) crystallization water may potentially be another water source for microorganisms on Mars.
Our interdisciplinary study of a physicochemical and microbiological approach to an astrobiological issue should help to evaluate cryobrines on Mars as potential habitats and thereby gain knowledge in order to support missions such as Mars Science Laboratory (MSL) and ExoMars/MicrOmega to recognize interesting habitable Martian sites. Very recently Martín-Torres et al. (Reference Martín-Torres2015) reported about evidence of night-time transient liquid brines (perchlorate based) in the uppermost subsurface of Gale crater in evaluating data of the Curiosity rover (MSL). The science team also found changes in the hydration state night/day of salts consistent with an active exchange of humidity between atmosphere and uppermost soil surface.
In this sense extremotolerant organisms are under current investigation with respect to survival of space and Mars conditions to gain knowledge about the boundaries of life beyond Earth. Therefore the extremotolerant organisms such as lichens and cyanobacteria are part of the current EXPOSE-R2 Biology and Mars experiment (BIOMEX) on the international space station (Baqué et al. Reference Baqué, de Vera, Rettberg and Billi2013; Böttger et al. Reference Böttger, de Vera, Fritz, Weber, Hübers and Schulze-Makuch2012; de Vera et al. Reference de Vera2012, Reference de Vera, Schulze-Makuch, Khan, Lorek, Koncz, Möhlmann and Spohn2014; Billi et al. Reference Billi, Baqué, Smith and McKay2013; Meeßen et al. Reference Meeßen, Sánchez, Brandt, Balzer, de la Torre, Sancho, de Vera and Ott2013a, Reference Meeßen, Sánchez, Sadowsky, de la Torre, Ott and de Verab). Finally, our study may contribute to a better understanding of the speciation of adsorbed H2O, hydrated and hydroxylated phase on the Martian surface (Jouglet et al. Reference Jouglet, Poulet, Milliken, Mustard, Bibring, Langevin, Gondet and Gomez2007; Vaniman et al. Reference Vaniman2014).
Materials and methods
Materials
Halite, a palm sized piece, from the hyper-arid core of the Atacama Desert (Yungay region, Chile, donor Alfonso F. Davila, Davila et al. Reference Davila, Gomez-Silva, de los Rios, Ascaso, Olivares, McKay and Wierzchos2008) have been employed in this study. For comparison, in particular for the hydration properties, very pure NaCl (Merck, 99.99%) and the smectite Ca-montmorillonite (STx, Gonzales County, Texas; source Clay Minerals Repository, 101 Geological Science Bldg., Columbia, MO 65211, USA) have been included in this study.
All experiments concerning N. commune were performed on natural colonies, collected dry from a concrete bridge deck in the national park ‘Unteres Odertal’ (53°8′2″N, 14°22′24″E; Brandenburg, Germany). For identification of associated bacteria, two different biofilm samples were investigated in addition: another sample, that was collected wet at the bridge site in the national park described above and dried for 20d in a sterile petri dish over silica gel prior to analysis and dry colonies from a flat rooftop (50°23′45″N, 9°1′32″E; Hesse, Germany). The dry colony material has been stored for 5 (national park) or 4 (rooftop) years in sterile glass flasks or polypropylene tubes at room temperature in the dark.
Methods
Sorption and thermal methods
The hydration/dehydration properties of Atacama halite, pure NaCl, montmorillonite and N. commune were investigated by means of isotherm measurements and thermoanalysis such as thermogravimetry (TG), differential thermogravimetry (DTG) and differential thermoanalysis (DTA). Sorption isotherms were measured gravimetrically from 256 to 293 K with a McBain–Bakr quartz spring balance (McBain & Bakr Reference McBain and Bakr1926) equipped with three MKS Instruments Inc. (MKS) Baratron pressure sensors covering a range of 10−5–103 mbar. The sensitivity of the quartz spring was 4 mg mm−1. The extension of the spring was measurable with a resolution of 0.01 mm giving a resolution of 0.04 mg for the quartz spring. In terms of ‘g water/g dry sorbent’ this results in a resolution of 0.0004 g g−1 for application of 100 mg sample or in the case of 400 mg to 0.0001 g g−1.
TG, DTG and DTA measurements were performed on a Netzsch STA 409 apparatus with a heating rate of 10 K min−1 up to 600 or 900 K and a purge gas stream (nitrogen) of 70 ml min−1. Prior to the experiments all samples had been stored in controlled atmosphere (evacuated desiccator): N. commune and montmorillonite at p/p s H2O = 0.79 (relative humidity (RH) = 79%) and halite or NaCl at p/p s H2O = 0.60 (RH = 60%) for several days.
Scanning electron microscopy (SEM, JOEL JSM640 and ZEISS Gemini Ultra Plus) combined with energy dispersive X-ray analysis (EDX) was applied to characterize the morphology and chemistry of the salt samples.
Before each sorption experiment, about 150 mg of sample (or 350 mg in case of the salts) had been degassed in high vacuum (p < 10−5 mbar) at 293 K overnight (N. commune and the salts for an extra run) as well as at 383 K (montmorillonite) and 413 K (salts) for several hours. The degassing temperatures were limited to individual adapted low value to prevent possible modification of the mineral- and biofilm-samples.
Recultivation
N. commune samples used in the survival experiment (5-year-old, Odertal) had been equilibrated in a desiccator over silica gel (RH = 30–40%). Samples were then incubated under the following conditions: in a desiccator over sterile saturated NaCl (RH = 75%) solution (19d at 20.2 ± 0.7 °C) or completely covered in sterile saturated NaCl solution (for 14d at 20.2 ± 0.8 °C). Three silica gel dried replicate samples served as a reference and five samples per treatment were analysed. One autoclaved (134 °C for 30 min) sample per treatment served as sterility control.
Survival of associated heterotrophic bacteria was determined by spiral plating (IUL EddyJet spiral plater) of homogenized sample material on low-nutrient Reasoner's 2A (R2A) agar plates. Samples were homogenized in sterile 1× phosphate buffered saline (PBS) for 2 min at 6000 rpm with an IKA ULTRA-TURRAX Tube Drive homogenizer. Three sub-samples of each homogenizate were serially diluted with 1× PBS. After incubation at 20.3 ± 0.7 °C for 7d, the colonies were counted.
Statistical analysis and plotting of heterotrophic plate counts was performed using the R statistics software environment (R Core Team 2013). A non-parametric test (Kruskal–Wallis) was used to check for significant variation of results among different treatment groups.
Microscopy and confocal laser scanning microscopy (CLSM)
Phase-contrast light microscopy was performed on dried biofilm pieces mounted with Citifluor AF2 (Citifluor Ltd.) with a Zeiss Axioplan microscope equipped with a 63× Plan-Apochromat objective. For CLSM, biofilms were prepared as follows: samples were covered in sterile ultrapure water (deionized (DI) water filtered with Satorius Sartopore 2, 0.2 μm) and allowed to swell for 10 min. After flattening the sample by squeezing between two object slides, 10 μl of 1 : 1000 diluted SYBR Green I fluorescent dye (Life Technologies) was applied followed by incubation for 5 min in the dark and twofold rinsing with ultrapure water. A Leica TCS SP5II CLSM equipped with an HCX PL APO CS 100× objective was used to acquire image data (excitation wavelengths: 458/496 nm). CLSM data were processed with the software distribution Fiji (Schindelin et al. Reference Schindelin2012).
Uptake of saturated NaCl and MgCl2 solutions
Silica gel dry biofilm material (four replicates per treatment) was weighed before and after incubation in brine for 24 h at 22.5 ± 0.2 °C. One parallel of samples was incubated in DI water. Samples were filter centrifuged in tube filters (Corning Costar Spin-X with 0.2 μm cellulose acetate membrane) at 9500 g for 1 min to remove loosely associated brine or water. After weighting the samples their content of soluble was determined as follows: homogenization in DI water as described above for the recultivation experiment and filtration through glass fibre filters (MACHEREY-NAGEL MN 85/70). After evaporating the liquid in a drying oven at 110 °C, the remaining solid phases were weighed. For calculation of salt contents, the average solid phase mass of the DI water parallel was subtracted from the values obtained from the salt containing samples.
16S rDNA clone libraries
Accompanying bacteria were extracted as follows: biofilm material (100–500 mg) was incubated in 10 ml sterile 1× Phosphate Buffered Saline (PBS) for 1 h and homogenized and filtered as described above for the determination of liquid uptake. Cells were pelleted by centrifugation at 4000 g for 10 min and washed once with 1× Phosphate Buffered Saline (PBS). Gene matrix Soil DNA Purification Kit (EURx) was used for DNA extraction. Cloning (TOPcloner™ TA kit, Enzynomics) and sequencing with primer M13F was carried out by Macrogen Inc., South Korea.
Vector sequences including primer region and low-quality ends were trimmed manually by means of DNA Baser (DNA Baser Sequence Assembler v4.x (2014), HeracleBioSoft SRL, www.DnaBaser.com). Clone libraries were checked for chimeric sequences using DECIPHER (Wright et al. Reference Wright, Yilmaz and Noguera2012) and suspicious sequences were removed. Ribosomal database project Classifier Version 2.8 was used for classification of clone sequences with an assignment confidence cut-off of 80% according to Bergey's Taxonomic Outline of the Prokaryotes (Wang et al. Reference Wang, Garrity, Tiedje and Cole2007).
Results and discussion
Characterization of the samples
Halite and sodium chloride consist of nonporous crystals different to the layered structure of the smectite montmoreillonite. Sorption of water vapour can occur in the inner pore system and/or on the outer surface of the particles. Montmorillonite is able to sorb bigger amounts of water (see later) in agreement with the ability of the polar water molecule to penetrate into the interlayer spaces. The salt crystals offer the outer surface for sorption only which is a few orders of magnitude less surface area for sorption of water molecules as for the smectite. The SEM images (Fig. 1) give information about the morphology and particle size of the samples. Halite (left) shows particles of irregular shape and the pure NaCl indicate more regular cube-shaped particles as expected. The particle size distribution for both samples seems to be roughly the same with 10–100 μm generating a surface area of about 0.06 m2 g−1 much less compared with 200 m2 g−1 of the smectite (related to water).

Fig. 1. SEM images of Atacama halite (left) with impurity CaSO4·2H2O (mark, identified by EDX) and NaCl, purity 99.99% (right).
The EDX analysis of NaCl shows exclusively Na and Cl. Halite is almost pure NaCl but with some impurities of gypsum (CaSO4) and an aluminosilicateas identified by EDX (mapping). The marked particle on the image (left part of Fig. 1) consists of the elements Ca, S, O forming most probably gypsum.
N. commune biofilms are shown in Fig. 2. Dry Biofilms are rather compact and brittle and swell to thin gelatinous layers after being wetted. Filaments of spherical cells typical for Nostoc species can be seen in wet biofilms. Single cells and micro colonies of smaller bacteria are located on the irregularly shaped EPS surface, which can be observed by light microscopy as well as by CLSM of fluorescent stained biofilms (Fig. 3). None of these cells are visible within the EPS whereas cavities are inhabited. These accompanying bacteria might regularly colonize surfaces of N. commune biofilms to take advantage of the protective function of their EPS against desiccation or UV radiation. A provision of nutrients excreted by cyanobacterial cells is also conceivable.

Fig. 2. N. commune: dry biofilm (A); stereo microscope micrograph of wet surface with visible N. commune cell filaments (B); phase contrast micrograph of bacterial microcolony (black arrow) on EPS surface (white arrow) (C).

Fig. 3. CLSM scans of N. commune biofilm shortly after wetting: top views at different depths and corresponding cross-sections (depth and position of sections are indicated by cross-hairs); SybrGreen I stained small bacterial cells are displayed in green and N. commune cells in red (chlorophyll autofluorescence), DNA-rich parts of cyanobacterial cells appear yellow due to mixing of both colours.
Water sorption properties of the materials at equilibrium
Thermoanalysis gives first of all a quick overview of the water release and at further rising temperatures about the dehydroxylation and decomposition of the samples. Figure 4 summarizes the TG/DTG/DTA data for N. commune and Fig. 5 the TG/DTG data for halite and NaCl. The initial mass loss (desorption of H2O) ranges from 12 wt.% for N. commune to tiny amounts of less than 0.3 wt.% for halite. Halite and NaCl (cf. Fig. 5) do not form hydrates above 273 K and therefore does not decompose such as other hydrate forming salts usually do. In particular the pure NaCl does not show any mass loss up to 600 K. Natural halite containing traces of gypsum behaves differently. Gypsum forms a dihydrate upon contact with water vapour. Halite in Fig. 5 shows mass loss of about 0.15 wt.% at 403 K very close to the temperature typical for mass losses of gypsum because of release of its crystallization water (Paulik et al. Reference Paulik, Paulik and Arnold1992).

Fig. 4. TG (solid green line), DTG (dashed-dotted blue line) and DTA (dotted red line) profiles for N. commune.

Fig. 5. TG (solid line) and DTG (dashed-dotted line) of halite (green) and NaCl, Merck 99,99% (solid red line, top) stored at RH = 60% prior to the experiments, note the TG scaling compared with Fig. 4
N. commune decomposes in two steps (Fig. 4) starting with weakly bonded water (endothermic step at 345 K) followed by a considerable mass loss between 500 and 600 K due to further dehydration and dehydroxylation of the organic polymers and, finally, anaerobe decomposition of the entire material. This process is partially exothermic (cf. DTA) different to the heat consuming dehydration at the beginning of the TG and DTA curve. The residual material looks black like carbon what can be explained by the occurrence of pyrolysis.
Because our paper focuses until now on the physical interaction of water vapour with the biofilm, the first TG-step is of special attention (the reversibly bonded water at T < 400 K, Fig. 4). Further, the question arises: Is there any water uptake of the halite under defined humid conditions and low temperatures? Isotherm measurements can give that information in terms of equilibrium data of physically bonded water. Fig. 6 shows the H2O sorption isotherms of halite at different low Mars relevant temperatures. Figure 7 compares the isotherms of halite and NaCl at 293 K in more detail. As can be seen the Atacama halite and NaCl do not sorb much water below the deliquescence RH (DRH) = 75%. Above this value both salts start gaining weight due to forming a wet skin of saturated solution on the crystal's surface (Davila et al. Reference Davila, Duport, Melchiorri, Jänchen, Valea, de los Rios, Fairén, Möhlmann, McKay, Ascaso and Wierzchos2010; Hansen-Goos et al. Reference Hansen-Goos, Thomson and Wettlaufer2014). Deliquescence continues at constant RH converting the solid salt surface step by step into a concentrated salt solution. Interestingly, the DRH depends somewhat on temperature in our experiments.

Fig. 6. Water isotherms of Atacama halite upon outgassing at 413 K at different temperatures: 256 K (squares), 273 K (triangles); filled symbols and dashed line denote dehydration.

Fig. 7. Water isotherms of halite (triangles, circles) and NaCl (diamonds, squares) at 293 K upon outgassing at room temperature in high vacuum overnight (triangles, diamonds) or outgassing at 413 K in high vacuum (circles, squares). Filled symbols and dashed lines denote dehydration. Note the extended view of the low sorbed amounts (RH < 70%) by logarithmic scaling.
A closer look to the water sorption below the deliquescence point is given for 293 K in Fig. 7 (note the logarithmic scale of the ordinate). Sorbed amounts of 0.0002–0.002 g g−1 for RH < 75% could be detected. Despite being close to the resolution of the method (see above) a tendency of higher sorbed amounts for halite degassed at elevated temperature is likely. Owing to the gypsum impurity of the halite the crystallization water of gypsum which had been removed at the elevated temperature is sorbed afterwards as extra amount by hydration of gypsum. In the case of degassing at room temperature the halite behaves like the pure NaCl taking some less water. This very small amount of water is due to the formation of pre-deliquescence layers on the crystal surfaces as reported by Bruzewicz et al. (Reference Bruzewicz, Checco, Ocko, Lewis, McGraw and Schwartz2011) and Hansen-Goos et al. (Reference Hansen-Goos, Thomson and Wettlaufer2014). Layer heights of some nm were calculated by Hansen-Goos in accordance with our findings taking into account the surface area estimated for the crystals in halite or the pure NaCl.
The hydration and dehydration isotherms of N. commune are displayed in Fig. 8 for temperatures of 257–293 K. The sorbed amount of about 0.17 g water g−1 vacuum dry sample at RH = 80% corresponds to the first TG-step (weakly bonded H2O below 400 K) of N. commune in Fig. 4. A detailed study of this ‘reversible’ water sorption was thus carried out by isotherm measurements. The amount of adsorbed water varies with the relative vapour pressure (0.001–0.9, corresponding to 0.1–90% RH in Fig. 8) and amounts to 0.01–0.25 g g−1 (corresponding to 1–25%). Thus about 25 wt.% water represents the somewhat less-strongly bonded water of the much higher total quantity bearing by the biofilm.

Fig. 8. Water sorption isotherms (water vapour uptake and release at equilibrium) for N. commune at 257 K (triangles), 273 K (squares) and 293 K (first run diamonds, second run squares), filled symbols and dashed lines denote dehydration.
Striking is the pronounced hysteresis between hydration and dehydration not observed for the lichen Xanthoria elegans or Leptothrix biofilms (cf. Jänchen et al. Reference Jänchen, Bauermeister, Feyh, de Vera, Rettberg, Flemming and Szewzyk2014). This may indicate a superior ability of N. commune to store water if compared with lichens and Leptothrix biofilms. Cyanobacterial photobionts as part of a lichen symbiosis are known to have an elevated water storage capacity and can contain more water in the saturated state than algal photobionts due to the formation of larger layers and the production of EPS (Gauslaa & Coxson Reference Gauslaa and Coxson2011). This has been explained by the observation, that lichenized algal photobionts can become photosynthetically active by uptake of humidity from the air, whereas lichens with a cyanobacterial photobiont and terrestrial N. commune colonies depend on the direct uptake of liquid water (e.g. from rain or by condensation of water vapour) to perform photosynthesis (Lange et al. Reference Lange, Kilian and Ziegler1986, Reference Lange, Büdel, Meyer and Kilian1993).
N. commune takes advantage of the ability to excrete large amounts of EPS and thus to save water in the biofilm for longer periods at lower RH. High moisture absorption and retention capacities have been reported as well for isolated polysaccharides of N. commune (Li et al. Reference Li, Xu, Liu, Ai, Qin, Li, Zhang and Huang2011). As a result, desiccation is slowed down and thus the period, when photosynthesis is possible is extended (Gauslaa et al. Reference Gauslaa, Coxson and Solhaug2012).
Figure 9 gives a comparison of the hydration and dehydration characteristics of N. commune with montmorillonite and halite as function of the RH. This comparison holds well for T ≪ 293 K and shows N. commune as much more hydrophilic than halite and similarly hydrophilic as the smectites. Thus halite can provide N. commune with liquid water because of deliquescence at the DRH = 75–80% even below 273 K. Application of the Dubinin equation on the isotherm data of N. commune (Fig. 6), as shown in (Jänchen et al. Reference Jänchen, Bish, Möhlmann and Stach2006) for the smectite, provides the data in a water amount/temperature plot at a certain water vapour pressure.

Fig. 9. Comparison of water isotherms at 273 K for montmorillonite (squares), N. commune (circles) and halite (triangles); filled symbols and dashed lines denote dehydration.
Thus detailed information about the physicochemical interaction of halite, montmorillonite and N. commune with water vapour and the deliquescence of halite forming a saturated solution on its surface has been obtained and documented in Fig. 9. However, it is also important to have information about the desiccation and saline tolerance of N. commune and the associated heterotrophic bacteria as part of the biofilm in contact with liquid phases. The following sections draw attention to this issue.
Recultivation of heterotrophic bacteria from N. commune samples
Heterotrophic plate counts for different treatments of biofilms are shown in Fig. 10. Colony-Forming Unit (CFU) numbers obtained from the silica gel dry controls, which have been in a dry state for at least 5 years were in a range of 6.2 × 106–2.3 × 107 g−1 dry weight, which is comparable with experimental data of various soil samples yielded under similar cultivation conditions (Iivanainen et al. Reference Iivanainen, Martikainen, Räisänen and Katila1997; Adesina et al. Reference Adesina, Lembke, Costa, Speksnijder and Smalla2007; Desai et al. Reference Desai, Parikh, Vaishnav, Shouche and Madamwar2009). Water uptake at RH = 75% (14.13 ± 0.76 wt.%) did not lead to a significant change in CFU number compared with the silica gel dry controls. A 24-fold decrease of CFU was observed for samples incubated in NaCl brine. All sterility controls showed no growth.

Fig. 10. Heterotrophic plate counts (logarithmic scale) per g silica gel dry sample for three different treatments: storage over silica gel until equilibration, at RH = 75% or in saturated NaCl solution.
Our results suggest a high desiccation tolerance of the associated heterotrophs. This could be explained by an adaptation of the biofilm's microbiota to the lifestyle of N. commune. As already mentioned in section ‘Water sorption properties of the materials at equilibrium’ the cyanobacterium needs to be in contact with liquid water to perform photosynthesis and thus has to be able to endure long periods of desiccation between events of rain or condensation of water (Lange et al. Reference Lange, Kilian and Ziegler1986, Reference Lange, Büdel, Meyer and Kilian1993; Gauslaa et al. Reference Gauslaa, Coxson and Solhaug2012). This would also explain the lacking benefit from uptake of small amounts of water for recultivability, since it would be unfavourable for the accompanying microbiota to become metabolically active under circumstances, where the biofilm host is not close to leaving dormancy. Survival of long periods in a desiccated state by the heterotrophs could also be enhanced by a protective function of the cyanobacterial EPS. Since the EPS surface has an irregular shape (especially, when the biofilm is dry, see Figs. 2 and 3), small bacterial cells are enclosed in cavities. Beside a protection against mechanical stress, this sheltered position can also help to avoid UV damage.
The brine treatment showed that only a fraction of 4% of the heterotrophs can grow under highly saline conditions. This is in agreement with the moderate tolerance of the biofilm forming host to NaCl resulting in an inhibition of photosynthesis at a concentration of 0.2 M or 30 g kg−1 (0.51 M) (Sakamoto et al. Reference Sakamoto, Yoshida, Arima, Hatanaka, Takani and Tamaru2009; Sand-Jensen & Jespersen Reference Sand-Jensen and Jespersen2012).
Uptake of water, NaCl and MgCl2 brines by N. commune biofilm
The uptake of DI water or saturated NaCl/MgCl2 solutions by dry biofilms within 24 h is listed in Table 1. While an amount of around 900% water was taken up, the salt solutions contributed to approximately a third of the final sample weight. The measured salt contents in the brines taken up were near the values of saturated solutions at 20 °C for NaCl (26.4 wt.%) and MgCl2 (35.3 wt.%).
Table 1. Liquid uptake by N. commune: weight percentages of liquids (taken up by whole biofilms), solubles/remnants (of wet homogenized biofilms after filtration) and calculated mass fractions of salts in the brine (values are means ± standard deviations)

As mentioned earlier, N. commune has been found to be only moderately salt tolerant and thus, the cyanobacterial colonies would not be viable in such a brine-soaked state. Nevertheless, it has been shown, that a community consisting of cyanobacteria and heterotrophs can be supported through exclusive water uptake from a brine formed by deliquescence on surrounding halite (Wierzchos et al. Reference Wierzchos, Ascaso and McKay2006; de los Ríos et al. Reference de los Rios, Valea, Ascaso, Davila, Kastovsky, McKay, Gómez-Silva and Wierzchos2010; Davila et al. Reference Davila, Hawes, Ascaso and Wierzchos2013; Robinson et al. Reference Robinson2015).
16S rDNA cloning
Affiliations of clone sequences to different phyla and classes are shown in Fig. 11 and Table 2. High proportions of sequences were assigned to the Alphaproteobacteria group (31.1–92.7%), to which Sphingomonadaceae constituted the major part (8.9–61.0%). This was more obvious for the long-time desiccated samples, indicating a high desiccation tolerance of both groups. Sequences of N. commune (assigned to genus GpI, see Table 2) were only present in the library of the short-time desiccated sample OT1. This result might be caused by cyanobacterial cells of long-time desiccated biofilms being less prone to damage during homogenization in the course of cell extraction.
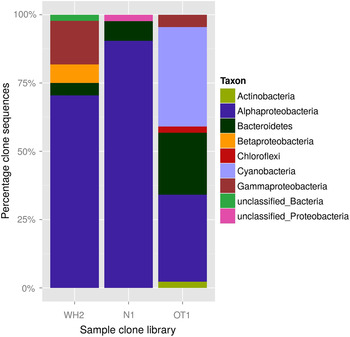
Fig. 11. Comparison of clone libraries from long-time desiccated samples WH2 and N1 together with the one from short-time desiccated sample OT1: percentage of clone sequences assigned to different taxa and unclassified groups.
Table 2. Clone sequences classified on genus level and the share of each phylum/class to the total clone sequence number in the sample library

Implications of the Martian surface conditions on habitability
A decade ago it was believed that the thin Martian atmosphere (about 6 mbar) with its very low water vapour pressure (about 0.001 mbar) cannot offer a liquid phase particularly in warmer equatorial latitudes serving as water source for possible biological activities. But the Martian soil should offer at least for some hours in a diurnal circle a liquid water phase (by condensation or hydration of minerals) for the organisms to become viable in the proposed biofilms being in contact with the ‘wet’ soil components. The biofilm may extend the presence of water because of its storage capability but the source must be the atmosphere. Möhlmann suggested three different sources of liquid water feed by the atmosphere in equatorial latitudes at the Martian surface: adsorption water on mineral surfaces, water from temporary melting processes in upper sub-surface parts of snow/icepacks and cryobrines formed by deliquescence of salts (Möhlmann Reference Möhlmann2008, Reference Möhlmann2010a, Reference Möhlmannb). The formation of so-called cryobrines on salts such as halite is one of the nominees to provide a liquid phase under close to Martian environmental conditions for possible biological activity beside geological and chemical processes (Möhlmann Reference Möhlmann2011).
Perchlorate measured at NASA's Phoenix Lander site and the formation of possibly liquid perchlorate at the lander's stud, as discussed by Renno et al. (Reference Renno2009a, Reference Rennob) and Chevrier et al. (Reference Chevrier, Hanley and Altheide2009) as well as the already mentioned results of Curiosity at Gale crater (Martín-Torres et al. Reference Martín-Torres2015) are in principle other examples for providing liquid phases on present Mars. Even though a strongly oxidizing perchlorate solution might be poisonous for many organisms members of the proteobacteria have been found to be able to reduce perchlorate in diluted solution (Coates et al. Reference Coates, Michaelidou, Bruce, O'Connor, Crespi and Achenbach1999).
Brines formed on halite would be probably more life supporting and a suitable source for water as found for cyanobacteria in the Atacama Desert. But, as observed in the Atacama Desert, a potential source of water should stay at least for some time in a diurnal circle liquid at Martian surface conditions in mid- and low-latitudes. Fig. 12 gives information about the stability of the NaCl brine at the Martian surface for equatorial latitudes. The straight (red) line in Fig. 12 characterizes the partial pressure of water vapour in the Martian atmosphere between 230 and 273 K. The progression of the effective vapour pressure of NaCl brine with temperature is documented by the concave line. The calculated pressure values are lower than the equilibrium data because the stability of the brine is better due to the presence of the CO2 atmosphere. Hence, approximately 6 mbar (0.06 Pa) CO2 of the Martian atmosphere reduces the vaporization rate in the diurnal circle (day vaporization/night condensation and deliquescence) significantly (based on Taylor et al. Reference Taylor, Baibakov, Brown and Hecht2006). Accordingly, NaCl brine should ‘survive’ below 265 K to sustain biological processes of halotolerant cyanobacterial biofilm communities.

Fig. 12. Atmospheric water vapour pressure (red straight line) and the effective water vapour pressure of NaCl solution on the Martian surface (calculations by D. Möhlmann).
According to our findings a hypothetical Mars bacterial community might be likely to succeed as life in microhabitats under present Martian surface conditions. This community could consist of a halotolerant primary producer, which had to be able, to gain enough energy to produce a large, hydrophilic EPS, similar to N. commune. As shown in section ‘Uptake of water, NaCl and MgCl2 brines by N. commune biofilm’, such an EPS is able to take up about 300% of saturated NaCl brine in direct contact to the liquid, which is then held back when the conditions get drier due to the water storing properties of the biofilm matrix. This reservoir can then be used by the primary producer as well as by likewise halotolerant heterotrophs inhabiting the biofilm as a protective niche. Clays, having similarly hydrophilic properties as N. commune (see Fig. 9), could add to enhanced water storage as a component of soil in contact to the biofilm-communities.
Conclusions
-
- Deliquescence of halite at low temperatures (<273 K) provides liquid water by forming a cryobrine at RH values<80% and might have an astrobiological potential for Mars.
-
- The stability of the NaCl cryobrine (concentrated halite solution) on the Martian surface at equatorial latitudes is better than equilibrium data suggest because of the presence of the CO2 atmosphere reducing the vaporization rate (day/night) significantly.
-
- Biofilms protecting microorganisms on Earth against desiccation show a rather high hydrophilic character compared with smectites. The biofilm of N. commune tends to be able to store water because of the hydrophilic EPS, documented by the hysteresis between the hydration and dehydration branch of the isotherms.
-
- The water content of the soil components and bio-materials changes with its nature and depends strongly on temperature and partial pressure. Knowing the local humidity of a planet's atmosphere the water content of these materials can be determined. Supply of this data may support the evaluation of spectroscopic results from orbit or rovers (MSL, ExoMars/MicrOmega).
-
- A high desiccation tolerance of heterotrophic bacteria associated to N. commune was emphasized by recultivation tests. The protective function of a large EPS against desiccation would be a benefit as well in a water-limited environment like Mars.
-
- The heterotrophic microbiota of the investigated N. commune biofilms is dominated by Alphaproteobacteria and might be adapted to their host's long-time desiccation enduring lifestyle. Water uptake from saturated NaCl solutions (either by uptake of brine or humidity) does not enhance recultivability of the heterotrophs. Part of the microbiota is far more tolerant to highly saline conditions, than the cyanobacterial host.
-
- Summarizing it can be stated that the current Martian surface conditions at equatorial latitudes might offer at least temporarily niches with habitable conditions for halotolerant organisms. Habitable means the presence of liquid water as brine at surface temperatures of about 255–265 K and RH < 80%. A N. commune-type biofilm together with montmorillonite could in this case serve as a habitat for heterotrophic bacteria by uptake and retention of briny water.
Acknowledgements
We thank Alfonso F. Davila for supply of the Atacama halite sample and Dirk Möhlmann (DLR, Berlin) for his contribution to section ‘Implications of the Martian surface conditions on habitability’. This research was supported by the Helmholtz Association through the research alliance ‘Planetary Evolution and Life’ as well as funded by a grant of the BMBF (50WB1151) and is part of the current BIOMEX mission (ESA call, 2009, Ref.-No. ILSRA-2009-0834).
















