The metabolic syndrome has become a major public health problem in the whole world. It is characterised by the clustering of risk factors, including insulin resistance, obesity or abdominal obesity, hypertension and dyslipidaemia in an individual which dramatically increases the risk of developing CVD and type 2 diabetes mellitusReference Plutzky1. Momordica charantia, the fruit of which is known as karella, bitter gourd or bitter melon (BM), is a common edible vegetable in Asia. Physiological benefits, including hypoglycaemia, hypolipidaemia, anti-virus and anti-carcinogenic effects, have been reported, but the mechanisms and functional components remain to be elucidatedReference Grover and Yadav2, Reference Krawinkel and Keding3.
Using a transactivation assay, we found that an ethyl acetate extract of BM activates both PPARα and PPARγReference Chao and Huang4. PPAR are ligand-activated transcription factors belonging to the nuclear receptor superfamily. Three subtypes (PPARα, β and γ) have been identified and shown to play a key role in the control of lipid and glucose homeostasis as transcription factors regulating genes encoding enzymes involved in these processesReference Desvergne and Wahli5. Fibrate-class hypolipidaemic drugs and thiazolidinedione (TZD)-class anti-diabetic drugs are, respectively, specific PPARα and PPARγ ligands, but efforts are now being made to screen for and develop PPARα and γ dual agonists, focusing on the metabolic syndrome, to resolve the problems of insulin resistance, hyperlipidaemia and central obesity simultaneouslyReference Berger, Akiyama and Meinke6. PPAR activators of food or diet origin may provide health benefits without toxicity concerns, as long as the food or diet is consumed in a reasonable amount on a regular basis. With the reported PPARα and PPARγ activation capabilityReference Chao and Huang4, BM is therefore implicated in a potential dietary intervention regimen for the prevention and amelioration of the metabolic syndrome.
BM has also been shown to reduce the accumulation of visceral fat in high-fat (HF) diet-fed rats, and it was suggested that the insulin-sensitising and glucose-lowering benefits of BM might be due to this anti-adiposity effectReference Chen, Chan and Li7. Circulating levels of catecholamine and NEFA, lipid oxidation in the liver and muscle and mitochondria uncoupling are reported to be all increased in BM-supplemented ratsReference Chen and Li8, Reference Chan, Chen, Go, Lam and Li9. However, morphometric and metabolic changes in adipocytes have never been explored. Since it is believed that hypertrophic adipocytes reduce insulin sensitivity by releasing cytokines that interfere with insulin signallingReference Arner10, the impact of BM on cell size and lipid metabolism in adipose tissues warrants evaluation. These effects were investigated in experiment 1 of the present study and compared with a selective PPARγ agonist, TZD, which ameliorates insulin resistance but is also well known for its adipogenic side effectReference Lambe and Tugwood11. As the results obtained in experiment 1 implied that lipogenesis in adipose tissues is decreased by BM supplementation, the gene expression of lipogenic enzymes was further examined in experiment 2.
Materials and methods
Preparation of lyophilised bitter melon powder
The wild BM (M. charantia L.) used in the present study was provided by the Hualien District Agricultural Research and Extension Station, Agricultural Council, Executive Yuan, Taiwan. After washing and slicing, the whole fruit (including seeds) was freeze-dried and powdered. In a previous study, although the ethyl acetate extract from seed or flesh activated PPARα in a transactivation assay, the extract from the whole fruit showed still higher activating potency than the seed and fleshReference Chao and Huang4. Therefore, we chose the whole fruit to be used in the present study. Proximate analysis showed that the lyophilised BM powder contained 3·8 % water, 38·2 % dietary fibre, 6·7 % minerals, 4·5 % crude protein and 2·7 % crude fat.
Animals and diets
Male Wistar rats (aged 6 weeks; purchased from the Animal Centre of the National Taiwan University) were housed individually in stainless-steel wire cages in a room maintained at 23 ± 2°C on a controlled 12 h light–dark cycle, with free access to feed and tap water. The feed intake was recorded every 2 d and the body-weight gain weekly. The protocols for animal care and handling were approved by the Institutional Animal Care and Use Committee of the China Medical University. Table 1 shows the compositions of the four test diets: a low-fat (LF) diet (5 % fat; AIN-76) and three HF diets (unsupplemented HF diet, HF and BM (HFB) diet, and HF and TZD (HFT) diet; 30 % fat), following Hsu & HuangReference Hsu and Huang12. In the HFB diet, 5 % (w/w) BM powder was substituted into the HF diet for the equivalent amount of protein, fat, cellulose and minerals. In the HFT diet, TZD was added to the HF diet as 0·01 % pioglitazone, kindly supplied by Takeda Pharmaceutical Company Ltd (Osaka, Japan); this dose is equivalent to about 5 mg/kg body weight per d.
Table 1 Composition of the test diets

LF, low-fat control diet; HF, high-fat control diet; HFB, high-fat diet containing 5 % bitter melon (Momordica charantia) powder; HFT, high-fat diet containing 0·01 % pioglitazone.
* AIN-76 mineral mixture and AIN-76 vitamin mixture.
† Proximal analysis showed that the lyophilised bitter melon powder contained 3·8 % water, 38·2 % fibre, 6·7 % ash, 4·5 % protein and 7 % fat.
Experiment 1
The aim of this experiment was to examine the anti-adiposity and insulin-sensitising effects of BM and compare them with those of TZD. Twenty-four rats were fed the HF diet for 3 weeks (weeks − 3 to − 1) to induce obesity (diet-induced obesity; DIO), and then separated into the HF, HFB and HFT groups (eight rats per group). As it was possible that the bitter taste of BM might cause a drop in feed intake, the HFB group underwent an adaptation period (week 0) in which they were given diet containing 1 % BM, which was raised to 3 % after 2 − d, then to 5 % after 3 − d. During this period, the HF and HFT groups received the HF diet. After this period, the rats were formally separated into three groups and fed their individual diets for 9 weeks (weeks 1–9). In addition, an LF control group (n 8) received the LF diet for the whole study period (weeks − 3 to 9). In experiment 1, bio-indexes for insulin sensitivity and tissue mass, cell size, lipid metabolism and mRNA levels of mature adipocyte markers in adipose were measured.
Experiment 2
The aim of this experiment was to test if lipogenic gene expression was reduced in the adipose tissue of BM-treated rats. Rats were initially fed an LF (n 6) or HF diet (DIO; n 12), then the DIO rats were divided into two groups of six to receive the HF or HFB diet for 9 weeks with the habituation process, exactly as described for experiment 1. At the end, rats were killed in the fed state. The retroperitoneal, epididymal and inguinal fats were excised and weighed. The adipose tissue was stored at − 70°C for RNA isolation. The mRNA levels of lipogenic genes and their transcriptional regulator, PPARγ and adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c (ADD1/SREBP-1c) were measured in retroperitoneal and epididymal fat.
Adipose cell size
The method used to measure adipose cell size was a slight modification of that of Hirsch & GallianReference Hirsch and Gallian13. Briefly, fat slices ( < 200 mg) cut from the epididymal and retroperitoneal fat were rinsed with saline at 37°C, then fixed in 2 % osmium tetroxide in collidine–HCl buffer (pH 7·4) at room temperature. After 3 d, the fixation solution was removed and replaced by saline for 24 h, then the saline was removed and 10 ml of 8 m-urea added for 24 h with occasional swirling to liberate the cells. Finally, the adipocytes were isolated by successive filtering through nylon mesh screens with diameters of 235 and 10 μm and washed with 0·01 % Triton X-100 in distilled water. The numbers of adipocytes with different diameter ranges were counted using a Coulter counter (Coulter Corporation, Miami, FL, USA).
Lipid content of adipose tissue
For the analysis of adipose lipid content, total lipid was extracted from the epididymal and retroperitoneal fat using the Folch methodReference Folch, Lees and Sloane Stanley14. The total lipid content of the adipose tissue samples was determined by weighing after complete removal of the organic solvent.
Activities of lipid metabolism enzymes
Glycerol-3-phosphate dehydrogenase (G3PDH), a key lipogenic enzyme in adipose tissue, was measured following Kozak & JensenReference Kozak and Jensen15. Briefly, the adipose tissue was homogenised in 4 volumes of ice-cold 50 mm-tri(hydroxymethyl)-aminomethane (tris) buffer (pH 7·5) containing 1 mm-EDTA, 1 mm-β-mercaptoethanol and 0·5 % Triton X-100. After sequential centrifugations at 10 000 g for 15 min and 100 000 g for 1 h (4°C), the final supernatant fraction was collected as the enzyme source. An appropriate amount of enzyme solution was incubated with 1 ml of substrate solution (100 mm-triethanolamine–HCl buffer (pH 7·5) containing 2·5 mm-EDTA, 0·12 mm-NADH, 0·2 mm-dihydroxyacetone phosphate and 0·1 mm-β-mercaptoethanol) and the decrease in the absorbance at 340 nm recorded over time (Hitachi U2001; Tokyo, Japan), and the enzyme activity expressed as nmol NADH consumed/min per mg protein.
The rate of lipolysis in adipose tissue was measured by the method of Morimoto et al. Reference Morimoto, Kameda, Tsujita and Okuda16. Adipose tissue (0·5 g) was minced and incubated in 2 ml of 25 mm-N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid buffer (pH 7·4) containing 135 mm-NaCl, 5 mm-KCl, 1 mm-MgCl2 and 2·5 % bovine serum albumin, with or without 1 μm-isoproterenol (a β-adrenol stimulator), at 37°C for 0, 1 or 2 h, then the glycerol released into the medium was measured using a commercial kit (Randox Laboratories, Crumlin, Co. Antrim, UK).
Oral glucose tolerance test
In experiment 1, an oral glucose tolerance test was performed on rats fed the experimental diets for 6 weeks. On the test days, after overnight food deprivation, blood was collected from the tail before (0 min), and at 30, 60, 90 and 120 min after, oral administration with a 2·5 m-glucose solution (1·5 g/kg body weight) and glucose measured as described below, then the area under the curve for serum glucose over the 2 h was calculated.
Serum measurements
Fasting serum obtained by tail bleeding was used for glucose and insulin measurements using, respectively, a glucose oxidase colorimetric assay (Randox Laboratories, Crumlin, UK) or an ELISA kit (Mercodia, Uppsala, Sweden). Fasting blood was collected from the abdominal vena cava, and serum TAG, cholesterol and adiponectin were determined. TAG and cholesterol were measured by enzymic methods (Randox Laboratories, Crumlin, UK), while adiponectin was measured using a mouse/rat adiponectin ELISA kit (B-Bridge, Sunnyvale, CA, USA).
RNA isolation and mRNA detection
Total RNA was extracted from homogenised adipose tissue using TRIZOL reagent according to the manufacturer's instructions (Invitrogen, New York, NY, USA). The quality of the extracted RNA was confirmed by a value of 2 for the 28S ribosomal RNA:18S ribosomal RNA ratio after ethidium bromide staining. In experiment 1, levels of mRNA for PPARγ and ADD1/SREBP-1c were measured by Northern blotting as previously describedReference Hsu and Huang12. In experiment 2, levels of mRNA for fatty acid synthase (FAS), acetyl-CoA carboxylase-1 (ACC-1), lipoprotein lipase (LPL), adipocyte fatty acid-binding protein (aP2), PPARγ and ADD1/SREBP-1c were measured by real-time PCR. Total RNA (1 μg) was reverse-transcribed into first-strand cDNA using 200 units of MMLV-RT (Promega, Medison, WI, USA). PCR was performed using 50 ng cDNA, 2 × SYBR® Green PCR Master Mix (Applied Biosystems, Foster, CA, USA) and 200 nm of the primer pair. The sequences of the PCR primers used are shown in Table 2. In this assay, 36B4 was used as an internal control. Amplification using forty cycles of two steps (95°C for 15 s and 60°C for 1 min) was performed on an ABI Prism® 7900HT sequence detection system (Applied Biosystems, Foster Ciry, CA, USA). To confirm the amplification of specific transcripts, melting-curve profiles were produced at the end of each run.
Table 2 Sequences of the polymerase chain reaction primers and GenBank accession numbers

F, forward; R, reverse; ADD1/SREBP-1c, adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c; FAS, fatty acid synthase; ACC-1, acetyl-CoA carboxylase-1; LPL, lipoprotein lipase; aP2, adipocyte fatty acid-binding protein; 36B4, acidic ribosomal phosphoprotein P0.
Statistical analysis
Data are expressed as mean values and standard deviations for the eight (experiment 1) or six (experiment 2) rats per group. The significance of differences between groups was analysed using one-way ANOVA and Duncan's multiple-range tests. The data were transformed to log values for the statistical analysis if the variances were not homogeneous. The general linear model of the SAS package (SAS Institute Inc., Cary, NC, USA) was used for both statistical analyses, and differences were considered significant at P < 0·05.
Results
Experiment 1
Body weight, feed intake and adipose tissue weight
Fig. 1 shows the growth curve for the rats over the entire study period (weeks − 3 to 9). From week 3 till the end of the study, the HFT group had a significantly higher body weight than the LF group (P < 0·05), while the HFB and HF groups had intermediate values. Table 3 shows the body-weight gain, feed intake, feed efficiency and adipose tissue weight of the four groups of rats. There was no difference in body-weight gain between the three high-fat diet-fed groups. Among the three groups, feed intake was significantly higher in the TZD-treated group (HFT), but not changed in the HFB group, indicating the bitter taste of BM did not affect food intake. The weights of the retroperitoneal and epididymal fat pads in the HF and HFT groups were significantly higher than those in the LF and HFB groups (P < 0·05). The inguinal fat pad weight was significantly higher in the HFT group than in the remaining three groups (P < 0·01).

Fig. 1 Growth curves for rats fed the low-fat control diet (○), high-fat control diet (●), high-fat diet containing bitter melon (Momordica charantia) powder (Δ) or high-fat diet containing pioglitazone (▲) (experiment 1). The three time periods are induction (weeks − 3 to − 1), adaptation (week 0) and treatment (weeks 1–9). Values are means, with their standard deviations represented by vertical bars. The significance of differences between the groups was analysed by one-way ANOVA and Duncan's multiple-range test. a,b,c Values with unlike letters were significantly different (P < 0·05).
Table 3 Body-weight gain, feed and energy intake, feed efficiency and adipose tissue weight of rats fed the low-fat control diet (LF), high-fat control diet (HF), high-fat diet containing bitter melon (Momordica charantia) powder (HFB) or high-fat diet containing pioglitazone (HFT) (experiment 1)*
(Mean values and standard deviations for eight rats per group)
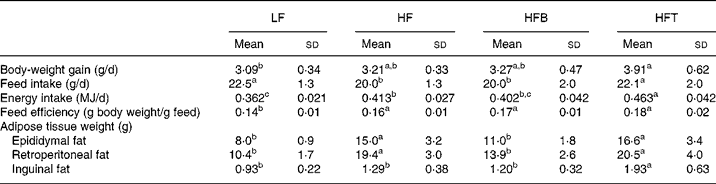
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* The significance of differences between the four groups was analysed by one-way ANOVA and Duncan's multiple-range test.
Oral glucose tolerance test and serum biochemical index
In experiment 1, an oral glucose tolerance test was performed at week 6. The area under the curve for serum glucose over the 2 h after an oral glucose dose was significantly increased by the HF diet (HF v. LF; P < 0·05). However, when BM or TZD was incorporated in the HF diet, the area under the curve for serum glucose was significantly lowered to a level comparable with that in the LF group (Table 4).
Table 4 The area under the curve for glucose (AUCglu) and serum glucose, insulin, adiponectin, triacylglycerol and cholesterol concentrations of rats fed the low-fat control diet (LF), high-fat control diet (HF), high-fat diet containing bitter melon (Momordica charantia) powder (HFB) or high-fat diet containing pioglitazone (HFT) (experiment 1)*
(Mean values and standard deviations for eight rats per group)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* The significance of differences between the four groups was analysed by one-way ANOVA and Duncan's multiple-range test.
† Area under the curve over the 2 h during an oral glucose tolerance test.
Fasting serum glucose and insulin levels were monitored at weeks 0, 3, 6 and 9. There was no difference in serum glucose level between the groups at basal (data not shown), and after dietary treatment for 3, 6 and 9 weeks (Table 4). However, hyperinsulinaemia was observed in the HF group from week 3 till the end of the study (HF v. LF; P < 0·05), but not in the DIO rats receiving BM or TZD. Table 4 also shows the serum adiponectin, TAG and cholesterol levels at the end of the study. Serum lipids did not differ between the four groups. Adiponectin levels in the HFT group were significantly higher than those in the LF group, but there was no significant difference between the three high-fat diet-fed groups.
Adipocyte size and triacylglycerol content
Figs. 2 (A) and (B) show the size distribution of adipocytes isolated from the retroperitoneal and epididymal fat pads of the rats in experiment 1. In the two fat depots, the HF diet resulted in a significantly higher number of large adipocytes (>180 μm) as compared with the LF group (P < 0·05). When TZD was incorporated in the HF diet, a marked reduction in the number of large cells (>180 μm) in retroperitoneal fat was observed. Compared with the HF group, the number of cells with a diameter >180 μm was significantly lower in the HFB group (P < 0·05) in both retroperitoneal and epididymal fat pads. In addition, the number of small cells (20–60 μm) in retroperitoneal fat was significantly higher in the HFB group than in the HF group (P < 0·05). As shown in Fig. 2 (C), TAG content of the adipose tissues in the HFB group was comparable with that in the LF group, but was significantly lower than that in the retroperitoneal fat in the HFT group (P < 0·005) and that in the epididymal fat in the HF group (P < 0·01). These results show that BM inhibits adipocyte hypertrophy in DIO rats.
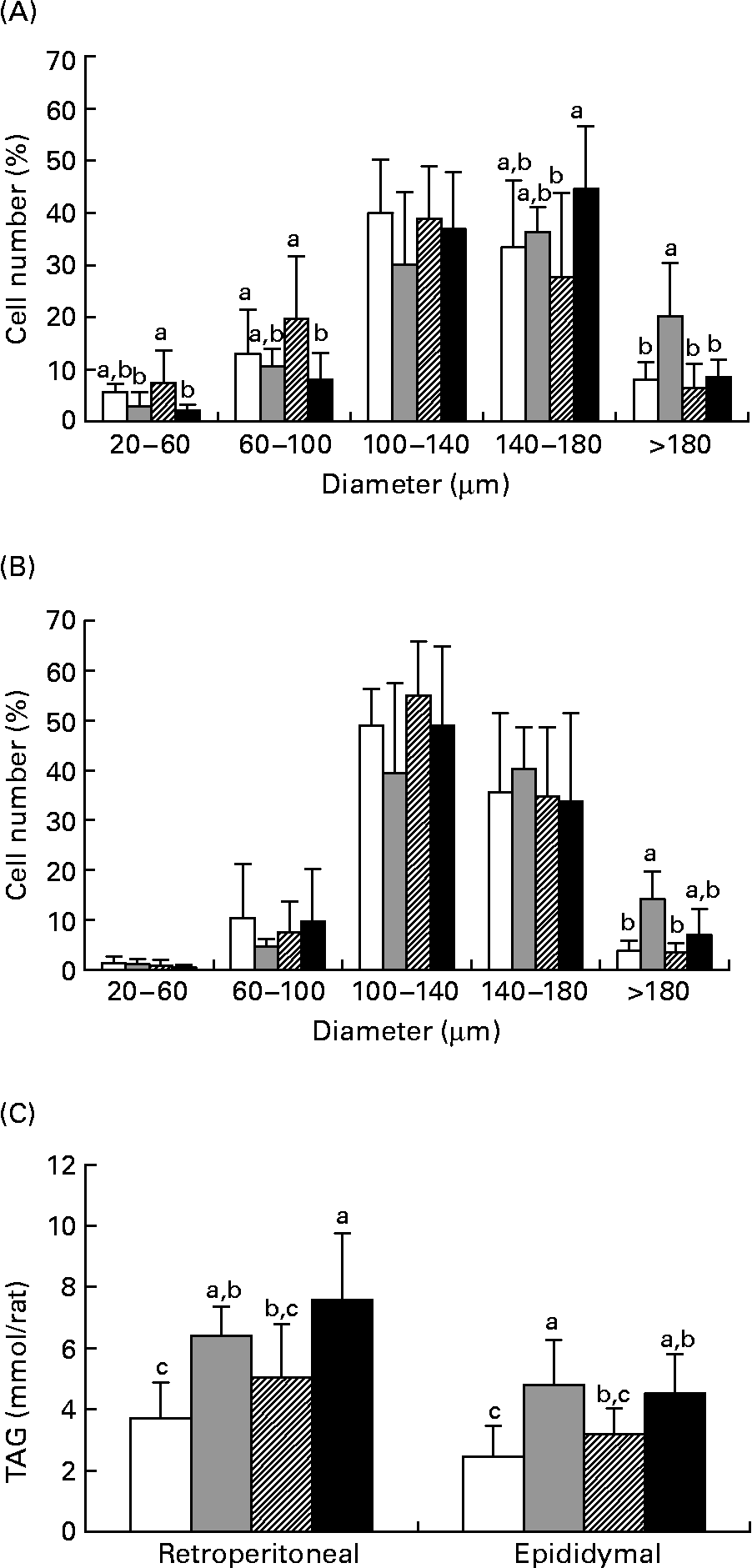
Fig. 2 Diameter distribution curves of adipocytes in the retroperitoneal (A) and epididymal (B) fat and TAG content (C) in the retroperitoneal and epididymal fat of rats fed the low-fat control diet (□), high-fat control diet (![]() ), high-fat diet containing bitter melon (Momordica charantia) powder (
), high-fat diet containing bitter melon (Momordica charantia) powder (![]() ) or high-fat diet containing pioglitazone (■) (experiment 1). Values are means, with their standard deviations represented by vertical bars. The significance of differences between the groups was analysed by one-way ANOVA and Duncan's multiple-range test. a,b,c Values with unlike letters were significantly different (P < 0·05).
) or high-fat diet containing pioglitazone (■) (experiment 1). Values are means, with their standard deviations represented by vertical bars. The significance of differences between the groups was analysed by one-way ANOVA and Duncan's multiple-range test. a,b,c Values with unlike letters were significantly different (P < 0·05).
Adipocyte lipid metabolism
The rates of lipogenesis and lipolysis in adipose tissue were measured in experiment 1. G3PDH is a lipogenic enzyme in adipose tissue responsible for directing the glycolytic intermediates into TAG synthesis. Fig. 3 (A) shows the G3PDH activity in the retroperitoneal and epididymal fat of the four groups and the result almost paralleled data of their TAG content (see Fig. 2 (C)). In both fat depots, the HFT group had the highest G3PDH activity, which was significantly higher than that in the HFB and LF group (P < 0·05), but was comparable with that in the HF group. Basal and stimulated lipolysis was also determined. The lipolysis rate on stimulation with 10 μm-isoproterenol is shown in Figs. 3 (B) and (C). Again, the HFT group had the highest value for the stimulated rate, while that in the HFB group did not differ from those in the HFT or HF group. The difference in basal lipolysis rate between the four groups was similar to that in the stimulated rate (data not shown).
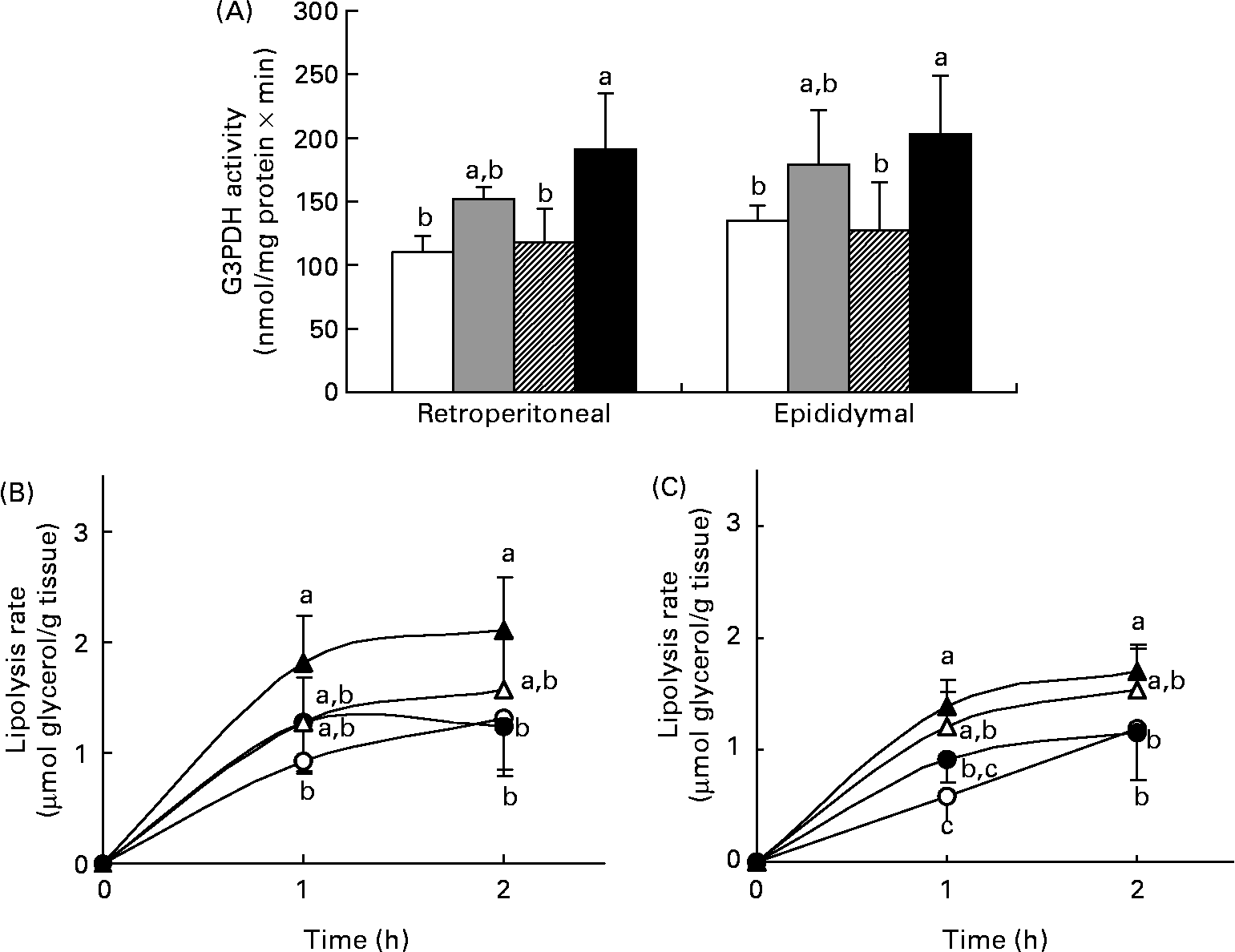
Fig. 3 Glycerol-3-phosphate dehydrogenase (G3PDH) activity (A) and lipolysis rate after stimulation with 10 μm-isoproterenol in the retroperitoneal (B) and epididymal (C) fat of rats fed the low-fat control diet (□, ○), high-fat control diet (![]() , ●), high-fat diet containing bitter melon (Momordica charantia) powder (
, ●), high-fat diet containing bitter melon (Momordica charantia) powder (![]() , Δ) or high-fat diet containing pioglitazone (■, ▲) (experiment 1). Values are means, with their standard deviations represented by vertical bars. The significance of differences between the groups was analysed by one-way ANOVA and Duncan's multiple range test. a,b,c Values with unlike letters were significantly different (P < 0·05).
, Δ) or high-fat diet containing pioglitazone (■, ▲) (experiment 1). Values are means, with their standard deviations represented by vertical bars. The significance of differences between the groups was analysed by one-way ANOVA and Duncan's multiple range test. a,b,c Values with unlike letters were significantly different (P < 0·05).
Peroxisome proliferator-activated receptor-γ and adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c mRNA levels
The differentiation of adipocytes is delicately controlled by a transcriptional cascade. To evaluate the effect of BM on adipogenesis, the mRNA levels of mature adipocyte markers, PPARγ and ADD1/SREBP-1c, were measured in experiment 1. There was no significant difference of the PPARγ gene expression between the four groups. However, in epididymal fat, the HFT group had the highest ADD1/SREBP-1c mRNA level, which was significantly higher than those of the HFB and LF groups (Fig 4; P < 0·05), but was comparable with the HF group. There was no significant difference between the HF and HFB groups. In retroperitoneal fat, a similar trend was also observed but did not reach statistical difference (data not shown).

Fig. 4 PPARγ and adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c (ADD1/SREBP) mRNA levels in the epididymal fat of rats fed the low-fat control diet (□), high-fat control diet (![]() ; HF), high-fat diet containing bitter melon (Momordica charantia) powder (
; HF), high-fat diet containing bitter melon (Momordica charantia) powder (![]() ) or high-fat diet containing pioglitazone (■) (experiment 1). The Northern blotting results were quantified by image analysis. Each value was normalised to that for 18S ribosomal RNA, then the relative mRNA abundance was calculated by taking the normalised value for the HF group as 1. Values are means, with their standard deviations represented by vertical bars. The significance of differences between the groups was analysed by one-way ANOVA and Duncan's multiple range test. a,b Values with unlike letters were significantly different (P < 0·05).
) or high-fat diet containing pioglitazone (■) (experiment 1). The Northern blotting results were quantified by image analysis. Each value was normalised to that for 18S ribosomal RNA, then the relative mRNA abundance was calculated by taking the normalised value for the HF group as 1. Values are means, with their standard deviations represented by vertical bars. The significance of differences between the groups was analysed by one-way ANOVA and Duncan's multiple range test. a,b Values with unlike letters were significantly different (P < 0·05).
The decreased G3PDH activity and the TAG content in adipose of BM-treated rats implied that the anti-adiposity effect of BM might be related to a suppression of lipogenesis in adipose tissue. Experiment 2 was thus carried out to examine the expressions of lipogenic genes.
Experiment 2
Body-weight gain and adipose weight
The body-weight gain and adipose tissue weight of rats in experiment 2 are shown in Table 5. Again, no difference in body-weight gain between the LF, HF and HFB groups was seen. However, the weight of the retroperitoneal and epididymal fat in the HFB group was significantly lower than that in the HF group (P < 0·005) and comparable with that in the LF group, as observed in experiment 1.
Table 5 Body-weight gain, adipose tissue weight and gene expression in the epididymal fat of rats fed the low-fat control diet (LF), high-fat control diet (HF) or high-fat diet containing bitter melon (Momordica charantia) powder (HFB) (experiment 2)*
(Mean values and standard deviations for six rats per group)

ADD1/SREBP-1c, adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c; FAS, fatty acid synthase; ACC-1, acetyl-CoA carboxylase-1; LPL, lipoprotein lipase; aP2, adipocyte fatty acid-binding protein.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* The significance of differences between the three groups was analysed by one-way ANOVA and Duncan's multiple-range test.
† The mRNA level is expressed as a fraction of that in the HF group assigned a value of 1.
Expression of lipogenic genes and their transcriptional regulators
To examine whether the anti-adiposity effect of BM might be related to a suppression of lipogenesis in adipose tissues, the expression of four lipogenic genes, FAS, ACC-1, LPL, and aP2, and their transcriptional regulators, ADD1/SREBP-1c and PPARγ, was compared in adipose tissues of the LF, HF and HFB groups. As shown in Table 5, the expression of all four lipogenic genes was down regulated by BM in the epididymal fat (P < 0·05; HFB v. HF), to a level that was about half of those in the HF group. There was no difference between the LF and HF groups, except that the FAS mRNA level tended to be lower in the LF than in the HF group. On the other hand, PPARγ and ADD1/SREBP-1c mRNA levels did not differ between the three groups. Similar results were also observed in the retroperitoneal fat (data not shown).
Discussion
Following the in vitro evidence that BM is a dual agonist of PPARα and PPARγReference Chao and Huang4, the present in vivo study confirmed that BM, just like the anti-diabetic drug TZD, ameliorated the hyperinsulinaemia and glucose intolerance induced by an HF diet. In contrast to the well-known adipogenic side effect of TZD, BM reduced visceral fat accretion and inhibited adipocyte hypertrophy by down regulating expression of the lipogenic genes FAS, ACC-1, LPL and aP2 in adipose tissue.
BM has been used for the treatment of diabetes throughout the worldReference Krawinkel and Keding3, Reference Yeh, Eisenberg, Kaptchuk and Phillips17. Its hypoglycaemic effect has been demonstrated in type 1 and type 2 diabetic rodentsReference Ahmed, Adeghate, Sharma, Pallot and Singh18, Reference Miura, Itoh and Iwamoto19 and in human subjects with type 2 diabetes mellitusReference Akhtar20. Numerous studies have been performed to determine the functional components and the mechanism of action of its anti-diabetic function. Its hypoglycaemic effect has been attributed to protection of β-cellsReference Ahmed, Adeghate, Sharma, Pallot and Singh18, alterations in glucose metabolismReference Shibib, Khan and Rahman21 and its insulin-like effectReference Ng, Wong, Li and Yeung22, Reference Cummings, Hundal, Wackerhage, Hope, Belle, Adeghate and Singh23. It is possible that different bioactive components are involved and that the hypoglycaemic effect is produced by more than one mechanism. Using a CHO-K1 cell clone stably transfected with a (UAS)4-tk-alkaline phosphatase reporter and a chimeric receptor of GAL4-rPPARα (or GAL4-rPPARγ) ligand-binding domain, Chao & HuangReference Chao and Huang4 found that wild BM contains PPARα and PPARγ activator(s) that can be extracted by non-polar organic solvents. This provides another explanation for the well-known hypoglycaemic, hypolipidaemic and anti-inflammatory effects and the possible anti-adiposity effect of BM.
The anti-adiposity effect of BM was first reported by Chen et al. Reference Chen, Chan and Li7, who subsequently showed a general decrease in the TAG content of the liver and muscle in rats fed an HF diet containing freeze-dried BM juiceReference Chen and Li8. In the present study, we focused on cell size and lipid metabolism in the adipose tissues of BM-fed rats, and compared these results with those obtained using TZD, a well-known insulin-sensitising agent that also increases adipogenesis at the cellular level by activation of PPARγReference Lambe and Tugwood11. To exclude the possible contribution of a decreased food or energy intake, the feeding experiment in the present study was started with an adaptation period (week 0) so that rats of the BM group got used to the bitter taste. In addition, the lyophilised BM powder was incorporated into the HF diet by substituting it for the equivalent amount of protein, fat, ash and fibre. These two strategies have successfully kept the feed and energy intake of the HFB group comparable with that of the HF group (Table 3).
The higher energy intake in the TZD-treated rats observed in the present study agrees with previous reports, and was attributed to lower leptin expression in adipose tissueReference De Vos, Lefebvre, Miller, Guerre-Millo, Wong, Saladin, Hamann, Staels, Briggs and Auwerx24, Reference Zhang, Graziano and Doebber25. Although the increased energy intake and adipogenic action of TZD favour energy storage in adipocytes, only subcutaneous fat (for example, inguinal fat), but not visceral fat (for example, retroperitoneal and epididymal fat), was increased in the HFT group as compared with the HF group. In the two visceral fat depots examined, the number of adipocytes with a diameter >180 μm was lower in the TZD-treated group than in the HF group. Okuno et al. Reference Okuno, Tamemoto and Tobe26 treated lean and obese (fa/fa) Zucker rats with troglitazone (a TZD) for 19 d and found that it caused an increase in the number of small adipocytes, decrease in the number of enlarged adipocytes and concomitantly normalised TNF-α and leptin expression, partially providing an explanation for the discrepancy that TZD relieves insulin resistance, but induces adipogenesis. In the present study, the TZD-treated group showed the highest G3PDH activity and also the highest basal and stimulated lipolysis in adipose tissue. This is in accordance with Bogacka et al. Reference Bogacka, Xie, Bray and Smith27, who reported that lipid storage genes, including those for G3PDH, LPL and FAS, are up regulated in the subcutaneous fat of pioglitazone-treated diabetic patients. A major lipolysis enzyme in adipose tissue, hormone-sensitive lipase, has been found to be up regulated by PPARγ agonists (rosiglitazone and pioglitazone) in differentiating preadipocytesReference Deng, Shan, Li, Shen, Lu, Cheng and Ning28.
When compared with the HF group, BM reduced fat pad weight, cell size and TAG content in the visceral fat of DIO rats. In human subjects, insulin resistance is strongly correlated with visceral fat accumulation. Interventions that reduce visceral adiposity could potentially improve insulin sensitivity. Recently, adipose tissue has been recognised to serve not only as an energy storage, but also as an endocrine organ by releasing adipocytokines (for example, TNF-α, leptin, adiponectin, resistin and NEFA) into the circulation to regulate both adipose tissue mass and the functions of other tissues by affecting systemic lipid and glucose metabolismReference Ahima and Flier29. The decrease in the number of large adipocytes is proposed to be an important mechanism for improving insulin sensitivity by reducing secretion of TNF-α, leptin and NEFA from enlarged fat cells, which are known to interfere with insulin signalling in several waysReference Arner10, Reference Okuno, Tamemoto and Tobe26, Reference Hotamisligil, Peraldi, Budavari, Ellis, White and Spiegelman30, Reference Griffin, Marcucci, Cline, Bell, Barucci, Lee, Goodyear, Kraegen, White and Shulman31. In addition, small adipocytes take up and oxidise more glucose than large adipocytes in the presence of insulinReference Olefsky32.
The inhibition of adipocyte hypertrophy by long-term consumption of BM may be mediated by increased lipolysis, fatty acid oxidation and/or decreased lipogenesis in adipose tissue. The former has been demonstrated by Chen & LiReference Chen and Li8, who reported higher circulation levels of catecholamines and NEFA in BM-treated rats. In the present study, although the lipolysis rate was not increased in the ex vivo adipose tissue from the HFB group (Figs. 3 (B) and (C)), we cannot eliminate the possibility of increased sympathetic activity in vivo, since we did not measure blood hormone concentrations. However, in the present study, serum NEFA levels were not increased by BM administration (data not shown).
Increased expression of PPARα target genes (i.e. coding for an enzyme or protein involved in fatty acid oxidation and transport) has been observed in the H4IIEC3 murine hepatoma cell line treated with a BM extractReference Chao and Huang4. A lower liver and muscle TAG content, accompanied by increased activity of enzymes involved in fatty acid β oxidation, was also observed by Chan et al. Reference Chan, Chen, Go, Lam and Li9. Results of the present study further demonstrated a reduction in lipogenesis in the adipose tissues of BM-treated rats. This evidence included a slightly lowered G3PDH activity, and a significant reduction in lipogenic gene expression in adipose.
Although the mRNA levels of lipogenic genes including FAS, ACC-1, LPL and aP2 were significantly lower in the adipose tissue of the HFB group than in the HF and LF groups, PPARγ and SREBP-1c, the two transcriptional factors controlling their expression, were not different at the mRNA level. The transcriptional activity of PPAR is mainly regulated by the presence of its ligand and may be further regulated by protein phosphorylation and dephosphrylationReference Hu, Kim, Sarraf and Spiegelman33, while that of ADD1/SREBP-1c is known to be regulated by proteolytic cleavage of ADD1/SREBP-1c precursor and the subsequent translocation of the released active form into the nucleusReference Brown and Goldstein34. Thus, the transcriptional activity of PPAR and ADD1/SREBP-1c might not be revealed by their own mRNA levels.
Despite that the BM extract has been shown to activate PPARγ in a transactivation assayReference Chao and Huang4, the mRNA level of its target genes, i.e. LPL and aP2, was not increased, but decreased in the adipose tissue. This unexpected result might partly be explained by the fact that there are multiple regulatory sites in the promoter region of the LPL and aP2 genes, indicating that these genes are under multiple regulationReference Yang, Christy, Cook, Kelly and Lane35, Reference Zhang, Repa, Gauthier and Mangelsdorf36, in addition to PPARγ. Besides, the potency of PPAR ligands existing in natural foods or herb materials is usually much lower than that of synthetic drugs. This can be seen from the much higher 50 % effective concentration (EC50) in the transactivation assay and the lower affinity in the ligand-binding assay. Furthermore, the co-activators and co-repressors expressed in liver and adipose tissue in rodents might be different from that of CHO cells used in the transactivation assay. In addition, the lack of concordance between the results of the transactivation assay and the in vivo study could partly be due to a metabolic or hormonal effect which does not exist in the in vitro study. For example, fish oil has been shown to be a PPARα and PPARγ activator, but the expression of LPL and PEPCK genes in adipose was down regulated, rather than up regulated, when tested in vivo Reference Raclot, Groscolas, Langin and Ferre37.
Based on the observed suppression of FAS and ACC-1 gene expression, a speculation that BM might antagonise the transcriptional activity of ADD1/SREBP-1c was raised. However, Nerurkar et al. Reference Nerurkar, Pearson, Efird, Adeli, Theriault and Nerurkar38 reported that nuclear SREBP-1c is increased in BM juice-treated HepG2 cells, although TAG is lowered. In the present study, the mRNA level of SREBP-1c in adipose was not changed by BM administration. The role of SREBP-1c in the regulation of lipogenic enzyme gene expression is well established in hepatocytes, but whether a similar role also exists in adipose is not clear, given that its importance in adipocyte differentiation has been characterisedReference Kim and Spiegelman39. Results of several reports have indicated that mRNA levels of SREBP-1c do not coincide with the changes in adipose lipogenic gene expressionReference Palmer, Rutter and Tavare40, Reference Bertile and Raclot41. Recently, Sekiya et al. Reference Sekiya, Yahagi and Matsuzaka42 reported that adipocyte lipogenesis is independent of control by SREBP-1c. Using the chromatin immunoprecipitation assay, they showed that the mature SREBP-1c failed to bind to the functional cis-element of FAS promoter in adipocytes, but could bind in hepatocytes. Recently, a role for liver X receptor in de novo lipogenesis and lipid accumulation in adipocytes has been proposed, but the evidence is still controversialReference Juvet, Andresen and Schuster43–Reference Stulnig, Steffensen, Gao, Reimers, Dahlman-Wright, Schuster and Gustafsson45. Therefore, the transcription factors that mediate the lipogenic gene expression in adipocytes through which BM exerts its anti-adiposity effect remain unclear and merit further study. On the other hand, the possibility that the lowered lipogenic gene expression in BM-supplemented rats was a direct effect of a lowered serum insulin level could not be excluded.
In spite of its bitter taste, which is unacceptable to some individuals, BM is a very common vegetable and has been consumed in Oriental societies for hundreds of years. The Chinese traditional conception is that diet cures more than the doctor. The present study shows that BM could ameliorate insulin resistance as effectively as the anti-diabetic drug TZD. Furthermore, BM could inhibit adipocyte hypertrophy by down regulating lipogenic gene expression in visceral fat, avoiding the adipogenic side effect of TZD. The potential of BM as part of the daily diet or as a supplement for ameliorating the metabolic syndrome is worth further exploration.
In conclusion, supplementation of BM to an HF diet significantly decreased the number of large adipocytes (>180 μm), increased the number of small adipocytes (20–60 μm) and down regulated the expression of lipogenic genes in adipose tissues of rats. It also ameliorated the glucose intolerance and hyperinsulinaemia in HF diet-fed rats.
Acknowledgements
The present study was supported by grants from the National Science Council (NSC93-2317B-039-004), Taipei, Taiwan. We thank the Takeda Pharmaceutical Company Ltd (Osaka, Japan) for kindly supplying pioglitazone (AD-4833).











