The pulp of jussara açaí, a fruit of the Euterpe edulis Martius palm tree, contains approximately 6 % protein, 49 % lipids and 43 % carbohydrates and is rich in anthocyanins (290 mg/100 g wet weight), mainly cyanidin-3-glucoside and cyanidin-3-rutinoside( Reference De Brito, de Araujo and Alves 1 ). Anthocyanins are important plant pigments that belong to a class of phenolic compounds collectively called flavonoids( Reference Kong, Chia and Goh 2 ). The anthocyanin phenolic structure can confer antioxidant properties through the donation or transfer of electrons from hydrogen atoms. The health-promoting properties of the açaí fruit have become more evident, as epidemiological studies suggest that increased consumption of anthocyanins lowers the risk of CVD, the most common cause of mortality among men and women( Reference Wallace 3 ).
Along with the genetic background, risk factors for CVD are associated with lifestyle and eating habits( Reference Mozaffarian, Wilson and Kannel 4 , 5 ) and contribute to the pathological frame of atherosclerosis( Reference Hansson 6 ). Potentially atherogenic lipoproteins, such as LDL, are associated with both functional and structural changes in the liver( Reference Targher, Bertolini and Padovani 7 ). These alterations are mainly caused by a combination of excessive production of reactive oxygen species (ROS) and changes in the antioxidant defence system( Reference Albano 8 ). Hepatic inflammation involves several chemokines, including monocyte chemotactic protein-1 (MCP-1)( Reference Baggiolini 9 ). The expression of MCP-1 is also thought to play an important role in hepatic steatosis and atherogenesis( Reference Kanda, Tateya and Tamori 10 ).
In addition to the therapeutic advances made with regard to atherosclerosis, non-pharmacological strategies for its prevention and treatment including eating habits and changes in lifestyle have been investigated. In this regard, the adoption of diets rich in fruits and vegetables and of active lifestyles has been recognised to have beneficial effects( Reference Smith, Allen and Blair 11 – Reference Xie, Kang and Burris 13 ). For instance, the ingestion of the pulp of Amazonian açaí (Euterpe oleracea Martius) has been shown to exert antioxidant and anti-inflammatory effects both in human subjects and in animal models( Reference Jensen, Wu and Patterson 12 – Reference Souza, Silva and Silva 14 ), as well as to exert atheroprotective effects in hyperlipidaemic ApoE-deficient (ApoE − / − ) mice fed a high-fat diet( Reference Xie, Kang and Burris 13 ). However, little is known about the atheroprotective effects of the jussara açaí fruit pulp. The recommended active lifestyle of regularly performing moderate-intensity aerobic exercise is thought to reduce oxidative stress in the skeletal muscle and liver and to promote atheroprotective effects and beneficial effects on lipid profile in both humans and animal models, including the ApoE − / − mouse model( Reference Radak, Chung and Naito 15 – Reference Teodoro, Natali and Fernandes 19 ).
In the present study, we hypothesised that the combination of a diet supplemented with açaí pulp and exercise training may potentiate the effects of jussara açaí pulp consumption on hepatic oxidative and inflammatory biomarkers. Therefore, we investigated the effects of jussara açaí pulp consumption, either alone or in combination with moderate-intensity aerobic exercise training, on the hepatic oxidative and inflammatory status of ApoE − / − mice.
Materials and methods
Animals
ApoE − / − mice (average body weight 25 g) aged 21 weeks were obtained from the Central Animal House at the Federal University of Viçosa, Brazil. During the experimental period, the mice were kept in collective cages in a temperature (22 ± 2°C)- and humidity (50 %)-controlled room with a 12 h photoperiod. The national guidelines for the care and use of animals were followed, and all experimental procedures were approved by the institutional ethics committee (Comissão de Ética no Uso de Animais/Federal University of Viçosa) under protocol number 19/2010.
Experimental design
The mice were divided into four groups as follows: control (C, no exercise training and fed the AIN-93M diet, n 5); control açaí (CA, no exercise training and fed the AIN-93M diet plus açaí, n 6); exercise açaí (EXA, exercise training and fed the AIN-93M diet plus açaí, n 6); exercise (EX, exercise training and fed the AIN-M93 diet, n 6). The mice were treated for twelve consecutive weeks and observed daily, and body weights and food intake were determined weekly.
Experimental diets
Fresh açaí berries were legally collected in a remaining area of the Atlantic Forest located in the ‘Zona da Mata’ of Minas Gerais state, Brazil. After harvesting, the fruits were threshed and transported to the Nutrition and Health Department at the Federal University of Viçosa in airtight plastic bags and were immediately submitted to processing. Fruit pulp was then obtained and lyophilised. The levels of moisture, ash, lipids, proteins and carbohydrates in the freeze-dried pulp were measured. Moisture content was determined by drying the sample in an oven at 105°C. Total lipid content in a diethyl ether extract was determined by drying the sample at 105°C followed by extraction using diethyl ether in a Soxhlet extractor and subsequent removal of the solvent by distillation( 20 ). Protein content was determined using the classic Kjeldahl method, and carbohydrate content was determined by calculating the percentage difference when subtracted from the total sum of moisture, ash, lipid and protein contents. Anthocyanin content was determined using the pH-differential method( Reference Giusti, Wrosltad and Wrolstad 21 ). The characterisation of the major anthocyanins in the açaí extract was performed using HPLC as described previously( Reference Schauss, Wu and Prior 22 ). Ash content was determined by incineration of the sample in a muffle furnace at 550°C and subsequent cooling in a desiccator at room temperature.
The freeze-dried açaí pulp had 8·45 % moisture, 5·28 % protein, 49·35 % lipids and 42·86 % carbohydrates. The total anthocyanin content of the freeze-dried açaí extract was 25·829 mg/g, with 9·524 mg/g being cyanidin-3-O-glucoside and 16·305 mg/g being cyanidin-3-O-rutinoside.
The diets were prepared according to the Association of Official Analytical Chemists( 20 ) and AIN-93M( Reference Reeves, Nielsen and Fahey 23 ) and were maintained under refrigeration (0–4°C) and protected from light until use. For the experimental groups to be treated with açaí, 2 % of the lyophilised fruit pulp was added and the diet composition was modified, considering the values of carbohydrates, protein, lipids and dietary fibre (Table 1). The amount of dietary fibre was based on the composition of the E. oleracea Martius fruit( Reference Schauss, Wu and Prior 22 ). The energy contents of the diets were 1590 kJ/100 g (380 kcal/100 g, AIN-93M) and 1611 kJ/100 g (385 kcal/100 g, AIN-93M plus açaí). The amount of freeze-dried fruit pulp added to the diets was, on average, 3·25 g/kg per d. The 2 % açaí pulp dose was chosen because of its relevance to human nutrition. In addition, this dose mimics the addition of a portion of açaí fruit pulp to human food( 24 ) and has been found to have effects in previous studies using rodents( Reference Souza, Silva and Silva 14 , Reference Souza, Silva and Magalhães 25 ).
Table 1 Composition (g/kg) of the diets fed to ApoE −/− mice
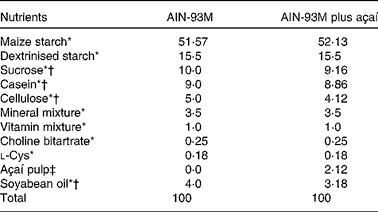
* AIN-93M rodent diet nutrients( Reference Reeves, Nielsen and Fahey 23 ).
† The concentration of the nutrient in the fruit was deducted.
‡ Freeze-dried açaí pulp contains 7 g protein, 41 g fat, 42 g carbohydrates and approximately 44·2 g dietary fibre/100 g. The total anthocyanin content is 25·829 mg/g (cyanidin-3-O-glucoside 9·524 mg/g and cyanidin-3-O-rutinoside 16·305 mg/g).
Exercise training protocol
Mice in the EXA and EX groups were subjected to a progressive treadmill (Insight Equipamentos Científicos) running programme, 5 d per week (Monday to Friday), for twelve consecutive weeks. Briefly, in the first week, the mice were made to run for 30 min daily at a speed of 12 m/min, 0 % incline. In the second week, the running time and speed were increased to 40 min/d and 14 m/min, respectively. From the third week onwards, the mice were made to run for 60 min/d at a speed of 16 m/min, 0 % incline. Exercise intensity was determined by adjusting the running speed to the oxygen consumption according to the method of Høydal et al. ( Reference Høydal, Wisløff and Kemi 26 ).
Sample preparation
The mice were anaesthetised with ketamine (10 mg/kg body weight) and xylazine (2 mg/kg body weight) and killed by increasing the anaesthetic dose followed by puncturing of the abdominal aorta 48 h after the last exercise session. To determine serum component levels, blood samples were collected in 5 ml test-tubes and centrifuged at 2938 g at 4°C for 10 min. The soleus muscle was harvested, immersed in liquid N2 and stored at − 80°C for subsequent analyses. After removal of all the adventitia from the aortic valve to the iliac bifurcation, the aorta was dissected and stored in 10 % formalin for subsequent analyses. The liver was removed, washed in saline and weighed. A fragment of the liver caudate lobe was immersed in Karnovsky solution for 24 h for histopathological analyses and the remaining fragments were immersed in liquid N2 and stored at − 80°C for subsequent analyses.
Measurement of muscle citrate synthase activity
Citrate synthase activity in the soleus muscle was assessed according to the method of Alp et al. ( Reference Alp, Newsholme and Zammit 27 ). The muscle was weighed and homogenised with a glass homogeniser on ice in a solution of Tris–HCl (100 mmol/l) at a constant weight:volume ratio. The homogenate was then added to a reaction mixture containing Tris–HCl (100 mmol/l), dithiobis(2-nitrobenzoic acid) (1·0 mmol/l) and acetyl-CoA (3·9 mmol/l). After the addition of oxaloacetate (1·0 mmol/l), absorbance was read at 412 nm for a 7 min period. Mean absorbance in variation per min was recorded for each sample, and citrate synthase activity was calculated using an extinction coefficient of 13 600 mol/l per cm.
Liver histopathological analyses
A fragment of the liver caudate lobe was immersed in Karnovsky solution for 24 h and was then dehydrated in ethanol and embedded in paraffin. The fragment was cut into 4 μm sections, stained with haematoxylin and eosin, and mounted on histology slides with Entellan® (Merck) for the quantification of lipid droplets, inflammatory infiltrates and sinusoidal capillaries. The slides were visualised and the images ( × 20) captured using a light microscope (Olympus BX-60®) connected to a digital camera (Olympus QColor-3®). A total of ten images from each mouse were used to count lipid droplets (expressed as percentage per histological area). An 810-point grid was imposed on the scanned images via the Image-Pro Plus 4.5® software system (Average Cybernetics).
Determination of antioxidant enzyme activity in the liver
Liver fragments were homogenised in 50 mm-phosphate buffer and the resulting suspension was centrifuged at 3000
g
at 4 C for 10 min. The supernatant was used to measure enzyme activity. Superoxide dismutase (SOD) activity was determined using a microplate reader (Asys UVM 340; Biochrom Ltda), at 570 nm, by evaluating the ability of SOD to remove superoxide (
![]() $$O _{2}^{ - } $$
) anions, thus decreasing the self-oxidation rate of pyrogallol(
Reference Dieterich, Bieligk and Beulich
28
). Protein content was measured using the method described by Lowry et al.
(
Reference Lowry, Rosebrough and Farr
29
). Catalase (CAT) activity was determined by the rate of decay of H2O2 read in a spectrophotometer (Pro-analise PAUV.1600; PRO-ANALISE Química e Diagnóstica Ltda) at 240 nm, as described previously(
Reference Aebi
30
).
$$O _{2}^{ - } $$
) anions, thus decreasing the self-oxidation rate of pyrogallol(
Reference Dieterich, Bieligk and Beulich
28
). Protein content was measured using the method described by Lowry et al.
(
Reference Lowry, Rosebrough and Farr
29
). Catalase (CAT) activity was determined by the rate of decay of H2O2 read in a spectrophotometer (Pro-analise PAUV.1600; PRO-ANALISE Química e Diagnóstica Ltda) at 240 nm, as described previously(
Reference Aebi
30
).
Real-time PCR analysis
Total mRNA was extracted from a 100 mg liver fragment. The tissue sample was treated with proteinase K (Sigma-Aldrich®), and the RNA was extracted with TRIzol (Invitrogen), according to the manufacturer's instructions. The purity and quantity of RNA were determined using a spectrophotometer (Thermo Scientific Evolution 60; Thermo Fisher Scientific).
The total RNA (2 μg) was used for synthesising complementary DNA using random primers (Promega®), buffer, dNTP (Sinapse®) and Moloney murine leukaemia virus RT (Fermentas®), according to the manufacturer's instructions. The relative expression of the MCP-1 gene was determined by quantitative real-time PCR. The PCR cycle involved an initial denaturation step at 95°C (2 min) followed by forty cycles with 15 s of denaturation (95°C), 30 s of annealing (60°C) and 1 min of extension (72°C), followed by the standard dissociation curve analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as a control endogenous gene, after finding that its expression was not significantly affected under the experimental conditions. Specific primers used for tissue factor, inducible NO synthase and β-actin were as follows: for MCP-1 – forward 5′-GGTCCCTGTCATGCTTCTGG-3′ and reverse 5′-CCTGCTGCTGGTGATCCTCT-3′; for GAPDH – forward 5′-CCACCCATGGCAAATTCC-3′ and reverse 5′-GATGGGATTTCCATTGATGACA-3′. The quantitative analyses were carried out in a thermocycler (Step One Plus; Applied Biosystems Real-Time PCR System) using the fluorescence quantification system (Platinum® SYBR® Green; Invitrogen). All samples were analysed in triplicate. Reaction efficiencies were determined as described previously( Reference Pfall 31 ), and amplification specificity was determined by the analysis of the dissociation curves( Reference Livak and Schmittgen 32 ).
Aortic lesion analysis
Lipid depositions in the aortic arch and in the thoracic aorta were measured using en face analysis with Sudan IV dye( Reference Palinski, Ord and Plump 33 ). The aortas were opened longitudinally and fixed for 12 h in a formalin–sucrose solution (4 % paraformaldehyde, 5 % sucrose, 20 μmol butylated hydroxytoluene/l and 2 μmol EDTA/l, pH 7·4) at 4°C. Later, the aortas were placed in a 70 % ethanol solution for 5 min. Subsequently, the aortas were stained with a solution containing 0·5 % Sudan IV dye, 35 % ethanol and 50 % acetone for 10 min under agitation and then bleached in an 80 % ethanol solution for 5 min. The stained aortas were photographed using an 8·1 megapixel digital camera with distance, zoom and luminosity being controlled. The analyses were carried out using the Image-Pro Plus® software package. Pixels were converted into cm2 using a standard microscopic scale under the same condition that the aortas were analysed. The sum of the areas of atherosclerotic lesions (where lipid accumulation was observed) was calculated using the software, and the results are expressed in cm2. To ensure that there were no differences in the total size of the aortas among the mice, their area was also measured. The analyses were carried out by two individuals blinded to the study design.
Determination of the serum lipid profile
The levels of total cholesterol, HDL-cholesterol and TAG were determined by the enzymatic colorimetric method (Cobas®, c 111 analyser; Roche) using commercial kits (Bioclin®; Quibasa Química Básica Ltda).
Statistical analysis
All data were submitted to the Kolmogorov–Smirnov test for symmetry and equality of variance. Data obtained for weights and SOD and CAT activities were analysed using the two-way ANOVA followed by Tukey's post hoc test when necessary. Data obtained for MCP-1 mRNA expression, lipid profile, area of atherosclerotic lesions and liver histopathology were analysed using the Kruskal–Wallis test followed by Dunn's post hoc test when necessary. Data obtained for muscle citrate synthase activity in mouse groups that were made to and not made to exercise were compared using unpaired Student's t test (SigmaStat version 3.5; Systat). Differences of P< 0·05 were considered to be statistically significant.
Results
Body and liver weights and food intake
There were no significant differences among the experimental groups with regard to their initial body weights and liver weights (Table 2). Mice that were made to exercise had higher final body weights compared with the C mice (26·6 (sd 3·3) v. 23·5 (sd 2·7) g, respectively). The average food intake was not different among the groups (data not shown).
Table 2 Body weight and liver weight of ApoE −/− mice fed a diet supplemented with açaí pulp and made to perform aerobic exercise (Mean values and standard deviations)
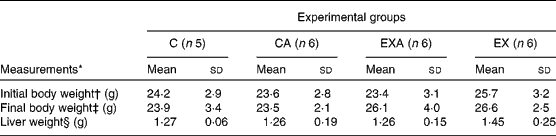
C, control; CA, control plus açaí; EXA, exercise plus açaí; EX, exercise.
* Data were analysed using two-way ANOVA.
† There were no significant main effects for exercise (P= 0·85), diet (P= 0·74) and interaction (P= 0·66).
‡ There was a significant main effect for exercise (P= 0·02), but no main effects for diet (P= 0·80) and interaction (P= 0·71).
§ There were no significant main effects for exercise (P= 0·28), diet (P= 0·22) and interaction (P= 0·29).
Muscle citrate synthase activity
Citrate synthase activity was measured to assess the impact of the employed aerobic exercise programme on the muscle oxidative capacity. Mice in the EXA (1·3 (sd 0·2) U/mg protein) and EX (1·3 (sd 0·1) U/mg protein) groups exhibited higher (P< 0·001) citrate synthase activity when compared with those in the C (0·7 (sd 0·2) U/mg protein) and CA (0·6 (sd 0·2) U/mg protein) groups, respectively.
Liver histopathology
Mice in the C group exhibited hepatic tissue disorganisation with higher percentages of lipid droplets (Fig. 1(a)) and intense areas of necrosis (mean data not shown). Mice in the EXA and EX groups exhibited lower percentages of lipid droplets when compared with those in the C group (Fig. 1(b)).

Fig. 1 Effects of açaí diet ingestion and aerobic exercise training on the percentage of lipid droplets in the liver of ApoE − / − mice. (a) Representative photomicrographs of hepatic tissue sections. Arrows indicate lipid droplets. The sections were stained with haematoxylin and eosin. All images were captured at the same magnification. Scale bar represents 65 μm. (b) Area of lipid droplets. Values are means (n 5–6 mice per group), with their standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (C) group (P< 0·05; Kruskal–Wallis test followed by Dunn's post hoc test). CA, control plus açaí; VC, vein; EXA, exercise plus açaí; EX, exercise.
Mice in the EX group had a higher number of sinusoidal capillaries in the liver when compared with those in the other groups (Table 3). However, there were no statistically significant differences among the experimental groups regarding the number of hepatocytes and inflammatory infiltrates.
Table 3 Hepatocyte, sinusoidal capillary and inflammatory infiltrate measurements in the liver of ApoE −/− mice fed a diet supplemented with açaí pulp and made to perform aerobic exercise (Mean values and standard deviations)

C, control; CA, control plus açaí; EXA, exercise plus açaí; EX, exercise.
* Mean value was significantly different from that of the C group (P< 0·05; Kruskal–Wallis test followed by Dunn's post hoc test).
† Mean value was significantly different from that of the CA group (P< 0·05; Kruskal–Wallis test followed by Dunn's post hoc test).
Antioxidant enzyme activity in the liver
Mice that were made to exercise exhibited a reduced SOD activity when compared with the C mice (Fig. 2), independent of the açaí diet (1·6 (sd 0·3) v. 1·0 (sd 0·2) U/mg protein, respectively). There was a significant main effect of exercise on SOD activity, but no main effect of diet and no interaction were observed. There was no statistically significant different among the experimental groups with regard to CAT activity, as no significant main effects of exercise or diet and no interaction were observed.

Fig. 2 Effects of açaí diet ingestion and aerobic exercise training on hepatic catalase (CAT) activity and hepatic superoxide dismutase (SOD) activity. The data were analysed using two-way ANOVA followed by Tukey's post hoc test. Values are means (n 5–6 mice per group), with their standard deviations represented by vertical bars. For CAT activity, there were no significant main effects for exercise (P= 0·06), diet (P= 0·57) and interaction (P= 0·12). There was a significant main effect of exercise (P= 0·01) for SOD activity, but no main effects for diet (P= 0·06) and interaction (P= 0·08). C, control; CA, control plus açaí; EXA, exercise plus açaí; EX, exercise.
Expression of monocyte chemotactic protein-1 mRNA in the liver
After 12 weeks of intervention, mice in the EX group exhibited a reduced mRNA expression of the inflammatory marker MCP-1 in the liver when compared with those in the C group (Fig. 3).
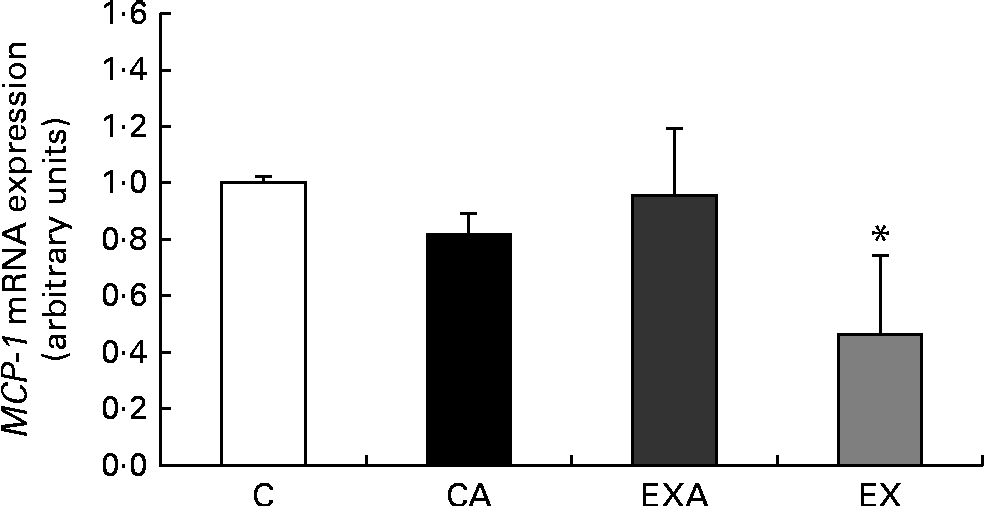
Fig. 3 Effects of açaí diet ingestion and aerobic exercise training on the mRNA expression of monocyte chemotactic protein-1 (MCP-1) in the liver of ApoE − / − mice. Values are means (n 5–6 mice per group), with their standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (C) group (P< 0·05; Kruskal–Wallis test followed by Dunn's post hoc test). CA, control plus açaí; EXA, exercise plus açaí; EX, exercise.
Area of atherosclerotic lesions
Figure 4(a) shows representative photomicrographs of the aortic lesion areas. There were no statistically significant differences (P>0·05) among the groups when the values of the total area of aorta were compared (data not shown). Given that the aortas had similar areas, it was possible to compare the total area of lesions directly as a percentage of the total area of the aorta (total injured area/total area of the aorta × 100). En face analysis of the aortas revealed that mice in the EX group had a reduced percentage of lesion area when compared with those in the other groups (Fig. 4(b)). However, no differences were found among the C, CA and EXA groups.

Fig. 4 Effects of açaí diet ingestion and aerobic exercise training on atherosclerotic lesion area in ApoE − / − mice. (a) Representative photomicrographs of lesions areas in the descending aorta of ApoE − / − mice in the experimental groups. Arrows indicate lesions. (b) Percentage of the lesion areas of mice in the experimental groups. Values are means (n 5–6 mice per group), with their standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (C) group (P< 0·05; Kruskal–Wallis test followed by Dunn's post hoc test). CA, control plus açaí; EXA, exercise plus açaí; EX, exercise. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Serum lipid profile
The serum levels of total cholesterol, TAG and HDL-cholesterol were not affected in ApoE − / − mice either by the ingestion of açaí pulp or by the aerobic exercise training programme (Table 4).
Table 4 Total cholesterol, TAG and HDL-cholesterol (HDL-C) levels in the serum of ApoE −/− mice fed a diet supplemented with açaí pulp and made to perform aerobic exercise (Mean values and standard deviations)
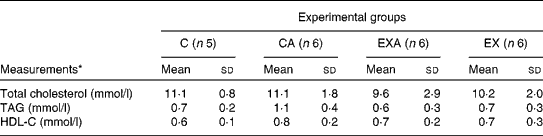
C, control; CA, control plus açaí; EXA, exercise plus açaí; EX, exercise.
* Data were analysed using the Kruskal–Wallis test.
Discussion
In the present study, we investigated the effects of jussara açaí pulp consumption, either alone or in combination with moderate-intensity aerobic exercise training, on the hepatic oxidative and inflammatory status of ApoE − / − mice. We found that exercise training improved the hepatic oxidative status, reduced the mRNA expression of the inflammatory marker MCP-1 and decreased hepatic steatosis as well as atherosclerotic lesion area in ApoE − / − mice. However, the ingestion of a diet containing 2 % jussara açaí pulp alone did not affect these parameters. We also found that the combination of diet and exercise training (i.e. EXA group) improved the hepatic oxidative status of the mice by reducing SOD activity and attenuated hepatic steatosis, which indicates that alterations in these parameters are mainly due to exercise training.
To determine the effects of jussara açaí pulp consumption and exercise training on the hepatic antioxidant enzymes, CAT and SOD activities were determined. In the present study, ApoE − / − mice exhibited high levels of CAT and SOD activities in the liver, which could be the result of counteracting the excess of superoxide anions formed in these animals( Reference Xie, Kang and Burris 13 ). CAT and SOD activities in the liver and skeletal muscle in response to aerobic exercise training have been found to increase, decrease or remain unchanged( Reference Brooks, Vasilaki and Larkin 16 , Reference Da Silva, Pinho and Rocha 18 , Reference Alessio and Goldfarb 34 ). These discordant results obtained for the activity of antioxidant enzymes in response to exercise training could be explained by the different exercise conditions (i.e. intensity and duration) used and the enzymes analysed, as well as the organ and animal model used. In the present study, moderate-intensity aerobic exercise training alone was found to reduce SOD activity in ApoE − / − mice (40·85 %), which suggests that the stress reduction induced by exercise diminishes the need for protective responses, although CAT activity was not altered. The exercise regimen used may have functioned to reduce the stressful environment present in this knockout mouse model. In this scenario, aerobic exercise training, a regular daily repetition of moderate-intensity exercise, contrary to exhaustive acute exercise, seems to decrease the production of ROS, which maintains the redox balance( Reference Radak, Chung and Goto 17 ). For example, aerobic exercise training has been found to induce adaptive responses, including attenuation of increases in ROS production, lipid peroxidation levels and nuclear factor-κB (NF-κB) activation and reduced glutathione:oxidised glutathione ratio in the liver of rats( Reference Radak, Chung and Naito 15 ). In addition, the skeletal muscle of mice subjected to endurance training has been reported to not release superoxide or NO and to exhibit no reductions in glutathione or protein thiol levels in response to in vitro isometric contractions. Furthermore, the contraction-induced activation of NF-κB and activator protein-1 DNA binding observed in the muscle of sedentary mice has not been observed in animals made to exercise( Reference Brooks, Vasilaki and Larkin 16 ). These findings suggest that aerobic training decreases contraction-induced ROS generation, activation of redox-sensitive signalling pathways and ROS stress. Thus, although we did not measure these parameters, it is possible that the exercise regimen that we employed enhanced the ability of the liver to reduce ROS generation, which results in lower SOD activity.
The results obtained for SOD activity in the present study differ from those obtained in previous studies using a different açaí species, E. oleracea, and experimental conditions, as a diet supplemented with 2 % of açaí pulp for 6 weeks was found to reduce SOD activity in the serum of a hypercholesterolaemic rat model( Reference Souza, Silva and Silva 14 ). In addition, SOD activity has been reported to be reduced in the brain of rats treated with açaí( Reference Spada, Dani and Bortolini 35 ). However, an increased hepatic activity of antioxidant enzymes (i.e. glutathione reductase and glutathione peroxidase) has been reported in ApoE − / − mice fed a diet with 5 % of açaí pulp for 20 weeks( Reference Xie, Kang and Burris 13 ). The results reported on the effects of polyphenols on CAT activity are also contradictory. For instance, unchanged( Reference Dragsted, Pedersen and Hermetter 36 ) or decreased activity( Reference Spada, Dani and Bortolini 35 ) has been demonstrated. These inconsistent results obtained for the activity of antioxidant enzymes could be explained by the different stress conditions used, the enzymes analysed, and the source of the dietary compounds.
To determine the effects of the applied treatments on the hepatic inflammatory condition, the mRNA expression of MCP-1 was analysed. The present results revealed a reduced (42 %) expression of MCP-1 mRNA in the liver of ApoE − / − mice in response to exercise training, but not as a result of açaí fruit pulp ingestion. Aerobic exercise training has been reported to decrease the levels of MCP-1 in the plasma of individuals with the metabolic syndrome( Reference Trøseid, Lappegard and Claudi 37 ) and normal individuals( Reference Garelnabi, Veledar and White-Welkley 38 ) and in the adipose tissue of obese mice( Reference Vieira, Valentine and Wilund 39 ). It is worth noting that in association with ROS production in the liver of rats, aerobic exercise attenuates the hepatic activity of the redox-sensitive transcription factor NF-κB, which is involved in the regulation of various cellular processes such as the transcription of inflammation-related proteins( Reference Radak, Chung and Naito 15 , Reference Radak, Chung and Goto 17 ). Thus, the reduced hepatic oxidative status observed in the present study (i.e. decreased SOD activity) may help to explain the decreased expression of MCP-1 mRNA in the liver of ApoE − / − mice that were made to exercise in the present study. In addition, MCP-1 is produced in, and secreted by, endothelial cells and its production is stimulated by mechanical stretch; thus, improved hepatic vascular function due to exercise might in part explain the inhibitory effect on chemokines( Reference Maiorana, O'Driscoll and Cheetham 40 ). In fact, we observed that exercise training augmented the numbers of hepatic sinusoidal capillaries in ApoE − / − mice in the present study. In these animals, free radicals induce the release of vasoconstrictor enzymes from sinusoidal endothelial cells, which stimulate hepatic cells to contract, thus decreasing sinusoidal perfusion and leading to regional hypoxia( Reference Daugherty 41 ). Unexpectedly, we did not find changes in the hepatic levels of MCP-1 mRNA in response to jussara açaí pulp ingestion either alone or in combination with aerobic exercise. Despite this, anthocyanins have been reported to exert anti-inflammatory effects by reducing the plasma levels of MCP-1 in human subjects( Reference Garcia-Alonso, Minihane and Rimbach 42 ) and E. oleracea fruit pulp has been reported to reduce pro-inflammatory cytokine levels in ApoE − / − mice( Reference Xie, Kang and Burris 13 ).
In the present study, ApoE − / − mice exhibited hepatic steatosis, as expected. Nevertheless, our data revealed that the percentage of hepatic lipid droplets in mice that were made to exercise was significantly reduced (70 and 56 %, respectively) when either treated or not treated with açaí, which indicates that such effects are mainly due to exercise training. Reduced hepatic steatosis in animals in response to regular exercise has been reported by others( Reference Rector, Thyfault and Morris 43 ). As a matter of fact, aerobic exercise is known to promote greater utilisation of fat by increasing its oxidation( Reference Helge 44 ), which reduces its accumulation in the hepatocytes. It is worth noting that there is a strong relationship between the increased expression of MCP-1 in the liver and the condition of severe steatosis( Reference Rull, Rodriguez and Aragones 45 ). This relationship can be explained by the fact that MCP-1 induces lipid accumulation in hepatocytes through the activation of the gene expression of PPARα ( Reference Gao 46 ), which is involved in the regulation of intracellular lipids( Reference Qin, Xie and Fan 47 ). Thus, the reduction of MCP-1 expression observed in mice that were made to exercise in the present study may help to explain the decreased deposition of hepatic lipid droplets in these animals.
On the other hand, the consumption of a diet containing 2 % jussara açaí pulp alone did not change the lipid droplet formation in the liver of these mice. Different results in other models and diets have been reported previously. For example, the increased amounts of hepatic lipid droplets and area of epididymal adipocytes caused by the Western diet in LDL receptor-deficient mice have been reported to be reduced by naringenin, a flavonoid found in citrus fruits( Reference Mulvihill, Assini and Sutherland 48 ). Moreover, the ingestion of melon juice extract, rich in SOD, has been found to attenuate the development of hepatic steatosis in hamsters fed an atherogenic diet( Reference Decorde, Ventura and Lacan 49 ). Despite the differences among experimental models and designs, our findings might also have been influenced by the high percentage of fat present in jussara açaí pulp (41 %).
In the present study, the serum lipid profile of ApoE − / − mice was not altered either by açaí pulp consumption or by aerobic exercise training or a combination of both. In line with these results, it has been reported that a diet containing anthocyanins (purple sweet potatoes, 1 %) does not affect the blood lipid profile in this animal model( Reference Miyazaki, Makino and Iwadate 50 ). It is worth noting that in ApoE − / − mice the plasma levels of cholesterol are approximately 5-fold higher than those in normal mice( Reference Piedrahita, Zhang and Hagaman 51 ); thus, the period of study employed by us might not have been sufficient to develop a significant change in the serum lipid profile. The results reported previously are not consensual inasmuch as there are studies showing that the serum lipid profile of ApoE − / − mice is either changed( Reference Shimada, Mikami and Murayama 52 ) or unchanged( Reference Meilhac, Ramachandran and Chiang 53 ) in response to exercise training.
Despite having no effect on the serum lipid profile of the experimental groups in the present study, the exercise protocol alone reduced the area of aortic atherosclerotic lesions by 58 %, while the combination of jussara açaí pulp diet and aerobic exercise reduced the total area of atherosclerotic lesions (4 %). These findings are in agreement with those of previous studies in that aerobic exercise training promotes the regression of atherosclerotic lesions in ApoE − / − mice and in other experimental models such as LDL receptor-deficient mice( Reference Da Silva, Pinho and Rocha 18 ). The exercise-induced expression of NO synthase( Reference Fukai, Siegfried and Ushio-Fukai 54 ) may contribute to the reduction of atherosclerotic lesion number as its inhibition accelerates atherosclerosis, although it was not assessed in the present study. Furthermore, exercise training has been reported to reduce atherosclerotic lesion number without changing blood lipid profile in ApoE − / − mice( Reference Shimada, Mikami and Murayama 52 ). We observed that the levels of serum total cholesterol and TAG have little or no influence on fat deposition in the coronary arteries of patients with severe atherosclerosis, which may help to explain the reduction of atherosclerotic lesion number by factors other than decreased total cholesterol levels, including reduced inflammation, endothelial dysfunction and oxidative stress( Reference Braz, Gutierrez and Da Luz 55 ). However, the consumption of a diet containing 2 % jussara açaí pulp alone did not affect the area of atherosclerotic lesions in ApoE − / − mice. In contrast to our findings, Xia et al. ( Reference Xia, Ling and Zhu 56 ) reported that an anthocyanin-rich extract from black rice reduced the size of advanced atherosclerotic lesions by 18 % after 20 weeks of intervention in 30-week-old ApoE − / − mice. Such different findings between studies may be related not only to the age of the animals (21 v. 30 weeks) and lesion stage (stable plaque v. vulnerable plaque), but also to treatment duration (12 v. 20 weeks). Indeed, studies using 20-week interventions have obtained different results( Reference Xie, Kang and Burris 13 , Reference Xia, Ling and Zhu 56 ).
In conclusion, while the ingestion of a diet containing 2 % jussara açaí pulp for 12 weeks did not affect the hepatic oxidative and inflammatory status of ApoE − / − mice, moderate-intensity aerobic exercise training improved the hepatic oxidative status, reduced the mRNA expression of the inflammatory marker MCP-1 and decreased hepatic steatosis as well as atherosclerotic lesion area in these animals. These findings do not support our hypothesis that the combination of a diet supplemented with açaí pulp and exercise training may potentiate the effects of jussara açaí pulp consumption on hepatic oxidative and inflammatory biomarkers.
Acknowledgements
M. C. G. P. and A. J. N. are CNPq fellows.
The present study was funded by the State of Minas Gerais Research Foundation (FAPEMIG). FAPEMIG had no role in the design and analysis of the study or in the writing of this article.
All authors contributed to the study design, outcome interpretation and manuscript preparation.
None of the authors has either financial or personal conflicts of interest to declare.










