It is well established that immune system stimulation (ISS) alters protein and amino acid (AA) metabolism and utilisation in animals and humans( Reference Reeds and Jahoor 1 ). During ISS, AA are redirected from growth and reproduction towards mounting an immune response, which influences AA requirements( Reference Obled 2 ). The latter may contribute to additional muscle proteolysis. Glutamine, arginine, aromatic AA, threonine, methionine and cysteine (Cys) putatively become more critical relative to other AA during ISS( Reference Reeds and Jahoor 1 , Reference Obled 2 ).
Special attention has been given to the metabolism of methionine and Cys (sulphur amino acids; SAA) during ISS due to their role as precursors for the synthesis of proteins and metabolites that are involved in the immune response( Reference Breuille, Obled, Furstand and Young 3 ). About half of the Cys flux in healthy adults and rats can be accounted for by the synthesis of glutathione (GSH), while the remainder is used primarily for protein synthesis( Reference Fukagawa, Ajami and Young 4 , Reference Malmezat, Breuille and Pouyet 5 ). It has been shown in rats that Cys flux increases substantially during disease due to enhanced GSH turnover( Reference Breuille, Obled, Furstand and Young 3 , Reference Malmezat, Breuille and Capitan 6 ). Moreover, Malmezat et al. ( Reference Malmezat, Breuille and Capitan 6 ) observed a fourfold increase in metabolic demand for Cys in septic rats due to an increased synthesis of GSH. An increase in erythrocyte GSH synthesis rate during ISS has also been observed in pigs and human subjects( Reference Reid and Jahoor 7 ). Furthermore, Cys is required for the synthesis of Cys-rich defensins, antimicrobial peptides and the acute-phase protein albumin, the synthesis of which increases substantially during ISS( Reference Denko, Purser and Johnson 8 , Reference Kluver, Schulz-Maronde and Scheid 9 ). Increases in methionine metabolism (i.e. transmethylation and remethylation) have been observed in patients with systemic inflammation, as a result of increased methyl group utilisation for the synthesis of polyamines, choline and carnitine( Reference Yu, Burke and Young 10 ). These observations indicate enhanced metabolic demands for SAA during ISS. However, quantitative estimates of the impact of ISS on dietary SAA requirements in animals for optimum biological performance have not been generated.
The gastrointestinal tract is also involved in defence activities during ISS. This involvement results in morphological and physiological changes, including oedema, as well as changes in gut motility, permeability, microflora( Reference Hang, Shi and Li 11 ), expression of digestive enzymes( Reference Jurjus, Barada and Khoury 12 ), mucin production( Reference Faure, Chone and Mettraux 13 ) and epithelial transport systems( Reference Hang, Shi and Sun 14 ). However, little is known about the impact of ISS on the measures of nutrient digestibility.
Therefore, in the present study, two experiments were conducted to determine the effect of chronic ISS on ileal nutrient digestibility and utilisation of SAA intake for whole-body protein deposition (PD) in growing pigs.
Materials and methods
General procedures
Institutional and national guidelines for the care and use of animals were followed, and all experimental procedures were reviewed and approved by the University of Guelph Animal Care Committee. In two experiments (Expt I and II), a total of sixty Yorkshire barrows were obtained from the University of Guelph Arkell Swine Research Center (Arkell, ON, Canada), housed individually in metabolism crates, as described by Nyachoti et al. ( Reference Nyachoti, de Lange and McBride 15 ). Across the two experiments, pigs were subjected to five different levels of dietary SAA that were lower than the established SAA requirements( 16 ). Pigs were allowed to acclimatise to environmental conditions for 10 d. During the first 3 d of the acclimatisation period, pigs were fed ad libitum on a commercial swine starter diet. Pigs were then assigned to experimental diets and feed restricted to approximately 38 g DM/kg body weight (BW) per d. The N-balance method was used to establish the PD of individual pigs before and during ISS. At the end of the experiment, pigs were euthanised by an intravenous injection of sodium pentobarbital (0·3 mg/kg BW; Virbac AH).
Treatments, experimental design, diets and feeding
Experiment I
A total of thirty-six barrows (initial BW 18·6 (se 0·70) kg) were blocked by time in two equal groups of eighteen pigs and randomly assigned to dietary treatments. Dietary treatments were arranged in a 3 × 2 factorial randomised complete block design with two main factors: (1) three levels of dietary SAA allowance (1·0, 2·4 or 3·6 g/d, representing 15, 37 and 55 %, respectively, of established SAA requirements for maximum growth for pigs of this BW( 16 )) and (2) ISS (ISS − v. ISS+). A total of three soya protein isolate and maize starch-based diets were formulated, in which SAA were among the first-limiting AA (diets 1 to 3; Table 1). The dietary SAA levels were achieved by altering the ratio of maize starch:soya protein isolate, which contains relatively low levels of SAA (Table 1). The ratios of essential AA:SAA in soya protein exceed the recommendations given by Wang & Fuller( Reference Wang and Fuller 17 ) and the National Research Council( 16 ) by 20 % (Table 1). The diets were calculated to contain 5·0 MJ/kg of metabolisable energy. The diets were fortified with vitamins and minerals to surpass the requirements recommended by the National Research Council (Table 1)( 16 ). Titanium dioxide (0·1 % TiO2; Sigma-Aldrich Limited) was included in all the diets as an indigestible marker for determining ileal digestibility of nutrients. Pigs were fed equal meals twice per d at 08.00 and 16.00 hours. Daily feed allowance was restricted to 800 g/d, resulting in daily metabolisable energy intakes of 12·0 MJ/d, to ensure constant energy intake levels across all the dietary treatments. Water intake was restricted to 2·5 litres/d to avoid water spillage and contamination of urine. A 5 d pre-ISS N balance was conducted to establish the PD of individual pigs as influenced by the SAA intake levels (n 12). At the end of the pre-ISS N-balance period, pigs at each SAA dietary level were injected intramuscularly with either lipopolysaccharide (LPS) (n 8; ISS+) or the same volume of a sterile saline solution (n 4; ISS − ). ISS was induced by an intramuscular repeated injection of increasing doses of Escherichia coli LPS every 48 h for 7 d. The initial dosage of 60 μg/kg BW was increased by 12 % at subsequent injections to avoid LPS tolerance( Reference Deitch 18 ). Subsequently, two consecutive N-balances (lasting 3 and 4 d, respectively) were conducted to monitor the pattern of PD during ISS.
Table 1 Ingredient composition and analysed nutrient contents of the experimental diets
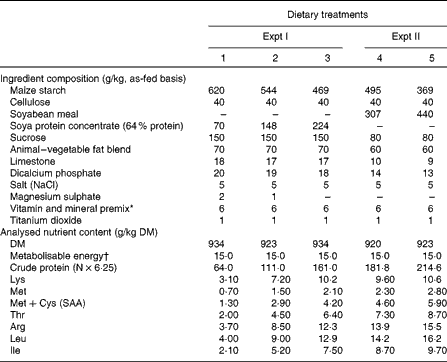
SAA, sulphur amino acids.
* Provided the following amounts of vitamins and trace minerals (per kg of diet): vitamin A (as retinyl acetate), 4·1 mg; vitamin D3 (as cholecalciferol), 0·03 mg; vitamin E (as dl-α-tocopherol acetate), 48 mg; vitamin K (as menadione), 3 mg; pantothenic acid, 180 mg; riboflavin, 6 mg; choline, 600 mg; folic acid, 2·4 mg; niacin, 30 mg; thiamin, 18 mg; pyridoxine, 1·8 mg; vitamin B12, 0·03 mg; biotin, 0·24 mg; Cu (as CuSO4·5H2O), 18 mg; Fe (as FeSO4), 120 mg; Mn (as MnO2), 124 mg; Zn (as ZnO), 126 mg; Se (as Na2SeO3), 0·36 mg; I (as KI), 0·6 mg (DSM Nutritional Products Canada, Inc.).
† Calculated value, MJ/kg.
Experiment II
A total of twenty-four barrows (initial BW 23·4 (se 0·43) kg) were blocked by time in two equal groups of twelve pigs and randomly assigned to dietary treatments. Dietary treatments were arranged in a 2 × 2 factorial randomised complete block design with the following factors: (1) two levels of dietary SAA allowance (4·0 or 5·0 g/d, representing 61 and 77 %, respectively, of established SAA requirements for maximum growth for pigs of this BW( 16 ) and (2) ISS (ISS − v. ISS+). Compositions of the experimental diets are presented in Table 1 (diets 4 and 5). The same principles were used for diet formulation as in Expt I, with the exception of using a soyabean meal as the sole source of dietary AA in Expt II. As per Expt I, a 5 d pre-ISS N-balance period (n 12 at each dietary SAA level) was followed by two N-balance periods during ISS. At the end of the first N-balance period, half of the pigs (n 6) at each dietary SAA level were injected intramuscularly with either LPS or the same volume of a saline solution, as described in Expt I.
Observations and sampling
Body weight and body temperature
BW was measured at the beginning and end of each N-balance period. Eye temperature (Expt I) or rectal temperature (Expt II) was monitored daily. Thermography of eye was performed using an IR camera (ThermaCam™ SC2000; FLIRSystems, Inc.( Reference Montanholi, Odongo and Swanson 19 , Reference Hughes, Patterson and Thornton 20 )). The camera was calibrated each day for room temperature and relative humidity( Reference Montanholi, Odongo and Swanson 19 ). The emissivity value was set at 0·98 according to the camera manufacturer's recommendation for biological tissues. Multiple IR pictures were taken approximately 0·8 m from the right eye and the best pictures, in terms of focus and precision, were selected for interpretation. The IR pictures were interpreted using ThermaCam™ Researcher 2001 software (FLIR Systems AB). An average of the best three pictures was taken and used for further analysis and interpretation. Due to the unavailability of the IR camera in Expt II, rectal temperature was measured( Reference Hughes, Patterson and Thornton 20 ).
Nitrogen balance
Urine was collected quantitatively and sampled daily as described by Zhu et al. ( Reference Zhu, Rademacher and de Lange 21 ). Urine was collected in tared bottles, containing a sufficient amount of 5 m-HCl to maintain a pH below 2·5. Faeces were collected twice daily and stored in sealed plastic bags at − 20°C until further processing. At the end of each N-balance period, faeces were thawed, pooled for each pig, homogenised using a Hobart mixer (Hobart Corporation) and kept in sealed plastic bags at − 20°C until further processing. Wasted feed, including vomit, was collected quantitatively using feed wastage trays, pooled for each pig and N-balance period, oven dried at 60°C, left overnight and then weighed to derive the actual daily N intake.
Blood collection and processing
Blood samples were taken by retro-orbital sinus bleeding at the end of the last N-balance period. For complete blood cell counts, blood was collected in tubes containing EDTA (BD Vacutainer; BD) kept on ice and analysed within 2 h after collection. For measuring serum albumin and haptoglobin levels, blood samples were collected in a serum tube containing clot activator (BD Vacutainer), left at room temperature for 1 h, centrifuged at 1500 g and stored at − 20°C. For determining plasma fibrinogen levels, blood samples was collected in tubes containing sodium citrate (BD Vacutainer), centrifuged immediately for 10 min at 1500 g at 4°C and stored at − 20°C.
Apparent ileal digestibility of nutrients and organ weights
The slaughter technique was used to measure the apparent ileal digestibility (AID) of nutrients( Reference Low 22 ). Between 6 and 7 h, after the morning meal and immediately after euthanasia, a ventral abdominal incision was made, spleen and liver were removed, rinsed with physiological saline, blotted dry and weighed. The last 100 cm of the small intestine was then isolated and clamped to prevent digesta movement. The ileum was then excised and the ileal digesta were gently expelled, collected and stored at − 20°C until further processing.
Chemical analysis
Blood levels of acute-phase proteins and complete blood cell counts were determined at the Animal Health Laboratory of the University of Guelph. Serum albumin and haptoglobin levels were analysed using a Roche cobas c501 biochemistry analyser (Roche Diagnostics)( Reference Doumas, Watson and Biggs 23 , Reference Makimura and Suzuki 24 ). Plasma fibrinogen was quantified using a KC4 Delta semi-automatic coagulation analyser (Trinity Biotech) and a TirinitClot kit (TriniCLOT™ Fibrinogen; Trinity Biotech). Complete blood count was performed using the ADIVA 120 Hematology System (Siemens Healthcare Diagnostics, Inc.).
Faecal samples along with ileal digesta and dietary samples were freeze-dried, ground and thoroughly mixed before analysis. TiO2 and DM were determined according to the standard Association of Official Analytical Chemists procedures( 25 ). N contents of the faeces and urine were measured using a LECO FP-428 Nitrogen Determinator combustion instrument (LECO Corporation) and analysed according to the standard Association of Official Analytical Chemists procedures( 25 ). Ileal digesta and dietary N contents were determined by LECO FP-2000 (LECO Corporation) at the laboratory of Evonik Degussa GmbH. Gross energy of the dietary and ileal digesta samples was determined as suggested by Widyaratne & Zijlstra( Reference Widyaratne and Zijlstra 26 ) using an IKA oxygen bomb calorimeter (model C 5003; IKA GmbH & Company KG).
AA analyses of the feed and digesta samples were performed at the laboratory of Evonik Degussa GmbH. AA contents in the dietary and ileal digesta samples were determined by ion-exchange chromatography with post-column derivatisation with ninhydrin. Before protein hydrolyses, AA were oxidised with performic acid, which was neutralised with sodium metabisulphite( Reference Llames and Fontanie 27 ). The samples were hydrolysed with 6 m-HCl for 24 h at 110°C. AA were quantified with the internal standard method by measuring the absorption of reaction products with ninhydrin at 570 nm.
Calculation and statistical analyses
Calculations
The AID of crude protein (CP; N × 6·25), AA and gross energy were calculated using the indicator method and TiO2 as an indigestible marker. Standardised ileal digestibility (SID) of AA was estimated as described by Stein et al. ( Reference Stein, Fuller and Moughan 28 ). The basal endogenous loss of each AA was calculated using the profile of basal endogenous AA in growing pigs as described by Jansman et al. ( Reference Jansman, Sminka and van Leeuwena 29 ).
SID SAA intake was calculated as follows:
where SID SAA is the SID of SAA (%) and SAAdiet and DMI are dietary SAA contents (g/kg DM) and DM intake (kg/d), respectively.
Whole-body PD (N retention × 6·25) was calculated as described by Zhu et al. ( Reference Zhu, Rademacher and de Lange 21 ). Faecal protein digestibility was measured, using TiO2 as an indigestible marker, to compute faecal N excretion from dietary N intake.
Linear-plateau (broken-line) regression models were used to relate PD (g/d) to SID SAA intake (g/d) and to estimate the cross-over point of dietary SAA intakes required to just reach plateau or maximum PD in ISS+ and ISS − pigs( Reference Robbins, Saxton and Southern 30 ).
At dietary SAA intakes below the cross-over point, the following linear regression model was used to estimate maintenance SID SAA requirements and the partial efficiency of SAA utilisation for PD:
The regression coefficients a and b were used to estimate maintenance SID SAA requirements (g/d), i.e. SID AA intake when PD equals zero, calculated as − a/b. The regression coefficient b (g PD/g SID SAA intake) represents the partial efficiency of the utilisation of SID SAA intake for PD( Reference Baker, Becker and Norton 31 ).
Statistical analyses
Statistical analyses were carried out using SAS software version 9.1 (SAS Institute, Inc.). One-time observations such as final BW, organ weights, plasma acute-phase protein concentrations and measures of digestibility were analysed using a factorial randomised complete block design (PROC MIXED) with dietary SAA allowance (D; three and two levels in Expt I and II, respectively), ISS (ISS − v. ISS+), interaction between D and ISS (D × ISS) and group of pigs (block) as fixed effects and pig within D and ISS as the random effect. Results for body temperature, PD and SID SAA intake were analysed separately for the two experiments as repeated measurements across the N-balance periods in a factorial randomised complete block design. This model included D, ISS, D × ISS, N-balance periods and block as fixed effects and pig within D and ISS as the random effect. Due to the sequential nature of the observations on each animal, a compound symmetry structure that yielded the model with the best fit was identified, according to the Akaike and Bayesian information criteria( Reference Burnham and Anderson 32 ). Values are reported as least-square means with their standard errors. The differences in the efficiency of SAA utilisation for PD (slopes) as well as SAA requirements for maintenance between the ISS − and ISS+ pigs were tested using the regression procedure (PROC REG). Treatment effects were considered significant at P≤ 0·05. A tendency towards a significant difference between treatment means was also considered at P≤ 0·10.
Results
General observations
Pigs readily consumed the experimental diets. The ISS+ pigs displayed some clinical signs of disease such as lethargy, vomiting and mild skin rashes. DM from vomit and wasted feed was less than 2·0 % of feed allowance. The analysed dietary nutrient contents were generally in agreement with anticipated values ( ± 10 %) that were derived from feed ingredient composition and nutrient levels in feed ingredients according to the National Research Council( 16 ). Only for diet 4 (Expt 2), the analysed CP and AA contents were higher than the anticipated values (>15 %). For the interpretation of the results, the analysed dietary nutrient contents and the observed AA digestibility values were used.
Measures of immune function
No interaction effects of D × ISS or effects of D on the measures of immune function were observed (P>0·10; Table 2). Repeated injection with increasing amounts of LPS increased the relative weights of spleen in Expt I and liver in Expt I and II (P< 0·01), as well as the eye (Expt I; Fig. 1) and rectal (Expt II) temperatures, by 1·2 and 0·4°C, respectively (P< 0·05). Plasma levels of haptoglobin and fibrinogen were also increased as a result of ISS (Expt I; P< 0·04). No treatment effect on serum albumin levels was observed (P>0·10). ISS increased leucocyte counts (P< 0·05), but had no effect on erythrocyte counts, Hb or haematocrit levels in Expt I (P>0·10).
Table 2 Impact of immune system stimulation (ISS) on the relative weight of organs, body temperature, levels of acute-phase proteins and blood cell counts* (Least-square means with their standard errors)
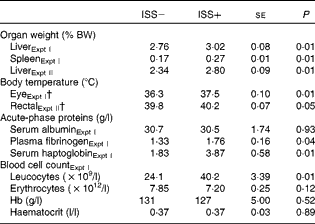
BW, body weight.
* Data are least-square means and represent the data obtained on day 7 after the start of ISS. Twenty-four out of thirty-six pigs in Expt I and twelve out of twenty-four pigs in Expt II were injected (intramuscularly) every 48 h over a 7 d period with increasing amounts of lipopolysaccharide (ISS+). The remaining pigs were injected with a sterile saline solution (ISS − ). In both experiments, responses were not influenced by the dietary treatments and there were no interaction effects of ISS × dietary treatment. Therefore, the results were pooled across the dietary treatments.
† Mean values of the 7 d period during ISS and based on the repeated-measures ANOVA.

Fig. 1 Impact of immune system stimulation (ISS) on the eye temperature of growing pigs during the 7 d ISS period (Expt I). Twenty-four out of thirty-six pigs were injected (intramuscularly) every 48 h for 7 d with increasing amounts of lipopolysaccharide (ISS+, ![]() ). The remaining pigs (n 12) were injected with a sterile saline solution (ISS − ,
). The remaining pigs (n 12) were injected with a sterile saline solution (ISS − , ![]() ). Eye temperature was determined by performing thermography of the eye using IR imaging.
). Eye temperature was determined by performing thermography of the eye using IR imaging.
Nutrient digestibility
In both Expt I and II, no effects of D or interaction effects of ISS × D on the AID of energy or the SID of CP and AA were observed (P>0·10; Table 3). Neither the AID of gross energy (measured only in Expt I), nor the SID of CP and key AA (i.e. essential AA that are among the first-limiting AA in practical pig diets) was affected by ISS (Table 3; P>0·20).
Table 3 Impact of immune system stimulation (ISS) on the apparent ileal digestibility (%, AID) of energy and standardised ileal digestibility (%, SID) of crude protein and some amino acids* (Least-square means with their standard errors)
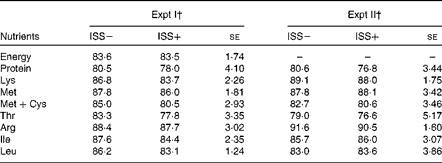
* Data are least-square means and represent the data obtained on day 7 after the start of ISS. AID was determined using an indigestible marker (TiO2) and the slaughter technique. SID was calculated as: AID %+((basal IAAend/AAdiet) × 100). Basal IAAend represents the basal endogenous loss of an amino acid (AA) expressed as g/kg DM intake as described by Jansman et al. ( Reference Jansman, Sminka and van Leeuwena 29 ) and AAdiet is the AA content in the diet (g/kg DM). There were no statistically significant differences.
† Pigs in Expt I and II were fed a maize starch and soya protein concentrate-based diet and a maize starch and soyabean meal-based diet, respectively. Twenty-four out of thirty-six pigs in Expt I and twelve out of twenty-four pigs in Expt II were injected (intramuscularly) every 48 h for 7 d with increasing amounts of LPS (ISS+). The remaining pigs were injected with a sterile saline solution (ISS − ). The dietary treatments did not influence the SID of amino acids and the AID of energy, and there were no interaction effects of ISS × dietary treatments. Therefore, the results were pooled across the dietary treatments.
Animal performance and nitrogen balance
The final BW of the pigs tended to increase as dietary SAA allowance was increased in Expt I and II (P>0·06; Tables 4 and 5). In Expt 1, the average daily feed intake tended to be lower for the ISS+ pigs (723 (se 3·8) g DM/d) than for the ISS − pigs (733 (se 4·0) g DM/d; P< 0·08), while these values did not differ in Expt 2 (792 (se 4·9) g DM/d for the ISS − pigs v. 784 (se 5·0) g DM/d for the ISS+ pigs; P>0·20). As expected, daily SID SAA intake was increased with dietary SAA allowance in both experiments (Tables 4 and 5). ISS reduced SID SAA intake in Expt I at all the three SAA intake levels (P< 0·01; Table 4), but not in Expt II (P>0·10; Table 5). In Expt I, the reduction in SID SAA intake due to ISS was largest at the lowest and highest levels of dietary SAA intake (P< 0·01; Table 4).
Table 4 Standardised ileal digestible (SID) intake of methionine plus cysteine (SAA), final body weight (BW) and whole-body protein deposition (PD) of pigs fed the varying amounts of SAA and exposed to immune system stimulation (ISS; Expt I)* (Least-square means with their standard errors)
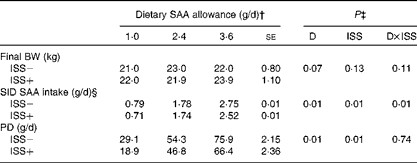
* Results for PD and SID SAA intake represent the best estimate of mean obtained during ISS based on the repeated-measures ANOVA. Immediately following the first N-balance period (pre-ISS; all pigs were considered ISS − ), twenty-four out of thirty-six pigs were injected (intramuscularly) every 48 h for 7 d with increasing amounts of lipopolysaccharide (ISS+). The remaining pigs were injected with a sterile saline solution (ISS − ).
† Pigs were fed soya protein concentrate and maize starch-based diets in which SAA were among the first-limiting amino acids.
‡ D and ISS represent diet (dietary SAA allowance) and ISS (ISS − v. ISS+) effects, respectively. D × ISS represents the interaction between D and ISS.
§ Daily SID SAA intake (g/d) was calculated as: SID of SAA % × (AAdiet× DMI). AAdiet and DMI represent the total amino acid in the diet (g/kg) and DM intake (kg/d), respectively.
Table 5 Standardised ileal digestible (SID) intake of methionine plus cysteine (SAA), final body weight (BW) and whole-body protein deposition (PD) of pigs fed the varying amounts of SAA and exposed to immune system stimulation (ISS; Expt II)* (Least-square means with their standard errors)
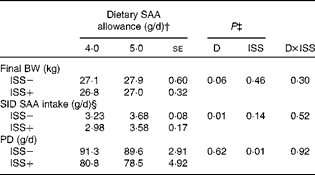
* Results for PD and SID SAA intake represent the best estimate of mean obtained during ISS based on the repeated-measures ANOVA. Immediately following the first N-balance period (pre-ISS; all pigs were considered ISS − ), twelve out of twenty-four pigs were injected (intramuscularly) every 48 h for 7 d with increasing amounts of lipopolysaccharide (ISS+). The remaining pigs were injected with a sterile saline solution (ISS − ).
† Pigs were fed soyabean meal and maize starch-based diets in which SAA were among the first-limiting amino acids.
‡ D and ISS represent diet and ISS (ISS − v. ISS+) effects, respectively. D × ISS represents the interaction between D and ISS.
§ See Table 4.
The daily PD increased linearly with SID SAA intake in Expt I (P< 0·01; Table 4 and Fig. 2). However, the SID SAA intake level did not influence PD in Expt II (P>0·60; Table 5 and Fig. 2). Within the two experiments and during ISS, the relative responses to the dietary treatments were similar for the two N-balance periods (P>0·40). In both experiments, ISS reduced PD at all the levels of SID SAA intake (P< 0·01; Tables 4 and 5). No interaction effects of ISS × D on PD were observed (P>0·74; Tables 4 and 5).
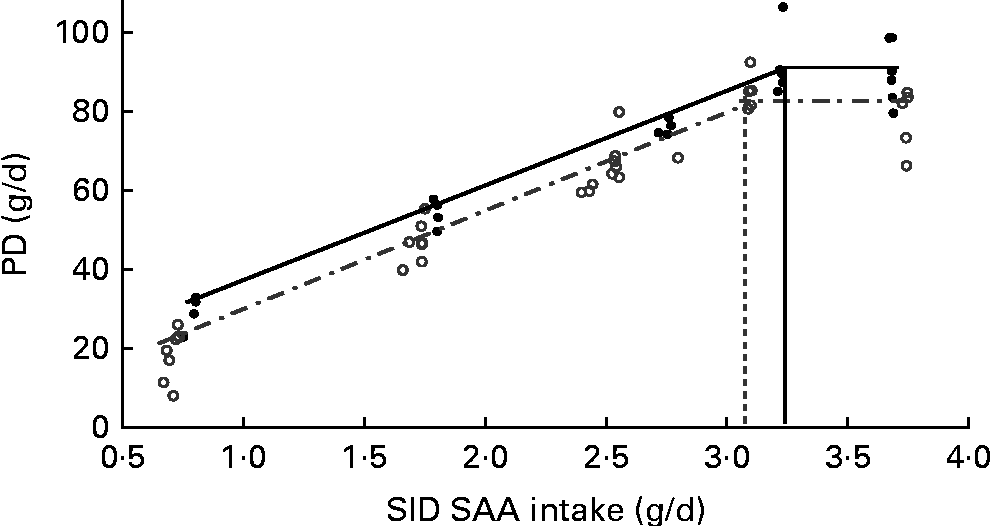
Fig. 2 Impact of immune system stimulation (ISS) and standardised ileal digestible (SID) methionine plus cysteine (SAA) intake on whole-body protein deposition (PD) in growing pigs. Results of the two experiments (Expt I and II) were combined for the linear-plateau regression analysis. Pigs in Expt I and II were fed a maize starch and soya protein concentrate-based diet, and a maize starch and soyabean meal-based diet, respectively. Three (1·0, 2·4 and 3·6 g/d) and two levels (4·0 and 5·0 g/d) of SAA intake were used in Expt I and II, respectively (see Tables 4 and 5). Twenty-four out of thirty-six pigs in Expt I and twelve out of twenty-four pigs in Expt II were injected (intramuscularly) every 48 h for 7 d with increasing amounts of lipopolysaccharide (ISS+, ![]() ). The remaining pigs were injected with a sterile saline solution (ISS − ,
). The remaining pigs were injected with a sterile saline solution (ISS − , ![]() ).
).
When the results of Expt I and II were combined, the PD response at varying amounts of SID SAA intake was accurately represented using the linear-plateau regression models for both ISS − and ISS+ pigs (R 2 0·94 and 0·95, respectively; Fig. 2). Break-point analyses of PD responses yielded different estimates of SID SAA requirements for maximum PD of 3·34 (se 0·11) g/d for the ISS − pigs and 3·08 (se 0·12) g/d for the ISS+ pigs (P< 0·056). At dietary SAA intakes below the estimated requirements, ISS had no effect on the partial efficiency of SID SAA utilisation for PD, represented by the slope for PD in Fig. 2 (P>0·27; Table 6). However, ISS increased the extrapolated maintenance SID SAA requirements, represented by the intercept at zero PD in Fig. 2 (P< 0·05; Table 6).
Table 6 Impact of immune system stimulation (ISS) on parameters representing the linear relationship between standardised ileal digestible (SID) sulphur amino acid (SAA) intake and protein deposition (PD) in growing pigs* † (Least-square means with their standard errors)
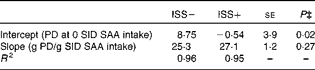
* Results of the two experiments (Expt I and II) were combined for the linear-plateau regression analysis (Fig. 2). Twenty-four out of thirty-six pigs in Expt I and twelve out of twenty-four pigs in Expt II were injected (intramuscularly) every 48 h for 7 d with increasing amounts of lipopolysaccharide (ISS+). The remaining pigs were injected with a sterile saline solution (ISS − ).
† One-slope model: y= a+b(x), where y is the PD (g/d), a (intercept) can be used for mathematical estimation of extrapolated maintenance requirement ( − a/b), b (slope) represents the partial efficiency of SAA utilisation for PD and x is the daily SAA intake (g/d) when SAA intake is below the requirements for maximum PD.
‡ Significant differences among the dietary treatments.
Discussion
The purpose of the present study was to evaluate the impact of chronic mild ISS on the ileal digestibility of nutrients and utilisation of SAA intake for PD. To our knowledge, this is the first attempt to evaluate SAA requirements and ileal digestibility of nutrients during ISS in growing pigs. In the present study, serial dilution of dietary protein sources was used to generate the dietary treatments and to avoid confounding of SAA intake levels with changes in dietary AA balance( Reference Gillis, Reijmers and Pluske 33 ). It has been shown that the efficiency of SAA utilisation for PD is affected by dietary AA balance( Reference Langer and Fuller 34 ). In a previous study( Reference Gillis, Reijmers and Pluske 33 ), it has been confirmed that SAA were among the first-limiting AA in the dietary AA profile that was used in the present study.
In an earlier study in our laboratory, we observed a persistent increase in plasma levels of cytokine IL-1β after repeated injection of pigs with increasing doses of LPS, which resulted in systemic inflammation( Reference Dinarello 35 , Reference Rakhshandeh and de Lange 36 ). Systemic inflammation leads to cell hypertrophy, and sometimes cell hyperplasia in organs that are involved in the immune response( Reference Kohl, Deutschman, Newman, Fleisher and Fink 37 ). In the present study, ISS resulted in increased relative weights of liver and spleen, the two major organs that are involved in the immune response, which is in agreement with the findings of Breuille and others( Reference Malmezat, Breuille and Capitan 6 , Reference Breuille, Arnal and Rambourdin 38 ). It has been suggested that a low nutrient intake compromises the immunocompetence of animals, including a reduced capability for maintaining plasma levels of acute-phase proteins( Reference Reeds and Jahoor 1 , Reference Jahoor, Wykes and Del Rosario 39 ). However, in the present study, SID SAA intake had no effect on the plasma or serum levels of albumin, fibrinogen and haptoglobin, suggesting that production of acute-phase proteins has a high priority during ISS, even when AA intake is below the requirements for maximum PD.
The acute-phase response to ISS is characterised by fever, anorexia, reduced serum albumin levels and increased acute-phase plasma protein levels( Reference Gabay and Kushner 40 ). In the present study, repeated injection of pigs with LPS resulted in an increase in eye and rectal temperatures as well as plasma levels of haptoglobin and fibrinogen, the two major acute-phase proteins. However, we did not observe a reduction in serum albumin levels, which is inconsistent with the findings of Jahoor et al. ( Reference Jahoor, Wykes and Del Rosario 39 ), possibly due to the induction of only a relatively mild immune reaction in our ISS model. Moreover, ISS increased the leucocytes, which is one of the common clinical manifestations of the systemic inflammatory response( Reference Brunkhorst, Wegscheider and Forycki 41 – Reference Baumann, Prowse and Marinkovic 43 ). Pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-α, as well as corticosteroids play an essential role in initiating the synthesis of hepatic acute-phase proteins and elevating body temperature; therefore, the increased plasma haptoglobin and fibrinogen levels as well as body temperature reflect ISS mediated by these molecules( Reference Dinarello 35 , Reference Baumann, Prowse and Marinkovic 43 ). Collectively, these results indicate that the immune system was successfully stimulated in the present study.
It has been suggested that ISS induces morphological and functional changes in the gastrointestinal tract, which may perturb nutrient digestion and absorption( Reference Hang, Shi and Li 11 , Reference Yamada, Yamada, Alpers, Laine, Owyang and Powell 44 ). However, this suggestion is not supported by experimental observations. In the present study, we measured the impact of ISS on the ileal digestibility of nutrients using a slaughter technique. We chose this technique to avoid additional stress caused by surgical procedures (e.g. cannulation), possible secondary infections and any interference with the digestive function of animals( Reference Fuller and Orskov 45 ). The impact of the three inherent technical problems with the slaughter method for measuring ileal nutrient digestibility was minimised by controlling the sampling time relative to the feeding schedule, clearly identifying the intestinal segment from which the samples were obtained, i.e. the distal ileum( Reference Donkoh, Moughan and Smith 46 ), and minimising mucosal cell shedding by using sodium pentobarbital for euthanising the pigs( Reference Badawy and Munro 47 ). In the present study, the observed AID AA and CP values were converted to SID AA and CP values by considering basal ileal endogenous losses, to correct for the known effects of dietary AA and CP levels on AID values( Reference Stein, Fuller and Moughan 28 ).
The SID of CP and AA were not affected by ISS in the present study, indicating that physiological changes in the gastrointestinal tract during LPS-induced ISS have minimal or no effect on overall digestive efficiency. Barker( Reference Barker 48 ) found that an abnormal mucosa in ISS lambs was not associated with a reduction in feed efficiency and growth performance. Williams et al. ( Reference Williams, Stahly and Zimmerman 49 ) reported that apparent faecal digestibility of CP was not affected by ISS in growing pigs at different stages of growth. Similar results were also observed in a study with Actinobacillus pleuropneumoniae-challenged pigs( Reference Zoric, Arvidsson and Melin 50 ). It remains possible that ISS-induced reductions in feed intake have an impact on the measures of digestibility; in the present study, feed allowance was controlled and DMI was similar in pigs on ISS − and ISS+( Reference Nyachoti, de Lange and McBride 51 ). It should be mentioned that in the present study, the digestibility values were numerically lower and appeared to be more variable in the ISS+ animals than in the ISS − animals. This could be due to either increased endogenous gut protein and AA losses induced by ISS or to a reduction in absorptive capacity( Reference Faure, Chone and Mettraux 13 , Reference Stein, Fuller and Moughan 28 ). Therefore, the impact of ISS on gut-specific endogenous losses and nutrient digestibility deserves further investigation. Reduced SID SAA intake in the ISS+ pigs in Expt I could be attributed to numerically lower SID values in the ISS+ animals and to some extent to the increased feed wastage and vomiting. The interaction effect of ISS × dietary treatment on SID SAA intake in Expt I could be linked mainly to numeric differences in DMI among the dietary treatments due to feed wastage and vomiting, especially for pigs on diets 1 and 3.
Based on the assumption that SID SAA intakes represent available AA intakes that are independent of dietary AA sources, PD responses to the dietary treatments were combined across the two experiments for the ISS − and ISS+ pigs (Fig. 2). In the present study, plateau or maximum PD (Fig. 2) was reached for both ISS − and ISS+ pigs at the two highest dietary SAA intake levels. In the ISS − pigs, the plateau PD appears to be determined by the level of energy intake as a result of the restriction in feed intake( Reference Mohn, Gillis and Moughan 52 ). The observed reduction in plateau PD in the ISS+ pigs is probably the result of a higher pro-inflammatory cytokine release and its associated endocrine changes( Reference Jahoor, Wykes and Del Rosario 39 , Reference Lecker, Goldberg and Mitch 53 ). Pro-inflammatory cytokines inhibit the release of anabolic hormones (e.g. somatotropin and insulin-like growth factor I) and increase protein degradation in skeletal muscles by activating the catabolic pathways, mainly through the ubiquitin–proteasome pathway( Reference Williams, Stahly and Zimmerman 49 , Reference Lecker, Goldberg and Mitch 53 ).
Based on the linear-plateau regression models, SID SAA requirement for maximum PD in the ISS+ pigs was 8 % lower than that in the ISS − pigs. The reduced requirements for SAA in the ISS+ pigs can be attributed largely to the reduced maximum PD in the ISS+ pigs( Reference Williams, Stahly and Zimmerman 49 ). It has been demonstrated that the efficiency of the utilisation of the first-limiting AA for PD is reduced at the levels required for maximum PD( Reference Williams, Stahly and Zimmerman 49 , Reference Mohn, Gillis and Moughan 52 ). Therefore, the marginal efficiency of the utilisation of SID SAA intake for PD was evaluated at the levels of SAA intake that are below the levels needed to maximise PD. In the present study, the marginal efficiency of the utilisation of SID SAA for PD was similar for the ISS+ and ISS − pigs; 1 g of additional SID SAA intake supported 25·3 (se 1·26) g/d for PD in the ISS − pigs and 27·1 (se 1·10) g/d in the ISS+ pigs, which is in agreement with N-balance observations reported by Fuller et al. ( Reference Fuller, McWilliam and Wang 54 ). Assuming that PD contains 3·6 % SAA and is similar for the ISS+ and ISS − pigs, the mean efficiency of the utilisation of SID SAA intake for SAA retention in PD can be estimated at 0·94 in the present study( 16 ). This value is somewhat higher than previous estimates( 16 ), and reflects the systematic overestimation of PD values that are established using conventional N-balance methodology( Reference Fuller, McWilliam and Wang 54 ). The assumption that PD in ISS − and ISS+ pigs contains the same amount of SAA may be questioned. We demonstrated previously that the whole-body sulphur content of pigs increases during ISS( Reference Rakhshandeh, Htoo and de Lange 55 ). Larsson et al. ( Reference Larsson, Liljedahl and Martensson 56 ) reported that N excretion was enhanced to a greater extent than sulphur excretion (end product of SAA metabolism) in patients with systemic inflammation. Moreover, a study with TNF-α-treated rats has shown a reduction in urinary inorganic sulphate and total sulphur excretion( Reference Hunter, Meakins and Grimble 57 ). These observations have suggested that the whole-body SAA content increases relative to other AA during ISS, which can be attributed to the preferential retention of SAA, specifically Cys, in non-protein nitrogenous compounds such as GSH( Reference Malmezat, Breuille and Pouyet 5 , Reference Rakhshandeh, Htoo and de Lange 55 , Reference Hou, Wykes and Hoffer 58 ).
SAA requirements of growing pigs can be divided into two main components: requirements for body maintenance functions and a requirement for PD( 16 ). The present study yields negative estimates of SID SAA requirements for maintenance in the ISS − pigs. The negative values can be attributed to the systematic bias when using N-balance methodology for measuring PD and the required extrapolation of the relationship between PD and SAA intakes to zero PD( Reference Mohn, Gillis and Moughan 52 ). However, a higher estimate of maintenance SID SAA requirements in the ISS+ pigs than in the ISS − pigs (300 (se 3·8) mg/d; using a common partial efficiency of SAA utilisation of 26·2 (se 1·18) for the ISS − and ISS+ pigs; average coefficients b in Table 6) directly contributes to the observed impact of ISS on PD at all the levels of dietary SAA intake. Maintenance requirements for absorbed SAA serves to replace endogenous gut and integument protein losses, replace SAA losses due to the minimum SAA catabolism and obligatory use for homeostasis of non-protein compounds such as GSH( Reference Baker, Becker and Norton 31 , Reference Fuller, McWilliam and Wang 54 ). In pigs, integument protein losses are only a minor contributor to SAA requirements, and the present study suggests that there is no incremental effect of ISS on endogenous gut SAA losses( Reference Fuller, McWilliam and Wang 54 ). Moreover, studies with human subjects and rats have shown that the rate of SAA catabolism remains unchanged or is reduced during ISS( Reference Malmezat, Breuille and Pouyet 59 ). Therefore, it appears that the substantial increase in extrapolated SAA maintenance requirements in the ISS+ pigs can be attributed largely to increased SAA utilisation for the synthesis of non-protein compounds that are involved in the immune response, such as GSH( Reference Breuille, Obled, Furstand and Young 3 ).
Conclusions and implications
Repeated injection with increasing amounts of LPS successfully stimulates the immune system and provides a chronic ISS model for studying nutrient utilisation in growing pigs. In the present study ISS per se did not change the SID of dietary AA or the AID of gross energy. However, the observed numerical changes in nutrient digestibility warrant further studies. Metabolic changes associated with ISS reduced the capacity of pigs for maximum whole-body PD at a fixed level of energy intake, which most probably contributed to reduced daily SAA requirements for maximum PD. The latter is in spite of ISS-induced increases in SAA requirements for body maintenance functions. The potential impact of ISS, and alternative disease models, on SAA utilisation should be considered when formulating diets for growing–finishing pigs.
Acknowledgements
The present study was presented in part in Proceedings of the 11th International Symposium on Digestive Physiology of Pigs, Montbrió del Camp, Costa Daurada, Spain, 20–22 May 2009( Reference Rakhshandeh, Htoo and de Lange 55 ).
We thank C. L. Zhu, G. Vandervoort and Y. R. Montanholi for technical assistance, and M. Chpak for editorial assistance. The present study was funded in part by the Natural Sciences and Engineering Research Council of Canada, the Ontario Ministry of Agriculture, Food and Rural Affairs, Ontario Pork and Evonik Degussa GmbH (all grants de Lange CFM). The FLIR camera and software were funded from the Canadian Foundation for Innovation and the Ontario Innovation Trust (Miller SP). This research is a publication from the Center for Nutrition Modeling, Department of Animal and Poultry Science, University of Guelph.
The authors' contributions were as follows: A. R. and C. F. M. d. L. designed the study; A. R. conducted the research; A. R. analysed the data; A. R., J. K. H., N. K., S. P. M. and C. F. M. d. L. wrote the paper; C. F. M. d. L. had primary responsibility for the final content. All authors read and approved of the final manuscript.
Author disclosure: A. R., J. K. H., N. K., S. P. M. and C. F. M. d. L. have no conflicts of interest.










