INTRODUCTION
In industrialized countries, acute infectious enteric diseases are a frequent cause of morbidity [Reference Wheeler1–Reference Ho4], particularly in children, but not of mortality. Because they are deemed preventable and often lend themselves to public health intervention, many countries have made infections with the major enteric pathogens statutorily notifiable. These surveillance data are important trend indicators, but because not all such infections are diagnosed and reported to public health authorities, these passive surveillance systems, unless supplemented by specific studies [Reference Scallan2, Reference Ruzante5], suffer from under-notification of unknown extent. This makes it difficult to use surveillance data directly for accurately assessing the burden of these illnesses in the population. However, they are still a valuable source of information on aspects of disease burden [Reference Ruzante5], particularly when analysed collectively with the purpose of inter-pathogen comparison.
Mortality is an important component of the disease burden, but disregards the fact that its social and economic consequences depend on age at death, and different enteric pathogens cause death at different ages. In contrast, ‘years of potential life lost’ (YPLL) involves estimating the average time a person would have lived had he or she not died prematurely. Thus, by weighting deaths among the young higher, it is a measure of premature mortality. Its use has been promoted to emphasize specific causes of death in proportion to their burden on society, and to identify areas for greatest health improvement [Reference Gardner and Sanborn6]. Various YPLL measures exist depending on definitions of ‘premature’ death [Reference Gardner and Sanborn6, Reference Perloff7].
The main objective of this study was to compare premature mortality among six major enteric infectious diseases notified in Germany, by use of three different YPLL measures. A secondary objective was to assess pathogen-specific differences in completeness of death notifications in the German surveillance system by comparing these data with those of the national cause-of-death statistic.
MATERIAL AND METHODS
Data source
Germany has an electronic surveillance system for statutorily notifiable infectious diseases. According to the German Protection against Infection Act, in effect since 1 January 2001, laboratory detection of most enteric infectious pathogens is notifiable. In addition, physicians are required to notify patients with clinical symptoms compatible with diarrhoea-associated haemolytic uraemic syndrome (HUS). For every notified person fulfilling the surveillance case definition (see below), a case report is filed by the local health department and transmitted (without the name and address of the patient) electronically, via state health departments, to the federal-level public health institute, the Robert Koch Institute (RKI). Although not required by law, the electronic case-form provides a field for ‘death associated with the notified disease’, and health departments are asked to mark the field if the infectious disease had contributed to the death. This is a broader concept than ‘underlying cause of death’, which is defined by the International Statistical Classification of Diseases and Related Health Problems (ICD-10) as the disease or injury which initiated the train of morbid events leading directly to death (or the circumstances of the accident or violence which produced the fatal injury). Since the beginning of 2004, every death marked on an enteric infectious disease report is subject to quality control. An email is sent to the local health department with the purpose of verifying the death notice and of assessing a (co-)causal contribution of the infectious disease.
Data for the years 2004–2008 were obtained from the national database of statutorily notifiable infectious diseases, hosted at the RKI. We selected those six notifiable enteric diseases for which the highest number of deaths was recorded in the database. These were illnesses due to infection with Campylobacter spp., Listeria monocytogenes, norovirus, rotavirus, non-typhoidal Salmonella spp., and Shiga toxin-producing E. coli (STEC) – of serogroup O157 and others. We obtained information on the German population size and, for comparison, on ICD-10-specific cause-of-death numbers for the period 2004–2008 from the websites of Germany's Federal Statistical Office [8].
Case definitions
Cases of these illnesses were included in the analysis if they fulfilled the respective national case definition that was established for surveillance purposes, and published by RKI [9]. The case definition generally comprised cases with compatible clinical symptoms (mostly gastroenteritis, except for listeriosis) with laboratory confirmation as well as those with compatible clinical symptoms without laboratory confirmation if they were reported in epidemiological conjunction with a laboratory-confirmed case. The latter facet was of particular importance for norovirus.
Laboratory confirmation for viral gastroenteritis pathogens was achieved by antigen-specific immunoassays or by pathogen-specific nucleic acid amplification. For bacterial enteric diseases, laboratory confirmation usually required a culture of the pathogen, except for STEC where detection of at least one of the Shiga toxins in enrichment broth or detection of their encoding genes sufficed. Additionally, an STEC infection could also be established in HUS cases by detecting anti-lipopolysacharide IgM antibodies against E. coli serogroups in the blood of these patients. For listeriosis, the case definition covered invasive illnesses where L. monocytogenes was isolated from a clinical sample of a normally sterile site or from any specimen in neonates.
Listeriosis was classified as pregnancy associated (PA) if illness occurred in a pregnant woman or an infant in its first month of life. For PA listeriosis, separate case reports for mothers and their infants were completed and linked by a unique identifier. We checked mother–infant pairs for completeness and added eight missing infant reports manually to the dataset where the information of a premature or neonatal death was noted in the annotation field of a mother-only case report and where this was additionally confirmed by quality control.
Statistical analysis
For each pathogen, we computed overall disease frequency, mean annual disease incidence, mean annual mortality (using the average German population size at that time), the case-fatality ratio (CFR), and three different variants of the YPLL measure (Table 1). The CFR was calculated by dividing the number of reported deaths by the total number of reported cases for each pathogen-specific illness and multiplying by 100. YPLL are generally computed by multiplying the number of deaths at each age by a weight for the potential years of life remaining at that age, and the terms are summed to get the total value. The three variants of YPLL differ by their specific weight, which largely depends on the view at what age social and economic losses begin and end:
(1) YPLL, using as weight the remaining life-expectancy at age of death according to probabilistic life-tables.
(2) Premature YPLL (P-YPLL), using as weight the time interval from age at death to an upper limit, which was set here at 70 years.
(3) Working YPLL (W-YPLL), using as weight the potential years of life lost in the age interval 15–65 years.
To assess pathogen-specific differences in completeness-of-death notifications in the German notification system, we compared these data with those of the national cause-of-death statistic, provided by Germany's Federal Statistical Office. The cause-of death census data are based on death certificates where a singular cause, i.e. the underlying cause of death, is ICD-10-coded. Thus, the two sources of pathogen-specific mortality rely on independent mechanisms of data collection.
Table 1. Formulas for years-of-life-lost measures used for six major enteric pathogens in Germany, 2004–2008
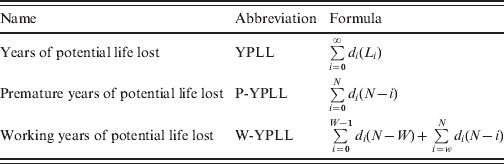
i, Age at death; d i, number of deaths at age i; L i, life-expectancy at age i; N, upper cut-off age; W, lower cut-off age.
RESULTS
From 2004 to 2008, the RKI received 1 481 807 case reports of the six illnesses, of which those caused by infection with norovirus were most frequent (617 437, 41%) followed by Campylobacter spp. (300 895, 20%), rotavirus (296 035, 20%), and non-typhoidal Salmonella spp. (260 207, 18%; Table 1). L. monocytogenes and STEC accounted for <1% of these illnesses. Proportion of case reports with laboratory confirmation was 49% for norovirus, 90% for rotavirus and at least 95% for enteric bacterial illnesses. The highest mean annual mortality was registered for Salmonella spp. (0·55 deaths/100 000 population per year), followed by norovirus (0·47) and L. monocytogenes (0·46), for which by far the highest CFR (∼10%) was noted, particularly for PA illnesses (16%). Campylobacteriosis, the most frequent enteric bacterial illness in Germany had the lowest mortality of the six pathogens (<10% of that of salmonellosis).
Apart from PA listeriosis and STEC (23 years), median age at death was >68 years for all pathogens and >80 years for viral enteric pathogens (Table 2). Regarding age at death, two different patterns were observed among the six pathogens. For non-typhoidal Salmonella spp., norovirus and principally also Campylobacter spp. (top row of Fig. 1), death was noted predominantly in elderly people, whereas for rotavirus, L. monocytogenes (through PA illnesses) and STEC (through HUS) a substantial proportion of death was also reported in young children, including newborns. STEC was the only pathogen for which a higher mortality was registered in young children compared to the elderly. Listeriosis caused the highest number of years potentially lost in all three different measures. Using YPLL measures that disregard life at advanced age (i.e. W-YPLL and P-YPLL), STEC and rotavirus had the highest YPLL, following listeriosis.
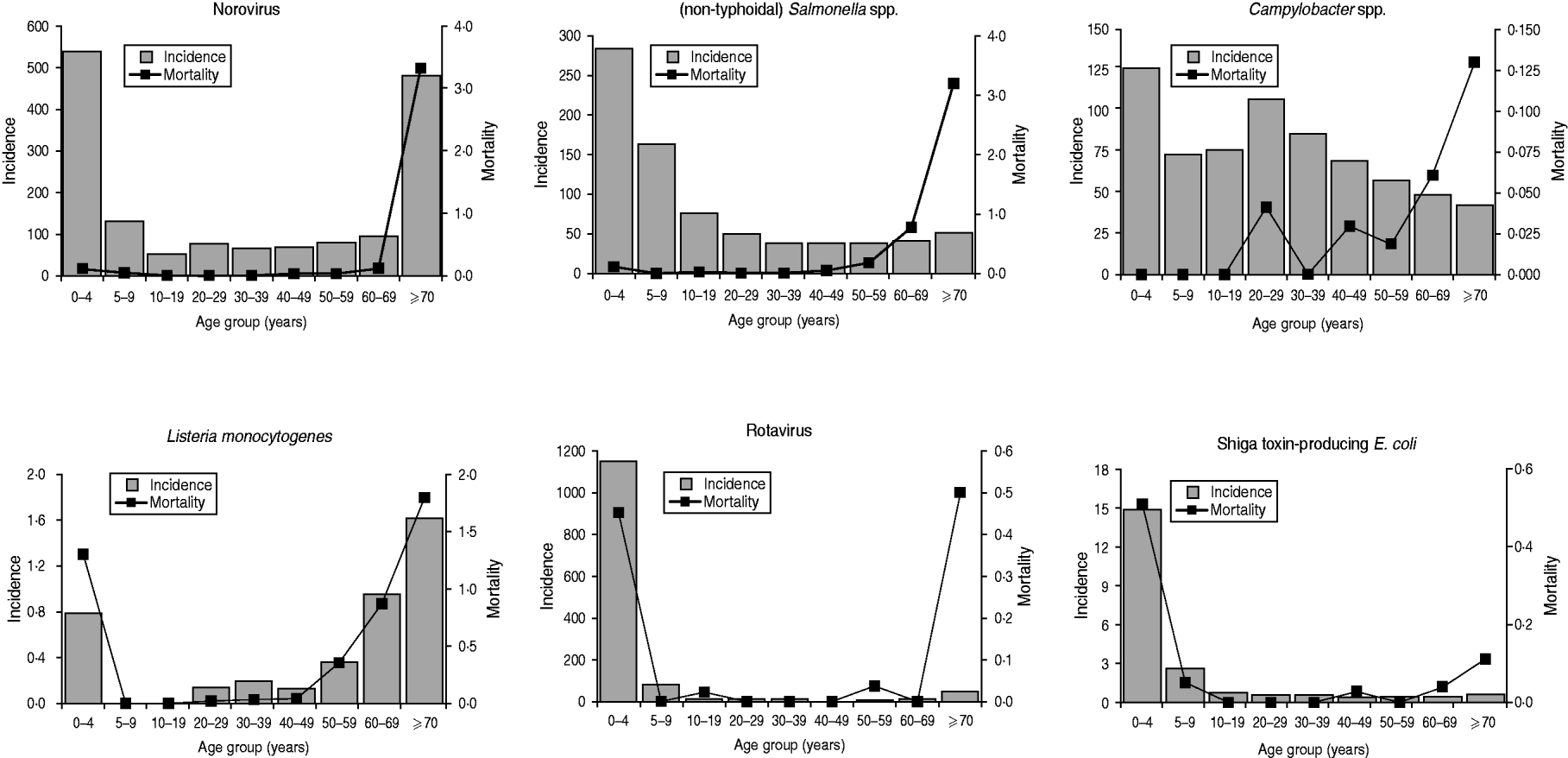
Fig. 1. Relationship of notification incidence and mortality with age for six major enteric pathogens in Germany, 2004–2008.
Table 2. Illnesses, deaths, and years of potential life lost for six major enteric pathogens, based on notification data, Germany, 2004–2008

CFR, case-fatality ratio; YPLL, years of potential life lost; P-YPLL, premature YPLL (<70 years); W-YPLL, working YPLL (15–65 years); PA, pregnancy-associated; non-PA, non-pregnancy-associated; STEC, Shiga toxin-producing E. coli.
* Per 100 000 population/year.
† Median.
‡ Incidence refers to persons aged <1 year.
Comparing pathogen-specific frequencies of death notified in the German surveillance system with those of the national cause-of-death statistic revealed an underassessment in the surveillance data – despite the more specific outcome definition of the cause-of-death statistic, which requires the infectious disease to be the underlying cause of death. The underassessment seemed somewhat proportional for most pathogens, except for norovirus where markedly more deaths were registered in the cause-of-death statistic, and for listeriosis where more deaths were captured in the surveillance system than in the national cause-of-death statistic (Fig. 2).

Fig. 2. Cause of death for six major enteric pathogens in Germany, 2004–2008 – comparison of data from notifiable infectious disease surveillance with the national cause-of-death statistic.
DISCUSSION
In this study, we assessed premature mortality of six major enteric pathogens notified in Germany by estimating the number of life years potentially lost to those infections – a measure infrequently used in infectious disease epidemiology thus far. Particularly the public health importance of preventing listeriosis is underscored, which caused by far the highest YPLL regardless of which variant of YPLL measure was used. Listeriosis, a rare but life-threatening foodborne illness, can present clinically as septicaemia, or meningitis/meningoencephalitis, mostly in older persons. During pregnancy, listeriosis may result in fetal loss, premature labour, neonatal infection and death [Reference Jackson, Iwamoto and Swerdlow10]. PA listeriosis accounted for ∼10% of notified listeriosis cases in Germany, and deaths in newborns contributed markedly to the pronounced premature mortality of the illness. Two other pathogens caused notable mortality in children: rotavirus and STEC. Seven deaths in children aged <1 year were registered for rotaviral disease in the 5-year period (data not shown), resulting in a higher ranking of the disease, compared to the other enteric pathogens, with respect to P-YPLL and W-YPLL. Besides other parameters, such as incidence, hospitalization rates, and associated costs [Reference Koch and Wiese-Posselt11], YPLL provides useful additional information to be considered in the decision as to whether rotavirus vaccination should be recommended in children in Germany. STEC infection can lead to life-threatening HUS, particularly in children [Reference Tarr, Gordon and Chandler12], which was the main driver for premature mortality in STEC illnesses. STEC infection in Germany is notifiable regardless of serotype and the virulent STEC serogroup O157 accounts usually for <20% of reported STEC infections, but for almost 80% of HUS cases. Importantly, STEC O157 incidence in Germany is low (∼0·2/100 000 population) compared to other countries, e.g. USA (0·99) [13], Canada [14], Ireland, and UK), suggesting an even more substantial effect of this serogroup on premature mortality in other countries. Non-typhoidal salmonellosis had the highest mortality rate during the study period. However, as almost no deaths were recorded for children, the illness ranked only fourth when considering YPLL. A similar situation was observed for illnesses due to norovirus infection.
Comparison of the surveillance mortality data with the national cause-of-death statistic revealed notable differences for norovirus and listeriosis. Compared to infectious disease surveillance data, more than twice the number of norovirus deaths was recorded in the cause-of-death statistic despite its more specific definition of cause of death (infections considered terminal co-factors are likely to go unrecognized in this statistic). Consequently, death contributed to by norovirus infection might be disproportionately under-ascertained in the German surveillance system (irrespective of age of death, data not shown). Conceivably, when large case numbers are notified to local health departments during the norovirus season, data quality may suffer in detail. In contrast, death associated with listeriosis is relatively overrepresented in the German surveillance system. Cases of listeriosis are more singular and often investigated in great depth by local health departments. Consequently, listeriosis-associated death is more completely ascertained in the surveillance system compared to other enteric illnesses, particularly as capturing death in the German surveillance system only requires the assessment that the infection contributed to the death – not that the infection was its underlying cause. Furthermore, the majority of notified listeriosis patients in Germany and elsewhere [Reference Mook, Patel and Gillespie15] have underlying conditions likely to lead to premature mortality even without the infection (which is also the reason why the term YPLL cannot be interpreted verbatim). It seems plausible that only a fraction of the fatalities will be causally attributed to the infectious disease on the death certificate even if it had initiated the train of morbid events leading directly to death, thereby biasing the cause-of-death statistic downwards.
At least two potential biases deserve mentioning that lead to under-ascertainment of mortality, and as a corollary also case-fatality and YPLL, in the German surveillance system. Follow-up of notified cases is rare. Consequently, death occurring after health departments contacted notified patients probably goes unrecognized. The median time interval between disease onset and notification was a week for bacterial pathogens and 4–5 days for viral enteric pathogens. Therefore, the surveillance system predominantly covers short-term mortality, but not long-term mortality. A large registry-based cohort study in Denmark, controlling for comorbidities, found an increased risk of dying up to 1 year post-infection for some bacterial enteric pathogens [Reference Helms16]. Second, causally attributing usually mild and self-limiting diseases to death is difficult in general and in particular in elderly persons that often have concurrent conditions. This has led some authors to disregard death at advanced age and advocate the use of P-YPLL and W-YPPL.
Burden-of-illness estimates combining indicators of mortality and morbidity into a single measure are increasingly used to inform public health policy [Reference Haagsma17]. For example, the World Health Organization has developed and is using the ‘disability-adjusted life year’, a single metric that sums up YPLL due to premature mortality and the years lost due to disability for incident cases of the health condition [Reference Murray and Lopez18]. The value of YPLL is, however, not limited to being an important building block of a composite burden-of-illness measure. It helps to identify areas that provide the greatest potential for health improvement.
Incidence of foodborne diseases is subject to secular trends and varies across countries. However, it is worth noting that mortality and relationship of the incidence of laboratory-confirmed bacterial enteric diseases in Germany is comparable to that of other countries (e.g. USA [Reference Scallan2, Reference Barton Behravesh19]) with the exception of STEC O157, which is more frequent in almost all countries, where English is the native language. By contrast, a marked difference was observed for viral illnesses, particularly for norovirus. A substantially higher number of annual cases than, for example in the USA, were captured in the German surveillance system that also includes illnesses that are epidemiologically linked to laboratory-confirmed cases. Surveillance of viral gastoenteritis varies widely across countries, and often estimates rather than actual reports of the disease are provided (e.g. in the USA), which at least partially accounts for the observed differences.
In conclusion, weighting death by age leads to a different view on the disease burden individual enteric pathogens cause. Although most deaths occurred in persons aged >65 years, L. monocytogenes caused by far the highest number of YPLL, predominantly due to its ability to infect the unborn or newborn via the mother. This underscores the public health importance of listeriosis prevention and to a lesser extent also that of rotavirus gastroenteritis and STEC illness. YPLL is simple to compute and easy to comprehend advocating its more frequent use in infectious disease epidemiology.
ACKNOWLEDGEMENTS
We are indebted to the staff of local health departments in Germany who diligently collect these data.
DECLARATION OF INTEREST
None.






