Red and processed meat consumption increases the risk of colorectal cancer, according to meta-analyses of epidemiological studies(Reference Larsson and Wolk1, Reference Norat, Lukanova, Ferrari and Riboli2). Several explanations have been given for this increase, involving fat, heterocyclic amines, N-nitrosated compounds and haem iron. Intake of dietary haem iron is associated with an increased risk of colon cancer among women of the Iowa Women's Health Study(Reference Lee, Anderson, Harnack, Folsom and Jacobs3). Intake of black pudding, a blood sausage high in haem iron, is associated with increased risk of colorectal cancer among women of the Swedish Mammography Cohort(Reference Larsson, Rafter, Holmberg, Bergkvist and Wolk4).
Experimental animal studies support the hypothesis that haem Fe promotes colorectal carcinogenesis(Reference Sesink, Termont, Kleibeuker and Vandermeer5). We have shown that dietary haem, in the form of haemin, Hb or red meat, promotes putative precancerous lesions, aberrant crypt foci (ACF) and mucin-depleted foci (MDF) in the colon of rats given a low-Ca diet(Reference Pierre, Tache, Petit, Van der Meer and Corpet6, Reference Pierre, Freeman, Tache, Van der Meer and Corpet7). Haemin is a free haem ring stabilized by a chlorine atom, in contrast with chlorine-free protein-bound haem in meat myoglobin. This haem-induced promotion is associated with increased lipoperoxidation and cytotoxicity of faecal water, and linked to urinary 1,4-dihydroxynonane mercapturic acid (DHN-MA) excretion, a lipid peroxidation biomarker(Reference Pierre, Peiro, Tache, Cross, Bingham, Gasc, Gottardi, Corpet and Gueraud8). The addition of Ca, antioxidant mix or olive oil to the diet inhibits haemin-induced lipoperoxidation, cytotoxicity and promotion of carcinogenesis in rats(Reference Pierre, Tache, Petit, Van der Meer and Corpet6), but no demonstration has already been given that these dietary factors can inhibit promotion by red meat. We thus suggested, after Sesink et al.(Reference Sesink, Termont, Kleibeuker and VanDerMeer9), that diets high in Ca and oxidation-resistant fat could prevent promotion by red meat.
The present study was designed to test the hypothesis that the addition of Ca, antioxidant mix or olive oil to the diet can inhibit the cancer-promoting effect of haem provided by beef meat.
Materials and methods
Animals and diets
Eighty Fischer 344 female rats were purchased at 4 weeks of age from Iffa Credo (St. Germain l'Arbresle, France). Animal care was in accordance with the guidelines of the European Council on animals used in experimental studies. The animal colony and staff got an official agreement #31-121 for in vivo rodent studies by the French government. Rats were housed by pairs in stainless steel wire-bottomed cages. The room was kept at a steady acclimatization to the animal colony and the rats were given control diet before being injected intraperitoneally with the carcinogen 1,2-dimethylhydrazine (Sigma Chemical, St. Quentin, France; 190 mg/kg body weight) in NaCl (9 g/l). Usually, several injections are given to rats. We reasoned that the first shot initiates carcinogenesis, and the following shots promote it, blurring diet-induced promotion. We thus chose a single-shot protocol, following Karkare et al.(Reference Karkare, Clark and Glauert10). Rats were randomly allocated to eight groups of ten 7 d later, and allowed free access to their respective diet for 100 d. We chose to initiate all rats with the carcinogen, since the study was designed to show dietary promotion, and because a 2·5 % Hb diet does not initiate ACF in rats (F Pierre and DE Corpet, unpublished results).
Eight experimental diets shown in Table 1 were based on the diet fed to control rats, a modified AIN-76 powdered diet(11) prepared and formulated in a powdered form by UPAE (INRA, Jouy-en-Josas, France). Dibasic calcium phosphate was included at a low concentration of 2·7 g/kg. Four beef meat diets were formulated to contain 60 % meat (w/w, freeze-dried). Beef meat contained 0·6 μmol/g haem. We chose this meat level, higher than what most people eat, to be able to detect protection against promotion. The effect of Ca and of an antioxidant mix was tested in two groups of rats given a beef meat diet supplemented either with calcium phosphate (31 g/kg) or antioxidant mix (butylated hydroxyanisole and rutin, 0·05 % each). Last, the effect of oil was investigated by replacing safflower oil by extra-virgin olive oil (5 %; Puget, France) in a beef meat diet. Because Ca, antioxidant mix and olive oil might be protective even in the absence of meat, three other groups received beef-free control diet, supplemented with calcium phosphate, antioxidant mix or olive oil. As shown in Table 1, all diets were balanced for protein (50 %), fat (20 %) and Fe (110 mg/kg) by addition of casein, lard and ferric citrate. The diets were made up every 14 d and maintained at − 20°C to reduce lipoperoxidation.
Table 1 Composition of diets (g/kg)

BHA, butylated hydroxyanisole.
* AIN76 mix, but 500 g/kg dibasic caloium phosphate replaced by sucrose in the mineral mix.
† All diets contained 110 mg/kg iron. Iron concentration was measured in freeze-dried beef before preparing the diets. Other nutrients were balanced: 50 % protein, 21 % fat, 20–24 % carbohydrate (based on added components, no analysis was done on whole diets).
Aberrant crypt foci assay
All eighty rats were killed by CO2 asphyxiation in a random order at day 99 or 100. Colons were excised from rats immediately post mortem, flushed with cold Krebs solution (Sigma Chemical), opened longitudinally and fixed flat between two sheets of filter paper in 10 % formalin (Sigma Chemical). Eighty colons picked up in random order were stained for 6 min in a 0·05 % filtered solution of methylene blue(Reference Bird12). The number of ACF per colon, and number of crypts in each ACF, were counted under light microscope at × 40 magnification in duplicate by two readers, blinded for the origin of the colon. Data from the two readers were pooled.
Mucin-depleted foci assay
MDF may predict colon carcinogenesis better than ACF, since Apc mutations are present in MDF with a frequency similar to that of tumours(Reference Femia, Dolara, Giannini, Salvadori, Biggeri and Caderni13). Colons, after being scored for ACF, were stained with a high Fe diamine-Alcian blue procedure to evaluate mucin production(Reference Caderni, Femia, Giannini, Favuzza, Luceri, Salvadori and Dolara14). Briefly, colons were rinsed in distilled water and left overnight in freshly prepared high iron diamine solution (50 ml distilled water with 120 mg N-N′-dimethyl-m-phenylene diamine, 20 mg N-N′-dimethyl-p-phenylene diamine and 1·4 ml 60 % ferric chloride). After rinsing, colons were counterstained in 1 % Alcian blue solution for 30 min. MDF number, and number of crypts per MDF, were scored blindly under light microscope at × 40 magnification by a single reader.
Preparation of faecal water
Faecal pellets were collected under each cage of two rats for 24 h, thus leading to five samples per group. Freeze-dried faeces were used to calculate dry faecal mass and to prepare faecal water by adding 1 ml sterilized water to 0·3 g faeces. Samples were then incubated at 37°C for 1 h, stirring thoroughly every 20 min, followed by centrifugation at 20 000 g for 10 min. The aqueous phase was re-centrifuged at the same speed and duration and the subsequent supernatant (faecal water) collected and conserved at − 20°C until use.
Thiobarbituric acid reactive substances and haem assay
Thiobarbituric acid reactive substances (TBARS) were measured in faecal water according to Ohkawa et al.(Reference Ohkawa, Ohishi and Yagi15), exactly as previously described(Reference Pierre, Freeman, Tache, Van der Meer and Corpet7). Haem contents of freeze-dried faeces and of faecal water were measured by fluorescence according to Van den Berg et al.(Reference Van den Berg, Koole-Lesuis, Edixhoven-Bosdijk and Brouwers16) and Sesink et al.(Reference Sesink, Termont, Kleibeuker and Vandermeer5), respectively, as already describedReference Pierre, Freeman, Tache, Van der Meer and Corpet7.
Cytotoxicity assay of faecal water
Cytotoxicity of faecal water was quantified on a cell line according to Bonneson et al.(Reference Bonneson, Eggleston and Hayes17), and as previously described(Reference Pierre, Freeman, Tache, Van der Meer and Corpet7). Briefly, a cancerous mouse colonic epithelial cell line, CMT93 (ECAC), was seeded in ninety-six-well microtitre plates (1·6 × 104 cells/well in 200 μl medium) and treated for 24 h with faecal water sample diluted at 10 % (v/v) in the culture medium. Cytotoxicity of each faecal water was quantified by the 3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyl tetrazolium bromide test.
Urinary 1,4-dihydroxynonane mercapturic acid assay
Each rat was placed alone in a metabolic cage for 2 d during the fifth week of the experimental diet. The 24 h urine was collected under each cage of one rat, thus leading to ten samples per group. DHN-MA assay was done by competitive enzyme immunoassay as previously described(Reference Gueraud, Peiro and Bernard18), using DHN-MA-linked acetylcholinesterase enzyme. Each urine sample was assayed in duplicate.
Statistical analysis
Results were analysed using Systat 10 software for Windows, and reported as means and standard deviations. Values were considered firstly using one-way ANOVA. If a significant difference was found between groups (P < 0·05) then each experimental group was compared to the control group using the Fisher's least significant difference test (ACF and MDF data), and the Dunnett multiple comparison test (all other data).
Results
Weight and food intake
Final body weight of rats was 198 (sd 3) g, without significant differences between groups at the end of the experimentation. Food intake was the same in all groups of rats (data not shown).
Aberrant crypt foci and mucin-depleted foci data
Beef meat diet doubled the number of MDF per colon compared to control rats, and increased the number of ACF per colon (both P < 0·01; Fig. 1). The number of crypts per lesion also was enhanced by beef meat diet (Table 2).
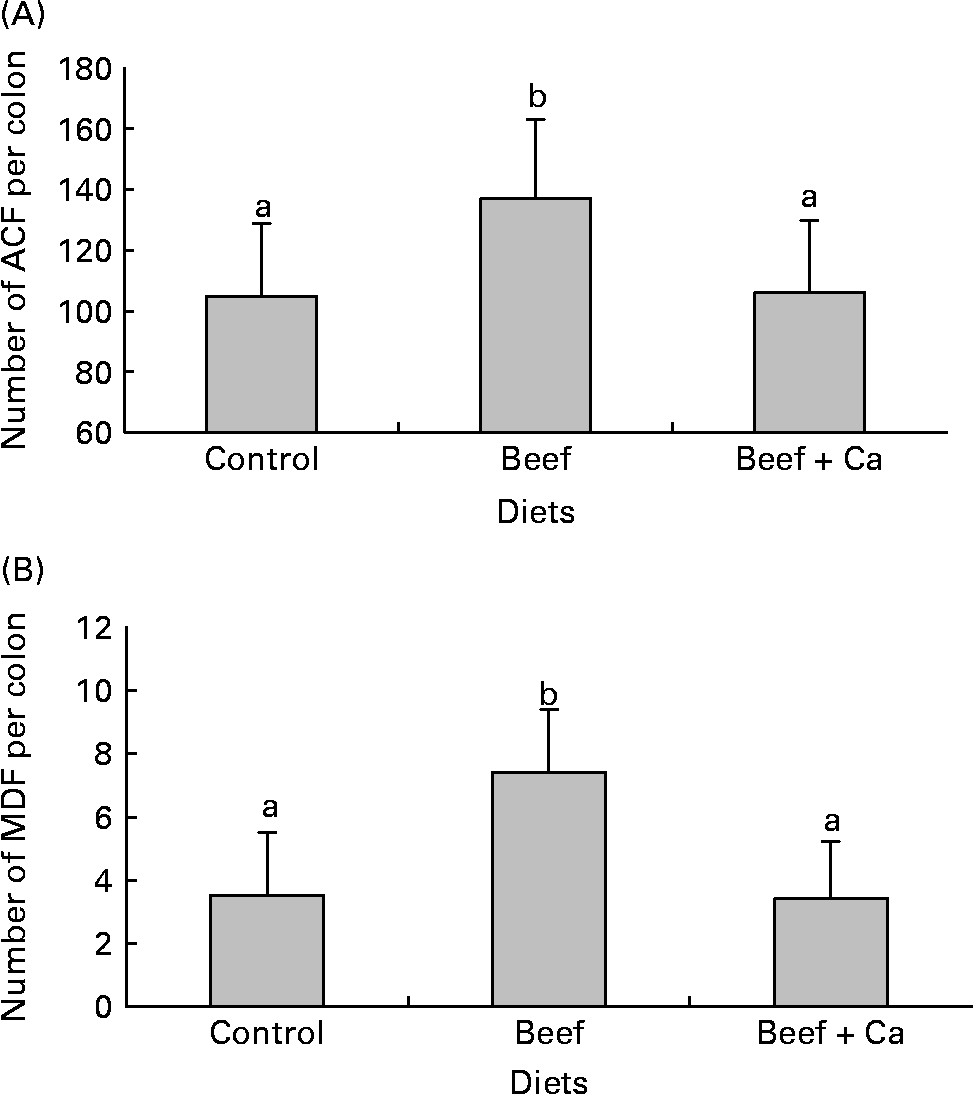
Fig. 1 Effect of beef meat and high-Ca beef meat diets on putative precancerous lesions per rat colon 107 d after the injection of dimethylhydrazine. (A), Number of aberrant crypt foci (ACF). (B), Number of mucin-depleted foci (MDF). Values are means with their standard deviations depicted by vertical bars (n 10). a,b Mean values with unlike letters were significantly different (P < 0·05).
Table 2 Effect of meat-based diets on aberrant crypt foci (ACF) and mucin-depleted foci (MDF) in the colon of rats 107 d after the injection of dimethylhydrazine* (Mean values and standard deviations)

AC, aberrant crypts; MDC, Mucin-depleted crypts.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Ten rats per dietary group. Diets were based on a low-Ca formula, and were balanced for Fe (110 mg/kg). See Table 1 for precise compositions.
Ca fully suppressed beef meat-induced promotion of ACF and MDF (Fig. 1), but did not reduce the mean number of crypts per lesion (Table 2). Antioxidant mix and olive oil significantly reduced meat-induced promotion of MDF number, but did not normalize it to control values (Table 2). In contrast, antioxidant mix and olive oil did not interfere significantly with beef meat-induced promotion of ACF number (Table 2). Thus, only Ca supplementation fully suppressed the beef-induced promotion of ACF and MDF.
Supplementation of no-meat control diet with antioxidant mix or olive oil did not modify ACF or MDF incidence (Table 2), as previously observedReference Pierre, Tache, Petit, Van der Meer and Corpet6. In contrast, surprisingly, high-Ca control diet-fed rats had more MDF and ACF in the colon than low-Ca control diet-fed rats, with more crypts per lesion (P < 0·01; Table 2).
Faecal haem, thiobarbituric acid reactive substances and cytotoxicity
Haem intake and faecal haem values matched the study design: as expected, little haem was detected in faeces of control rats, in contrast with beef meat-fed rats (Table 3). We also measured haem concentrations in faecal water because, according to bile acid studies, the soluble fraction of colonic contents would interact more strongly with the mucosa than the insoluble fraction. As expected, haem concentration in faecal water depended directly on haem level in beef-based diets (Table 3). However, rats fed the beef meat high-Ca diet had little haem in faecal water, as previously observed(Reference Pierre, Tache, Petit, Van der Meer and Corpet6).
Table 3 Effect of meat-based diets on faecal values in rats, notably haem, lipoperoxides and cytotoxicity of faecal water* (Mean values and standard deviations)

DHN-MA, 1,4-dihydroxynonane mercapturic acid; MDA, malondialdehyde; TBARS, thiobarbituric acid reactive substances.
a–d Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Faecal values, five cages per group; urine values, ten rats per group. Diets were based on a low-Ca formula, and were balanced for Fe (110 mg/kg). See Table 1 for precise compositions.
Haem can induce the formation of peroxyl radicals in fats, which may be cytotoxic and cleave DNA in vivo. Lipid peroxidation was thus measured in faecal water by the TBARS assay. Lipid peroxidation correlated with haem concentration in faecal water (Table 3); r 0·95, P = 0·0002). Ca almost normalized beef-induced lipid peroxidation to control level (66 and 33 μm-malondialdehyde equivalent, respectively), but antioxidant mix or olive oil did not reduce peroxide values (130 and 148 μm-malondialdehyde equivalent, similar to 141 μm in beef meat-fed rats). Furthermore, as already seen, faecal water of beef meat-fed rats was cytotoxic to CMT93 cells. Ca fully inhibited beef meat-induced cytotoxicity of faecal water, but antioxidant mix and olive oil did not.
Urinary 1,4-dihydroxynonane mercapturic acid excretion
Table 3 shows that beef meat-based diet increased urinary DHN-MA excretion 15-fold compared with control diet (P < 0·001). Ca and olive oil did not reduce DHN-MA excretion in beef meat-fed rats, while antioxidant mix gave a slight decrease, though statistically significant.
Discussion
The present data confirm that a red meat diet promotes colon carcinogenesis in rats. The results also establish that red meat promotion can be suppressed by dietary Ca. In addition, faecal water cytotoxicity and lipoperoxide level were linked with haem-induced promotion.
Red meat promoted ACF and MDF in the colon of carcinogen-induced rats, and meat promotion was associated with faecal water haem, cytotoxicity and TBARS, in line with our previous study(Reference Pierre, Freeman, Tache, Van der Meer and Corpet7). The mechanism of haem promotion is not known, and may be linked to the stimulation of the endogenous production of N-nitroso compounds(Reference Bingham, Pignatelli, Pollock, Ellul, Malaveille, Gross, Runswick, Cummings and Oneill19, Reference Lunn, Kuhnle, Mai, Frankenfeld, Shuker, Glen, Goodman, Pollock and Bingham20). Alternatively, we suggest promotion is linked to peroxidation and cytotoxicity. We have explored the effect of faecal water from beef-fed rats on normal (Apc +/+) and premalignant colonic cells (Apc Min/+). Results show that Apc mutated cells survive haem-induced faecal lipoperoxides, notably 4-hydroxynonenal (HNE) that is toxic to normal cells. Selection of mutated cells by cytotoxic lipoperoxides may explain haem promotion of colon carcinogenesis(Reference Pierre, Tache, Gueraud, Rerole, Jourdan and Petit21). Beef diet reduces caspase-3 activity in the rat colonic mucosa, thus decreasing apoptosis induction(Reference Yang, Mutanen, Cheng and Duan22). In addition, loss of adenomatous Polyposis coli protein induces histone deacetylase 2: it is overexpressed in polyps of adenomatous Polyposis coli protein-deficient mice, and it prevents apoptosis of colonic cells(Reference Huang and Guo23, Reference Zhu, Martin, Mengwasser, Schlag, Janssen and Gottlicher24). Furthermore, HNE alters histone acetylation(Reference Rahman, Marwick and Kirkham25). Therefore, the modification of histone acetylation by meat-induced HNE may reduce apoptosis induction and explain the selection of Apc-mutated cells. This speculation on the meat-promotion mechanism is supported by the present study: ACF and MDF promotion in beef meat-fed rats was associated with high faecal lipoperoxides and cytotoxicity, and, as discussed later, dietary changes that normalized faecal lipoperoxides and cytotoxicity also suppressed beef meat- promotion.
Ca added on top of the beef meat diet normalized the number of ACF and MDF at the same low level as the control group, a protection already observed against haemin-induced promotion(Reference Pierre, Tache, Petit, Van der Meer and Corpet6). In faeces from rats given high-Ca beef meat diet, haem concentration was high (similar to beef meat-fed rats), but faecal water haem level was low (similar to no-meat control rats) (Table 3). Calcium phosphate precipitates haem in the gut, and little haem remains available to induce lipoperoxidation, as previously showed with haemin(Reference Pierre, Tache, Petit, Van der Meer and Corpet6, Reference Sesink, Termont, Kleibeuker and VanDerMeer9). Faecal water thus contained little lipid peroxide and showed little cytotoxic activity (Table 3). The trapping of haem by Ca abolished carcinogenesis promotion. This confirms that haem is the cause of red meat promotion. The results also suggest that Ca can reduce colorectal cancer risk in meat-eaters. It might explain discrepancies between clinical trials with Ca supplements, since results were not analysed based on meat consumption by volunteers. This could be looked for in a prospective cohort study, to find out whether the protective effect of Ca against red meat promotion can also be seen in man. Similarly, an epidemiological study shows that chlorophyll from vegetables attenuates the elevated risk of colon cancer associated with red meat intake(Reference Balder, Vogel, Jansen, Weijenberg, van den Brandt, Westenbrink, van der Meer and Goldbohm26).
No protection against beef meat promotion was afforded by the antioxidant mix or by olive oil (Table 2). These supplements did not normalize faecal values of haem lipoperoxides and cytotoxicity either (Table 3). The present results conflict with a previous study in which the antioxidant mix or olive oil had been effective against haemin-induced promotion(Reference Pierre, Tache, Petit, Van der Meer and Corpet6). This conflict suggests that dietary haemin is not a suitable model for red meat to test preventive strategies in rodents. We speculate that antioxidant molecules added to the diet can directly prevent the effect of haemin powder on dietary oil(Reference Pierre, Tache, Petit, Van der Meer and Corpet6). Here, in contrast, protein-bound haem in red meat might have been protected from the antioxidant effect. Furthermore, since faecal water lipoperoxides and cytotoxicity are associated with haem-induced carcinogenesis, here and in previous studies(Reference Pierre, Tache, Petit, Van der Meer and Corpet6, Reference Pierre, Freeman, Tache, Van der Meer and Corpet7), we suggest they can be used as short-term biomarkers to screen preventive strategies against red meat-induced promotion of colon cancer.
An unexpected result of the present study was that rats eating a high-Ca control diet had more ACF and more MDF than low-Ca control diet-fed control rats. This promotion by calcium phosphate in a high-fat context was not seen in a low-fat context, in our previous haemin study(Reference Pierre, Tache, Petit, Van der Meer and Corpet6). It is generally agreed that Ca reduces the risk of colorectal cancer(Reference Newmark and Lipkin27), and a meta-analysis study shows that Ca modestly decreases tumour incidence in rats(Reference Corpet and Pierre28). However, in fourteen studies out of thirty-two, a non-significant tumour incidence increase was seen in Ca-fed rats. The meta-analysis also shows that some Ca salts are more protective than others, and that calcium phosphate affords no protection(Reference Corpet and Pierre28). Calcium phosphate (which we used here) sometimes promotes ACF or tumours(Reference Weisburger, Braley, Reinhardt, Aliaga, Rivenson, Hard, Zhang, Takahashi, Esumi and Sugimura29, Reference Bull, Bird, Bruce, Nigro and Medline30). For instance, Bull et al.(Reference Bull, Bird, Bruce, Nigro and Medline30) showed that calcium phosphate promotes colon tumourigenesis in two genetic contexts (Sprague-Dawley and Fischer 344 rats). In both types of rat the promotion was more important in 30 % fat diet-fed rats than in 3 % fat diet-fed rats(Reference Bull, Bird, Bruce, Nigro and Medline30). Phosphate, not Ca, might be the promoting nutrient, an issue discussed by Bruce et al.(Reference Bruce, Giacca and Medline31). To conclude, promotion by calcium phosphate in a high-fat context was unexpected, but has already been reported.
Another surprise here is that all beef meat diets increased urinary DHN-MA excretion more than 10-fold. We have recently proposed urinary DHN-MA as a non-invasive biomarker of haem Fe-induced promotion of carcinogenesis. Indeed, DHN-MA excretion increases dramatically in rats given high-haem diets, and the excretion parallels ACF and MDF numbers in azoxymethane-initiated rats(Reference Pierre, Peiro, Tache, Cross, Bingham, Gasc, Gottardi, Corpet and Gueraud8). However, in the present study, the addition of Ca to the meat diet did not reduce DHN-MA excretion while it normalized ACF and MDF promotion. DHN-MA is the major urinary metabolite of HNE, a lipid peroxidation product. DHN-MA excretion reflects endogenous HNE formation in the body, together with formation in the diet(Reference Pierre, Peiro, Tache, Cross, Bingham, Gasc, Gottardi, Corpet and Gueraud8), the latter being probably quantitatively the most important(Reference Gasc, Tache, Rathahao, Bertrand-Michel, Roques and Gueraud32), while preneoplastic lesions such as ACF and MDF may be due to lipid peroxidation occurring locally in the colon lumen. One can hypothesize that Ca blocks haem in the intestine(Reference Sesink, Termont, Kleibeuker and VanDerMeer9) but not in the diet, because of its powdered form, so the overall DHN-MA excretion is not significantly reduced by the addition of Ca although HNE formation is reduced in the colon lumen. Indeed, in a previous work, we have shown that HNE was found three times less in the faeces of rats fed beef+calcium diet as compared to beef diet(Reference Pierre, Tache, Gueraud, Rerole, Jourdan and Petit21). It thus seems that DHN-MA is a biomarker of exposure to a promoting dose of haem in the diet, but DHN-MA is not a risk factor. However, measurement of HNE in faeces, which can reflect HNE in colon lumen, could be an indicator of the colon cancer risk associated with diets.
In conclusion, the present study shows for the first time Ca prevention of red meat promotion of colon carcinogenesis. The present study supports the concept that toxicity associated with the excess of a useful nutrient like haeminic Fe may be prevented by another nutrient like Ca. Yoghurt or cheese can be eaten after red meat, provided portions are small enough to avoid fat overload. We found hardly any examples of a similar antidote effect, except maybe the lactulose prevention of protein-induced hepatic encephalopathy(Reference Blei and Cordoba33). This Ca preventive effect should be looked for in a cohort study by crossing haem and Ca intake with adenoma or cancer risk. Furthermore, different haem forms do not have the same promoting potency and cannot be antagonized by identical agents, since the haemin effect is not identical to the red meat effect. Lastly, faecal water lipoperoxidation and cytotoxicity may be responsible, at least in part, for haem-induced promotion of colon carcinogenesis.
Acknowledgements
This study was supported in part by the INRA, the DGER and the French region Midi-Pyrénées. We thank Xavier Blanc (UPAE) for the preparation of experimental diets, Marie-Claude Nicot for the assay of Ca and Fe in meats, and Raymond Gazel and Florence Blas Y Estrada for the care of the animals.






