Introduction
Surgical site infection (SSI) is one of the most common forms of healthcare-associated infections, accounting for 16–22% of all such infections [1, Reference Magill2]. Approximately 157 000 SSIs per year are estimated to occur in the USA in about 1% of all patients who have undergone surgery [Reference Magill2, 3].
In Japan, approximately 60 000 thoracic cardiovascular operations are performed per year, and about 10 000 of those are on the thoracic aorta for the treatment of aortic aneurysm and dissection [4]. Although endovascular surgery is growing rapidly, a substantial proportion of operations on the aorta are still performed by open surgery [5].
SSI following cardiovascular surgery is relatively common, possibly due to its highly invasive nature [Reference Inui and Bandyk6], and the potential to develop into mediastinitis which can be fatal. It is also costly. A cardiac SSI costs approximately £17 000 in the UK [Reference Jenks7], whereas in the USA, a mediastinitis costs about $63 000 [Reference Speir8]. Therefore, prevention, early detection and treatment of SSI following cardiovascular surgery are of paramount importance, and better knowledge of specific SSI risk factors associated with such surgery is needed.
There is a wealth of literature on the epidemiology and risk factors of SSI following open heart surgery and coronary artery bypass surgery [Reference Morikane9]; however, few have addressed those following surgery on the thoracic aorta. A possible reason for this may be that the nationwide SSI surveillance system in the USA, the National Healthcare Safety Network (NHSN), does not specifically include surgery on thoracic aorta in its targeted operative procedure categories. Using nationwide surveillance data is the best way to describe the epidemiology and investigate risk factors of SSI following specific procedures [Reference Mu10].
In Japan, surgery on the thoracic aorta is targeted in the Japan Nosocomial Infections Surveillance (JANIS) system. By using this database, this study sets out to describe the incidence of SSI and to identify pertinent associated risk factors in patients who have undergone thoracic aorta surgery.
Methods
Data on SSI in patients who underwent surgery on the thoracic aorta between 2012 and 2014 were extracted from the JANIS database and 3538 operations were identified. Approval for data extraction was granted by the Japanese government's Ministry of Health, Labour and Welfare, and the project was approved by the institutional review board of Yamagata University School of Medicine.
The methodology of the JANIS has been described in detail elsewhere [Reference Morikane9, Reference Morikane11]. As of January 2018, it collects SSI surveillance data from more than 700 institutions and is the largest SSI database in Japan. Variables collected include age, American Society of Anesthesiologists (ASA) score, duration of operation, emergency, gender, use of implant and wound class. JANIS definition of SSI comprises superficial, deep incisional and organ/space SSIs, and is consistent with what appears in NHSN. All SSIs detected during hospitalisation, readmission and post-discharge outpatient visit were included. In cases with SSI, causative pathogens, date of diagnosis and specific site of infection are also recorded.
Prior to performing statistical analysis, non-dichotomous variables were examined and modified to fit the statistical model. Continuous variables such as the duration of operations and age were converted to four quartiles, and the incidence of SSI in each quartile was compared. The variable was then either dichotomised or trichotomised if there was a definite cut-off point; in the absence of this, the variable was regarded as continuous. Categorical variables such as ASA score and wound class were examined in the same way.
Potential risk factors associated with SSI were assessed using univariate modelling analysis. Categorical variables were compared by χ 2 test with values of P < 0.2 considered to be potential independent variables, and entered into the logistic regression model. The multivariate model was developed using forward stepwise logistic regression, and variables of two-tailed P < 0.05 were retained in the final model. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Incidence of SSI over time
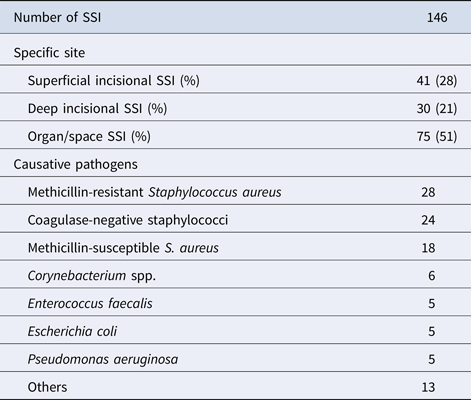
The cumulative incidence of SSI in surgeries on thoracic aorta during the study period was 4.1% (146/3538); the lowest incidence was recorded in 2014, but this was not significantly different from the preceding 2 years (Table 1). Organ/space infections accounted for approximately half of all SSI (Table 2). The causative pathogens were identified in 104 cases and methicillin-resistant Staphylococcus aureus was the most common, followed by coagulase-negative staphylococci.
Table 1. Incidence of SSI following surgery on thoracic aorta

CI, confidence interval.
Table 2. Characteristics of SSI following surgery on thoracic aorta
Modification of non-dichotomous variables
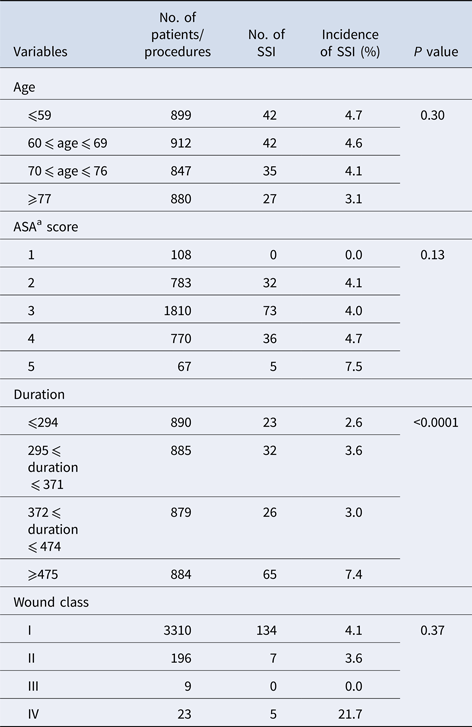
Non-dichotomous variables such as age, ASA score, duration and wound class were evaluated (Table 3). There was an inverse relationship between age and incidence of SSI, and a substantial difference in the incidence of SSI between adjacent age groups, except ‘age ⩽ 59’ and ‘60 ⩽ age ⩽ 69’; as a consequence, the age variable was trichotomised as ‘age ⩽ 69, 70 ⩽ age ⩽ 76, age ⩾ 77’.
Table 3. Evaluation of non-dichotomous variables
a ASA, American Society of Anesthesiologists.
A marked difference in the incidence of SSI was recorded between each ASA score group, except for ASA2 and ASA3. As the proportion of operations with ASA1 and ASA5 were very small (3.1% and 1.9%, respectively), this variable was dichotomised as ‘ASA ⩽ 3, ASA ⩾ 4’.
The incidence of SSI increased with the duration of operation but no definite cut-off point at which to rationalise dichotomisation or trichotomisation with duration of operation was evident. The latter value was divided by 10 and hence regarded as a continuous variable. The majority of operations were classified as wound class I or II, and the incidence of SSI in both classes was similar (4.1% and 3.6%, respectively); as this was not statistically different, this variable was not evaluated further.
Risk factors for SSI
The risk factors associated with SSI by univariate modelling analysis are shown in Table 4. All collected variables except ‘implant’ fulfilled the criteria for inclusion into the multivariate logistic regression models (c-index = 0.65) where duration of operation (increase in 10 min) (adjusted OR = 1.03; 95% CI 1.02–1.03), emergency operation (adjusted OR = 1.58; 95% CI 1.13–2.18) and male gender (adjusted OR = 1.50; 95% CI 1.03–2.18) remained significant.
Table 4. Univariate analysis for risk factors associated with SSI

a ASA, American Society of Anesthesiologists.
Discussion
Among SSI associated with cardiovascular surgery, those following surgery on the heart and abdominal aorta have been relatively well investigated [Reference Inui and Bandyk6, Reference Morikane9], but such studies are lacking following surgery on thoracic aorta. Several SSI surveillance systems have been implemented in various developed countries, including the USA, UK, the European countries and Japan [12–14]. However, with the exception of Japan, none has specifically included surgery on thoracic aorta, leading to a poor understanding of the epidemiology and predisposing risk factors attendant with the latter procedure. A significant strength of the current study is that review of the JANIS database on thoracic aorta SSI enabled the identification of 3538 operations from as many as 76 hospitals over a 2-year period. This is reflected by the substantially higher rate of SSI following open thoracic aortic repair found in this study (4.1%) through active, prospective surveillance data compared with a hospital administration database in the USA with a reported rate of 1.3% for the same type of surgery [Reference Vogel15]. We have previously reported incidences of SSI following open heart surgery and coronary artery bypass surgery in Japanese patients of 2.6% and 4.1%, respectively [Reference Morikane9]. Given the several common features attendant with these procedures and surgery on thoracic aorta, such as median sternotomy, cardiopulmonary bypass and longer duration of operation, the similarity of SSI rates is unsurprising.
Similar to our earlier study on open heart and coronary artery bypass surgery [Reference Morikane9], the skin bacteria S. aureus and coagulase-negative staphylococci accounted for the majority (67%) of the 104 cases of SSI with identified causative pathogens.
The incidence of SSI is an important quality indicator of the hospital settings. However, when it is used to compare hospital performance, unmodifiable patient characteristics need to be taken into account. The National Nosocomial Infections Surveillance (NNIS), the predecessor of NHSN, provides a simple and convenient risk stratification system (NNIS Risk Index) that utilises three risk factors (wound class, ASA score and duration of operation), which are also included in the JANIS [Reference Morikane11]. The value of the Risk Index has been questioned when applied to cardiovascular surgeries [Reference Roy16, Reference Gaynes17], which are generally considered as clean wound (class I) and patients with cardiovascular disease are often classified into ASA score 3 or higher.
In the current study, 94% of the operations had class I wounds and therefore this was not a significant risk factor. This was consistent with our earlier finding that 99% of coronary artery bypass graft (CABG) surgeries had class I wounds, but not with open heart surgery (CARD), possibly due to a substantial number of patients in CARD operated on for infectious endocarditis [Reference Morikane9].
The ASA score did not remain significant in the final model, a finding also in contrast to the earlier study where both CARD and CABG, ASA score (4 vs. 3 vs. 2 vs. 1), remained significant in the final model, with adjusted odds ratios of 1.58 and 1.75, respectively [Reference Morikane9].
The influence of gender as a risk factor has been widely investigated in different types of surgeries [Reference Mu10, Reference Morikane11], with varying results. Here, male gender emerged as a risk factor of SSI in contrast to earlier findings for CABG and CARD where crude SSI rates were highest in females (4.97%) than in males (3.84%) following CABG [Reference Morikane9].
Patients who undergo emergency surgery have no time for the necessary preparation, and are at a risk of various post-operative complications. As previously found for emergency cardiac surgery overall [Reference Morikane9], the variable ‘emergency’ remained significant in the final model with high odds ratios and, here, such patients accounted for up to 41% of the study group. One can therefore speculate that if stringent infection control practice is applied to such a high-risk population, this could result in a substantial decrease in the number of SSI recorded.
This study has a number of limitations. First, the JANIS is a national surveillance programme with voluntary reporting, and not all hospitals in Japan reported their data and so our findings may not therefore be applicable to all Japanese hospitals. Second, the c-index of 0.65, which is a measure of goodness of fit for binary outcomes in a logistic regression model, was not high. Lastly, as many similar studies have noted, variables such as diabetes and body mass index might also be significant risk factors for SSI and should be recorded in the JANIS system.
In conclusion, we have described predisposing risk factors for SSI in surgery on thoracic aorta, using the Japanese SSI surveillance database. This showed marked differences between two types of cardiac surgery, and surgery on thoracic aorta. Also, our current risk stratification based on the NNIS Risk Index in surgery on thoracic aorta was suboptimal, and in order to better adjust the risk level and allow meaningful comparisons of SSI incidence between hospitals, some significant variables already collected in our surveillance system, and possibly additional variables, should be incorporated into the risk stratification. Lastly, our findings may help hospitals to identify high-risk patients who require more intense infection control measures for the prevention of SSI.
Acknowledgements
The authors thank all hospitals participating in the JANIS system.
Financial support
This work was supported by grants H24-Shinkou-Ippan- 010 from the Ministry of Health, Labour and Welfare, Japan.
Conflict of interest
None.







