Conjugated linoleic acid (CLA) is a class of isomers of linoleic acid that occur naturally in dairy products and meat from ruminant animals due to bacterial biohydrogenation of ingested PUFA in the rumen( Reference Bhattacharya, Banu and Rahman 1 ). CLA has been shown to be produced in vitro and in vivo by different species of bacteria( Reference Barrett, Ross and Fitzgerald 2 – Reference Wall, Ross and Shanahan 5 ). Although cis-9, trans-11-CLA (rumenic acid) is the major natural form/isomer of CLA in foods, accounting for more than 90 % of dietary CLA, mixtures containing equal amounts of cis-9, trans-11-CLA and trans-10, cis-12-CLA (t10c12-CLA) isomers are produced industrially and sold as supplements. Both the isomers exhibit significant biological activities, which may exert synergistic or antagonistic effects( Reference Bhattacharya, Banu and Rahman 1 ). CLA has been shown to inhibit carcinogenesis( Reference Kelley, Bartolini and Newman 6 ), prevent atherosclerosis in different animal models( Reference Kritchevsky, Tepper and Wright 7 – Reference Wilson, Nicolosi and Chrysam 9 ), modulate the immune system( Reference Yang and Cook 10 , Reference Yu, Correll and Vanden Heuvel 11 ), and affect body composition, by reducing body fat and increasing lean body mass( Reference Clément, Poirier and Niot 12 , Reference Liu, Joseph and Wakefield 13 ). Also, t10c12-CLA has been shown to be the isomer responsible for the anti-obesity effect attributed to CLA, and over the past years, its impact on body fat modulation has been largely studied in different animal models and in human subjects( Reference Clément, Poirier and Niot 12 – Reference Salas-Salvado, Marquez-Sandoval and Bullo 14 ). There is evidence to suggest that fat mass reduction is the result of multiple interactions of t10c12-CLA with numerous metabolic signalling pathways, leading to decreased energy intake and increased energy expenditure, inhibition of adipogenesis and lipogenesis, modulation of adipokines and cytokines, and increased fatty acid (FA) β-oxidation in skeletal muscle( Reference Park and Pariza 15 ).
The response to CLA supplementation appears to be highly species-specific, with mice generally being more sensitive than other animal models and human subjects. These differences are attributed to the dose levels used (human trials use lower doses), age (animal trials usually use young subjects), rate of body fat turnover (small animals have faster metabolism) and dietary regimens (ad libitum in animal models v. energy restriction in human trials)( Reference Park and Pariza 15 , Reference Wang and Jones 16 ). In most studies using mice, body fat reduction induced by t10c12-CLA supplementation is accompanied by adverse effects such as hepatic steatosis and hyperinsulinaemia( Reference Clément, Poirier and Niot 12 , Reference Liu, Joseph and Wakefield 13 ). These are features frequently associated with the metabolic syndrome, and commonly observed in obese and diabetic individuals( Reference Le Roy, Llopis and Lepage 17 , Reference Tamura and Shimomura 18 ). Hepatic steatosis is characterised by an increase in liver mass with the accumulation of intracellular lipids, primarily in the form of TAG. Increased uptake of circulating FA, increased hepatic de novo lipogenesis, reduced rate of FA oxidation and reduced FA secretion are the multiple mechanisms leading to increased accumulation of lipids in the liver( Reference Vyas, Kadegowda and Erdman 19 ).
Moreover, recent studies have identified the gut microbiota as an environmental factor with an important role in host fat metabolism, and in the development of hepatic steatosis( Reference Le Roy, Llopis and Lepage 17 ). In a continuous bidirectional communication between the gut and the liver, hepatic products can directly influence the microbiota composition, whereas bacterial metabolites may have both direct and indirect effects on liver function and physiology( Reference Quigley and Monsour 20 , Reference Bajaj, Hylemon and Younossi 21 ). Thus, in the present study, we investigated the impact of dietary t10c12-CLA supplementation on intestinal microbiota composition and SCFA production.
Materials and methods
Animals and diets
Experiments involving animals were carried out in accordance with the protocols approved by the Ethics Committee, University College Cork, under a license issued by the Department of Health and Children (Cruelty to Animal Act 1876, Directive for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (89/609/EEC)). Male C57BL/6 mice (7–8 weeks of age) were obtained from Charles River, and housed under barrier-maintained conditions within the Biological Services Unit, University College Cork. After 1 week of acclimatisation, the animals were divided into two groups (n 8 per group). Both groups were fed ad libitum with a Teklad Global rodent standard diet (#2018S; Harlan Laboratories) and allowed free access to water. The FA present in the diet included palmitic acid, stearic acid, oleic acid, linoleic acid and linolenic acid. The intervention group received the standard diet supplemented with 0·5 % (w/w) t10c12-CLA (Matreya LLC; Table 1). Body weight was assessed weekly. After 8 weeks of dietary intervention, fasted animals were killed by decapitation, and blood samples were collected, allowed to clot for 2 h at 4°C and centrifuged at 2000 g for 20 min. Liver, brain, fat pads (epididymal, perirenal and mesenteric), heart, kidneys and intestines were removed, blotted dry on a filter paper, weighed and flash-frozen in liquid N2. Caecal content was collected for the analysis of microbiota composition and SCFA concentrations. All samples were stored at − 80°C before analyses.
Table 1 Diet composition (g/100 g)
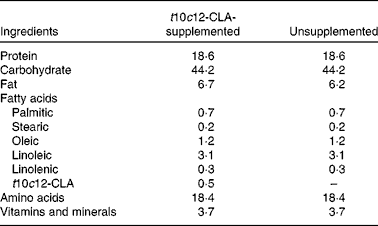
t10c12-CLA, trans-10, cis-12-conjugated linoleic acid.
Lipid extraction and fatty acid analysis
FA profiles of the liver, brain, epididymal adipose tissue, heart and kidneys were analysed. Lipids were extracted with chloroform–methanol (Fisher Scientific), according to the method of Folch et al. ( Reference Folch, Lees and Sloane-Stanley 22 ). After extraction, samples were methylated by using 0·5 m-NaOH (Sigma-Aldrich) in methanol for 10 min at 90°C, followed by 10 min incubation at 90°C with 14 % (w/v) boron trifluoride in methanol (Sigma-Aldrich)( Reference Park and Goins 23 ). FA methyl esters were recovered with hexane (Fisher Scientific). Before GLC analysis, samples were dried over anhydrous sodium sulphate (Sigma-Aldrich) for 1 h, and stored at − 20°C. FA methyl esters were separated using a Varian 3800 GC flame-ionisation system, fitted with a Chrompack CP Sil 88 column (Chrompack; 100 m × 0·25 mm internal diameter, 0·20 μm film thickness), and He gas as a carrier. The column oven was programmed initially at 80°C for 8 min, and then increased by 8·5°C/min to a final column temperature of 200°C. The injection volume used was 0·6 μl, with automatic sample injection on an SPI 1093 splitless on-column temperature-programmable injector. Peaks were integrated using the Varian Star Chromatography Workstation version 6.0 software, and identified by comparison of retention times with pure FA methyl ester standards (Nu-Chek Prep, Inc.). The percentage of individual FA was calculated according to the peak areas, relative to the total area (total FA were set at 100 %). All FA results are expressed as g/100 g FA methyl ester.
SCFA concentration analysis
Caecal content was vortex-mixed with Milli-Q water, incubated at room temperature for 10 min, and centrifuged at 10 000 g to pellet bacteria and other solids. The supernatant was filtered and transferred to a clear GC vial. 2-Ethylbutyric acid (Sigma) was used as the internal standard. The concentration of SCFA was analysed using a Varian 3500 GC flame-ionisation system, fitted with a Nukol-FFAP column (30 m × 0·32 mm × 0·25 μm; Sigma). The initial oven temperature was set at 100°C for 0·5 min, raised to 180°C at 8°C/min and held for 1 min, then increased to 200°C at 20°C/min, and finally held at 200°C for 5 min. The temperatures of the injector and the detector were set at 240 and 250°C, respectively. He gas was used as a carrier at a flow rate of 1·3 ml/min. A standard curve was built with different concentrations of a standard mix containing acetate, propionate, isobutyrate and n-butyrate (Sigma). Peaks were integrated by using the Varian Star Chromatography Workstation version 6.0 software. All SCFA data are expressed as μmol/g.
Measurement of hepatic TAG concentrations
Hepatic lipids were extracted according to the method of Folch et al. ( Reference Folch, Lees and Sloane-Stanley 22 ). After extraction, samples were dried under a stream of N2, and resuspended in a 5 % (v/v) solution of Triton X-100 in distilled water. TAG concentrations were measured using a commercial kit (EnzyChrom Triglyceride Assay; BioAssay Systems).
Measurement of serum metabolic markers
Commercial kits were used for the measurement of metabolic markers in serum. Glucose concentration was measured using the QuantiChrom Glucose Assay Kit (BioAssay Systems), TAG concentration using the EnzyChrom Triglyceride Assay Kit (BioAssay Systems), insulin concentration using the Ultra-Sensitive Mouse ELISA Kit (Crystal Chem, Inc.), and leptin concentration using the Mouse Leptin ELISA Kit (Crystal Chem, Inc.).
Amplicon sequencing of the gut microbiota
For high-throughput amplicon sequencing, DNA was extracted from faecal samples using the QIAmp DNA Stool Mini Kit (Qiagen), according to the manufacturer's instructions, with the addition of a bead-beating step (30 s, × 3), and stored at –20oC. The microbiota composition of the samples was established by amplicon sequencing of the 16S rRNA gene V4; universal 16S rRNA primers estimated to bind to 94·6 % of all 16S genes (i.e. the forward primer (F1: 5′-AYTGGGYDTAAAGNG) and a combination of four reverse primers (R1: 5′-TACCRGGGTHTCTAAAGNG, R2: TACCAGAGTATCTAATTC, R3: 5′-CTACDSRGGTMTCTAATC and R4: 5′-TACNVGGGTATCTAATC); RDP'S Pyrosequencing Pipeline: http://pyro.cme.msu.edu/pyro/help.jsp) were employed for PCR amplification. Molecular identifier tags were attached between the 454 adaptor sequence and the target-specific primer sequence, allowing for the identification of individual sequences from the pooled amplicons. The Ampure Purification System (Beckman Coulter) was used to clean the amplicons, before being sequenced on a 454 Genome Sequencer FLX platform (Roche Diagnostics Limited), in line with 454 protocols at the Teagasc high-throughput sequencing centre.
Sequence analysis
Raw sequences were quality-trimmed and filtered, using the QIIME Suite of programs( Reference Caporaso, Kuczynski and Stombaugh 24 ). The resulting trimmed FASTA sequences were assigned to taxa through BLAST analysis, against the SILVA database (version 106) for 16S reads. BLAST outputs were parsed using MEGAN( Reference Huson, Auch and Qi 25 ) with a bit-score of 86; taxonomy was assigned to phylum, family and genus levels. Sequence reads were clustered, aligned and chimera-checked with Qiime, and phylogenetic analysis was implemented with FastTreeMP( Reference Price, Dehal and Arkin 26 ). The relevant α- and β-diversities were calculated again using Qiime, and subsequently principal coordinate analysis was performed on distance matrices. Principal coordinate analysis plots were visualised with the KiNG viewer( Reference Chen, Davis and Richardson 27 ).
Statistical analysis
To assess whether differences between treatment groups were significant, statistical analysis was performed by Student's t test (Graph-Pad Software). Treatment effects with P< 0·05 were considered significant. Kruskal–Wallis and Mann–Whitney tests, as implemented in the SPSS statistical software package, were used to find significant differences in microbial taxa and α-diversity. Significance was considered as P< 0·05. Data are presented as means with their standard errors.
Results
Effects of dietary trans-10, cis-12-conjugated linoleic acid on fat storage, liver weight, host metabolism and body weight
After 8 weeks of dietary supplementation with t10c12-CLA, a 2-fold decrease in visceral body fat (sum of epididymal, mesenteric and perirenal fat pads; P< 0·001) and a significant increase in liver mass (P< 0·01; Table 2) were found in the intervention group, compared with the unsupplemented control group. Body-weight gain and final body weight did not differ between the groups (Table 2). In addition, no difference in food intake was observed between the groups (Table 2). The greater liver weight observed in mice supplemented with t10c12-CLA was accompanied by a 7-fold increase in hepatic TAG concentrations (P< 0·001; Table 2). In contrast, serum TAG concentrations were lower in mice fed t10c12-CLA, compared with the unsupplemented control mice (P< 0·01). Also, t10c12-CLA supplementation was associated with higher serum glucose (P< 0·01) and serum insulin (P< 0·05) concentrations. Serum leptin concentration, which is proportional to the amount of fat in the body, was lower in the t10c12-CLA-supplemented group than in the unsupplemented group (P< 0·05; Table 2).
Table 2 Effects of trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) intake on body mass, liver mass and visceral fat mass, and on metabolic markers (Mean values with their standard errors; n 8)
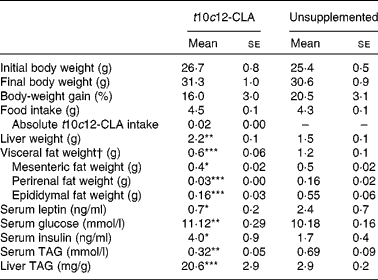
Mean value was significantly different from that of the unsupplemented control group: * P< 0·05, ** P< 0·01, *** P< 0·001; Student's t test.
† Visceral fat = sum of epididymal, perirenal and mesenteric fat pads.
Effects of dietary trans-10, cis-12-conjugated linoleic acid intake on host fatty acid composition
FA profiles of the brain, epididymal adipose tissue, liver, heart and kidneys were analysed after 8 weeks of dietary t10c12-CLA supplementation. The analysis revealed that t10c12-CLA was incorporated into the epididymal adipose tissue, liver, heart and kidney tissues, but was not detected in the brain (Table 3). We did not detect t10c12-CLA in any tissues of the unsupplemented control group. The FA composition of all tissues tested was significantly altered in the t10c12-CLA-supplemented group compared with the unsupplemented control group, the greatest impact being observed on the brain, epididymal adipose tissue and liver (Table 3; Fig. 1(a)–(c)). The t10c12-CLA-supplemented group had significantly higher amounts of total SFA in the brain and epididymal adipose tissue than the unsupplemented control group (P< 0·001; Fig. 1), including a 1·6-fold increase in myristic acid (14 : 0; P< 0·05) and a 1·2-fold increase in palmitic acid (16 : 0; P< 0·001) in the brain, and a 2-fold increase in myristic acid (P< 0·01) and palmitic acid (P< 0·001) in the epididymal adipose tissue (Table 3).
Table 3 Effects of dietary trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) intake on tissue fatty acid composition (g/100 g fatty acid methyl ester (FAME)) in mice (Mean values with their standard errors; n 8)

Mean value was significantly different from that of the unsupplemented control group: * P< 0·05, ** P< 0·01, *** P< 0·001; Student's t test.

Fig. 1 Tissue fatty acid composition in mice fed a diet supplemented with trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) or with no supplementation. The fatty acid composition was altered by t10c12-CLA supplementation, with a greater impact on the (a) brain, (b) epididymal adipose tissue, (c) liver, (d) heart and (e) kidneys. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the unsupplemented control group (* P< 0·05, ** P< 0·01, *** P< 0·001; Student's t test). FAME, fatty acid methyl esters. ![]() , SFA;
, SFA; ![]() , MUFA;
, MUFA; ![]() , PUFA n-3;
, PUFA n-3; ![]() , PUFA n-6;
, PUFA n-6; ![]() , others.
, others.
Moreover, the t10c12-CLA-supplemented group had significantly lower amounts of myristic acid in the heart (57 %; P< 0·05) and lower amounts of stearic acid (18 : 0) in the epididymal adipose tissue (17 %; P< 0·05) and liver (41 %; P< 0·001), whereas palmitic acid was higher in the liver (13 %; P< 0·001). No differences were observed in the levels of SFA in the kidney of the t10c12-CLA-supplemented group compared with the unsupplemented control group. Furthermore, t10c12-CLA supplementation was associated with reduced levels of MUFA in both epididymal adipose tissue and kidneys, while increased levels were found in the liver and brain (Table 3; Fig. 1). Higher amounts of palmitoleic acid (16 : 1-c9) were detected in the brain (11 %; P< 0·05) and lower amounts in the liver (31 %; P< 0·05), heart (74 %; P< 0·05) and kidneys (56 %; P< 0·001) of the t10c12-CLA-supplemented group. CLA supplementation also led to increased concentrations of oleic acid (18 : 1c9) in the liver (50 %; P< 0·001) and brain (8 %; P< 0·01), and decreased concentrations in the epididymal adipose tissue (30 %; P< 0·01) and kidneys (19 %; P< 0·05). n-3 PUFA concentrations were also affected by t10c12-CLA supplementation, with the brain of the intervention group having 24 % less amounts of DHA (22 : 6n-3; P< 0·001), and epididymal adipose tissue having 58 % less amounts of linolenic acid (18 : 3n-3; P< 0·001), 32 % less amounts of docosapentaenoic acid (22 : 5n-3; P< 0·05) and 67 % less amounts of DHA (P< 0·001) than those of the unsupplemented control group. The amount of EPA (20 : 5n-3) was 50 % lower (P< 0·001), DHA 55 % lower (P< 0·001) and docosapentaenoic acid 33 % higher (P< 0·05) in the liver of the t10c12-CLA-supplemented group compared with those of the unsupplemented control group. Moreover, the heart of the t10c12-CLA-supplemented group had 57 % less amounts of linolenic acid (P< 0·05) and 60 % more amounts of docosapentaenoic acid (P< 0·001), whereas the kidneys had 43 % less amounts of linolenic acid (P< 0·01) and 45 % more amounts of docosapentaenoic acid (P< 0·01) compared with the same tissues from the unsupplemented control group.
Furthermore, significant differences in n-6 PUFA composition were observed in the tissues of the intervention group. In the brain of these mice, arachidonic acid (20 : 4n-6) content was decreased by 21 % (P< 0·001), while dihomo-γ-linolenic acid (20 : 3n-6) content was increased by 67 % (P< 0·001).
Linoleic acid (18 : 2n-6), dihomo-γ-linolenic acid and arachidonic acid concentrations in the epididymal adipose tissue of the intervention group were decreased by 32 % (P< 0·001), 50 % (P< 0·001) and 29 % (P< 0·01), respectively. Significantly lower amounts of γ-linolenic acid (18 : 3n-6) (33 %; P< 0·001), dihomo-γ-linolenic acid (22 %; P< 0·001) and arachidonic acid (49 %; P< 0·001) were also detected in the liver of these mice; less amounts of linoleic acid was detected in the heart (14 %; P< 0·05) and kidneys (17 %; P< 0·05), whereas higher amounts of dihomo-γ-linolenic acid was detected in the kidneys (50 %; P< 0·01). Furthermore, the relative proportion of SFA:MUFA, an important aspect of phospholipid composition and membrane fluidity, was altered in all tissues of these mice except the heart, and the ratio of n-6:n-3 PUFA was also changed, with higher (P< 0·05) proportions of n-6 PUFA in the brain, epididymal adipose tissue and liver (Table 3).
Effects of trans-10, cis-12-conjugated linoleic acid intake on SCFA production
Microbial fermentation in the caecum was enhanced by dietary supplementation with t10c12-CLA. Acetate, propionate and isobutyrate levels were significantly higher (P< 0·05) in the t10c12-CLA-supplemented group than in the unsupplemented control group, whereas no difference between the two groups was observed for n-butyrate. Moreover, total SCFA concentration (sum of acetate, propionate, isobutyrate and n-butyrate) was 34 % higher in the caecal content of the t10c12-CLA-supplemented group than that of the unsupplemented control group (P< 0·05; Fig. 2).

Fig. 2 Caecal SCFA concentrations in mice fed a diet supplemented with trans-10, cis-12-conjugated linoleic acid (![]() ) or with no supplementation (
) or with no supplementation (![]() , control group). Values are means (n 8), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the unsupplemented control group (P< 0·05; Student's t test).
, control group). Values are means (n 8), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the unsupplemented control group (P< 0·05; Student's t test).
Effects of dietary trans-10, cis-12-conjugated linoleic acid intake on gut microbiota composition
A total of 416 309 V4 16S amplicon sequence reads were generated, corresponding to a mean of 23 200 reads per mouse post-quality checking. All rarefaction curves were approaching parallel (data not shown), indicating sufficient depth of sequencing. Reads were clustered into operational taxonomical units of 97 % identity, and diversity metrics estimated. Of the five α-diversity metrics used (Shannon, Simpson, chao1, phylogenetic diversity and observed species), no significant differences were observed between the CLA-supplemented and unsupplemented control groups. The β-diversity value was calculated with both weighted and unweighted Unifrac distance matrices. The subsequent principal coordinate analysis revealed a distinct separation of the two groups for both measures (Fig. 3(a) and (b)).
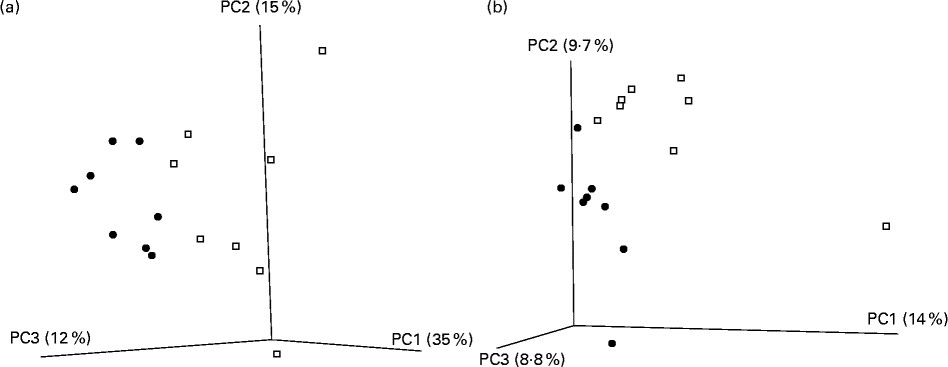
Fig. 3 Principal coordinate (PC) analysis based on (a) weighted Unifrac and (b) unweighted Unifrac distance matrices in mice (n 8 per group) fed a diet supplemented with trans-10, cis-12-conjugated linoleic acid (![]() ) or with no supplementation (
) or with no supplementation (![]() ).
).
The mouse gut microbiota was dominated by members of the Firmicutes and Bacteroidetes phyla. Microbial composition assignment revealed several significant differences between the CLA-supplemented and the unsupplemented control groups (Fig. 4 (a) and (b)). At the phylum level, t10c12-CLA supplementation significantly reduced the proportions of Firmicutes (P= 0·003) and increased the amounts of Bacteroidetes (P= 0·027), when compared with no supplementation. At the family level, the members of Desulfovibrionaceae (P= 0·027), Lachnospiraceae (P= 0·006), Family XIII Incertae Sedis (P= 0·016) and Peptococcaceae (P= 0·009) were all significantly decreased in the t10c12-CLA-supplemented group, compared with the unsupplemented control group, while the members of Porphyromonadaceae (P= 0·002) were significantly increased in the t10c12-CLA-supplemented group. At the genus level, the populations of Desulfovibrio (P= 0·021), Lachnospiraceae Incertae Sedis (P= 0·006) and Ruminococcus Incertae Sedis (P= 0·027) were significantly decreased, and the populations of Odoribacter (P= 0·002) were significantly increased in the t10c12-CLA-supplemented group when compared with the unsupplemented control group.

Fig. 4 Gut microbiota composition in mice fed a diet supplemented with trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) (a) or with no supplementation (b). The microbiota composition was altered by t10c12-CLA intake, as determined by pyrosequencing of 16S rRNA (n 8). Pie charts represent the mean percentage read number for the corresponding colour-coded family (only reads ≥ 1 % are shown). Bacteroidetes: ![]() , S24-7;
, S24-7; ![]() , Bacteroidaceae;
, Bacteroidaceae; ![]() , Rikenellaceae;
, Rikenellaceae; ![]() , Porphyromonadaceae. Firmicutes:
, Porphyromonadaceae. Firmicutes: ![]() , Ruminococcaceae;
, Ruminococcaceae; ![]() , Lactobacillaceae;
, Lactobacillaceae; ![]() , Erysipelotrichaceae;
, Erysipelotrichaceae; ![]() , Peptococcaceae;
, Peptococcaceae; ![]() , Lachnospiraceae;
, Lachnospiraceae; ![]() , others.
, others.
Discussion
The effects of dietary CLA supplementation on body fat have largely been studied in mice and, although the effects of t10c12-CLA seem to be independent of genetic strain( Reference House, Cassady and Eisen 28 ), the C57BL/6 mouse has been shown to be very sensitive, and it constitutes a useful model for studying lipid metabolism dysfunction( Reference Degrace, Moindrot and Mohamed 29 ). Indeed, in the present study, C57BL/6 mice developed severe lipoatrophy with concomitant liver steatosis after 8 weeks of dietary supplementation with 0·5 % (w/w) t10c12-CLA. Moreover, supplementation with t10c12-CLA had a marked effect on the composition of the murine gut microbiota, when compared with no supplementation, despite no significant differences in diversity between groups (data not shown). Perturbations of the gut microbiota composition may play an important role in the development of diseases associated with altered metabolism such as obesity, diabetes and CVD( Reference Tremaroli and Bäckhed 30 ). For example, studies using germ-free animals have shown that the absence of the microbiota is accompanied by increased FA oxidation and decreased lipogenesis, making these animals resistant to diet-induced obesity, steatosis and insulin resistance( Reference Bäckhed, Ding and Wang 31 , Reference Bäckhed, Manchester and Semenkovich 32 ).
Furthermore, some evidence suggests that body-weight gain is associated with higher proportions of Firmicutes and lower proportions of Bacteroidetes in the gut microbiota( Reference Bäckhed, Ding and Wang 31 , Reference Ley, Bäckhed and Turnbaugh 33 , Reference Turnbaugh, Hamady and Yatsunenko 34 ), while body-weight loss has been correlated with increased abundance of Bacteroidetes( Reference Ley, Turnbaugh and Klein 35 ). In the present study, we observed that decreased body fat in mice receiving t10c12-CLA was associated with higher proportions of Bacteroidetes and lower abundance of Firmicutes. Interestingly, Larsen et al. ( Reference Larsen, Vogensen and van den Berg 36 ) demonstrated the same compositional changes in the intestinal microbiota of human subjects with type 2 diabetes. Moreover, they showed that a higher Bacteroidetes:Firmicutes ratio correlates positively with higher blood glucose levels and lower body mass. As type 2 diabetes is usually associated with increased body weight, these findings led to the suggestion that overweight and diabetes are associated with different groups of the intestinal microbiota, and that levels of glucose tolerance should be considered when linking the microbiota with obesity and other metabolic diseases.
Membrez et al. ( Reference Membrez, Blancher and Jaquet 37 ) further demonstrated that modulation of the gut microbiota with antibiotics influences whole-body glucose homeostasis, independent of body weight/body fat mass. In antibiotic-treated mice, reduced liver TAG levels correlated with improved insulin resistance, suggesting that the influence of the gut microbiota on glucose and liver metabolism may have similar mechanisms. The mechanism suggested for these changes was an increase in lipopolysaccharides, the main compound of the outer membrane of Gram-negative bacteria, such as bacteria from the Bacteroidetes phylum. Lipopolysaccharides have been shown to cause acute whole-body insulin resistance( Reference Virkamäki and Yki-Järvinen 38 ), and are a potent stimulator of inflammation. Lipopolysaccharides activate Toll-like receptor 4 in the gut, leading to the expression of TNF-α( Reference Bajaj, Ridlon and Hylemon 39 ). Increased TNF-α has been shown to exert potent antiadipogenic effects( Reference Petruschke and Hauner 40 ) and induce hepatic steatosis( Reference Yang, Lin and Lane 41 ).
In a study by Le Roy et al. ( Reference Le Roy, Llopis and Lepage 17 ), it was further confirmed that insulin resistance develops separately from obesity. However, they did not observe differences in TNF-α levels, and suggested that the gut microbiota may affect hepatic metabolism through other mechanisms, independent of the immune system (Fig. 5). As an example of a different mechanism, in a lipidomic study, Velagapudi et al. ( Reference Velagapudi, Hezaveh and Reigstad 42 ) suggested that the increase in phosphatidylcholine (16 : 0/18 : 1) induced by the microbiota may activate lipoprotein lipase, resulting in reduced serum TAG levels together with increased storage of lipids in the adipose tissue and liver. Although we did not examine single lipid classes in the present study, we observed an increase in 16 : 0 and 18 : 1 FA in the liver of mice fed with t10c12-CLA; therefore, there is a possibility that these animals could have more amounts of 16 : 0/18 : 1 phospholipids.
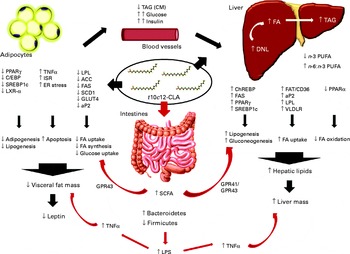
Fig. 5 Schematic summary of all the mechanisms of action of trans-10, cis-12-conjugated linoleic acid (t10c12-CLA), as proposed by the different studies cited in the present paper. There are a variety of proposed mechanisms through which t10c12-CLA supplementation may cause hepatic steatosis and lipoatrophy in mice. Also, t10c12-CLA up- and down-regulates genes involved in fatty acid (FA) synthesis, uptake and oxidation in the adipose tissue and liver in a direct and an indirect manner (black arrows). In the present study, we propose that the gut microbiota and its products are extra environmental factors affecting host lipid metabolism (red arrows). ACC, acetyl-CoA carboxylase; aP2, adipocyte-specific fatty acid binding protein; C/EBP, CAAT/enhancer-binding protein; ChREBP, carbohydrate response element binding protein; CM, chylomicron; DNL, de novo lipogenesis; ER, endoplasmic reticulum; FAS, fatty acid synthase; FAT/CD36, fatty acid translocase; GPR, G-protein coupled receptor; ISR, integrated stress response; LPL, lipoprotein lipase; LPS, lipopolysaccharide; LXR-α, liver X receptor-α; SCD1, stearoyl-CoA desaturase; SREBP-1c, sterol regulatory element-binding protein 1c; VLDLR, VLDL receptor.
We also observed other important changes associated with dietary supplementation of t10c12-CLA. There were increased proportions of Porphyromonadaceae and decreased abundance of Lachnospiraceae and Desulfovibrionaceae. Porphyromonadaceae have previously been associated with non-alcoholic fatty liver disease, atherosclerosis and diabetes( Reference Henao-Mejia, Elinav and Jin 43 ). Members of the family Lachnospiraceae have been shown to protect mice against colonisation by Clostridium difficile ( Reference Reeves, Koenigsknecht and Bergin 44 ), whereas enhanced levels of bacteria from the Desulfovibrionaceae family were associated with impaired glucose tolerance and a serious metabolic syndrome phenotype( Reference Zhang, Zhang and Wang 45 ). Moreover, in a study by Bajaj et al. ( Reference Bajaj, Ridlon and Hylemon 39 ), Lachnospiraceae abundance was reported to be lower in patients with cirrhosis, while Porphyromonadaceae abundance was positively correlated with cognitive impairment and inflammation in patients with hepatic encephalopathy.
SCFA are the end products of bacterial fermentation, with acetate, propionate and butyrate being the major SCFA in the mammalian gut. The type and amount of SCFA produced depends on diet, intestinal transit and microbiota composition( Reference Macfarlane and Macfarlane 46 ). In the present study, SCFA levels were found to be altered by t10c12-CLA supplementation with higher levels of acetate, propionate and isobutyrate detected in the caecal content, even though both groups received a similar amount of carbohydrates and proteins. Thus, the higher amount of SCFA detected in the intervention group was probably due to the marked changes in the microbiota composition induced by dietary t10c12-CLA. SCFA are considered important energy sources for the host, and a few studies have suggested a link between increased levels of SCFA and obesity( Reference Turnbaugh, Hamady and Yatsunenko 34 , Reference Turnbaugh, Ley and Mahowald 47 ). However, in the present study, the higher levels of SCFA were associated with lower fat mass. The precise mechanism involved in SCFA modulation of host metabolism is not clear, and the results reported in the literature are conflicting. Thus, in the present study, we propose three explanations for the possible role of SCFA in t10c12-CLA-induced lipoatrophy/liver steatosis: (1) increased propionate induces gluconeogenesis( Reference Wolever, Spadafora and Eshuis 48 ); (2) SCFA activate G-protein-coupled receptor 41, stimulating hepatic lipogenesis( Reference Samuel, Shaito and Motoike 49 ); (3) SCFA activate G-protein-coupled receptor 43 that regulates energy uptake through the adipose tissue, and promotes the utilisation of excess energy in other tissues instead of storage in the adipocytes( Reference Kimura, Ozawa and Inoue 50 ) (Fig. 5).
The fat-lowering effect of t10c12-CLA is complex, involving multiple mechanisms, and previous studies have shown that feeding t10c12-CLA triggers changes in the pattern of gene expression, reducing FA uptake and storage in the adipocytes, and favouring lipid accumulation in the liver of mice( Reference Clément, Poirier and Niot 12 , Reference Vyas, Kadegowda and Erdman 19 , Reference Jourdan, Djaouti and Demizieux 51 ). In the adipose tissue, t10c12-CLA has been reported to increase (pre)adipocyte apoptosis and reduce adipogenesis and lipogenesis, by the inhibition of key transcription factors( Reference Kennedy, Martinez and Schmidt 52 ). The nearly complete absence of adipose tissue reduces the production of leptin by adipocytes, and the release of NEFA, thereby reducing lipid flux to the liver and subsequent VLDL secretion rates( Reference Degrace, Moindrot and Mohamed 29 ). Indeed, in the present study, lower serum TAG and leptin levels were observed in the t10c12-CLA-supplemented group when compared with the unsupplemented control group. Moreover, t10c12-CLA supplementation markedly increased glucose and insulin concentrations in the serum, compared with no supplementation. Thus, t10c12-CLA supplementation may lead to insulin resistance and decreased glucose uptake by adipocytes, as inflammatory cytokines such as TNF-α and IL-6 down-regulate the expression of lipogenic proteins and the insulin-dependent GLUT 4 (for a review, see Bäckhed et al. ( Reference Bäckhed, Ding and Wang 31 )). High levels of circulating insulin and glucose have been reported to induce the expression of sterol regulatory element-binding protein 1c (SREBP-1c) and carbohydrate response element-binding protein in the liver, and, therefore, stimulate hepatic de novo lipogenesis( Reference Shimomura, Bashmakov and Horton 53 , Reference Yamashita, Takenoshita and Sakurai 54 ).
Another factor that may contribute to the development of liver steatosis is the profound change in the composition of long-chain fatty acids in the liver, induced by t10c12-CLA supplementation. The present study showed that t10c12-CLA intake was associated with an increased n-6 PUFA:n-3 PUFA ratio in the liver. Reduced availability of n-3 PUFA may increase SREBP-1 expression, and reduce PPARα expression, stimulating lipogenesis over lipid oxidation, with accumulation of TAG in the liver( Reference El-Badry, Graf and Clavien 55 ). Furthermore, t10c12-CLA supplementation was associated with altered FA composition of other tissues, besides changing liver FA profiles. Our observations confirmed previous reports demonstrating that t10c12-CLA supplementation decreased arachidonic acid, and altered the ratio of SFA:MUFA, especially increasing the amount of palmitic acid over palmitoleic acid( Reference Kelley, Bartolini and Newman 6 , Reference House, Cassady and Eisen 28 , Reference Evans, Brown and McIntosh 56 ). Although the exact mechanism of CLA action on tissue FA composition has not been elucidated, a few studies have suggested that the reduction in stearoyl-CoA desaturase activity may impair the conversion of SFA to MUFA( Reference House, Cassady and Eisen 28 , Reference Ley, Turnbaugh and Klein 35 ), whereas the inhibition of linoleic acid elongation and desaturation may have an impact on n-6 PUFA synthesis( Reference Eder, Slomma and Becker 57 , Reference Lin, Bo and Oliver 58 ).
Several studies investigating the anti-obesity effect of CLA supplements have focused on gene regulation in the liver and adipose tissue; however, to our knowledge, this is the first study to show the impact of dietary t10c12-CLA on the gut microbiota composition and SCFA production. Long dietary exposure to t10c12-CLA transformed the gut microbiota, favouring the growth of harmful bacteria, thus increasing host susceptibility to a variety of diseases. The greater proportions of Bacteroidetes and Porphyromonadaceae bacteria found in the t10c12-CLA-supplemented group most probably had an influence on lipid metabolism and induction of hepatic steatosis, with higher levels of SCFA contributing to enhanced lipogenesis and gluconeogenesis in the liver.
Thus, diet plays an important role in the modulation of the gut microbiota composition, and as observed in the present study, a single dietary FA is capable of inducing a systemic effect on the host. Although data on the effects of CLA in human subjects are contradictory, few studies that used purified isomers instead of CLA mixtures have shown a detrimental influence of t10c12-CLA, raising concerns about the safety of supplements containing higher amounts of this isomer( Reference Risérus, Arner and Brismar 59 , Reference Tricon, Burdge and Kew 60 ). Considering the fact that non-prescription pills for weight loss are frequently used as an easier alternative to diet and physical activities( Reference Saper, Eisenberg and Phillips 61 ), more clinical studies of longer duration with larger sample sizes are needed.
Dietary approaches targeting beneficial bacteria and suppressing harmful species may be a new strategy to prevent or treat hepatic steatosis and associated metabolic disorders. The use of FA mixtures with equal proportions of CLA isomers, or the use of probiotics and prebiotics to balance the potentially negative effects of t10c12-CLA on the microbiota composition may be alternatives for individuals seeking anti-obesity dietary solutions.
Acknowledgements
The authors acknowledge Eoin Barrett, Alan Hennessy and Talia Huffe for their technical assistance.
The present study was supported by the Science Foundation of Ireland-funded Centre for Science, Engineering and Technology, the Alimentary Pharmabiotic Centre (grant no. SFI/12/RC/2273).
The authors' responsibilities are as follows: C. S., R. P. R., F. S. and E. M. Q. designed the research; T. M. M. and R. W. conducted the research; T. M. M., R. W. and O. O. analysed the data; T. M. M., R. W., O. O. and C. S. wrote the manuscript; G. F. F., P. D. C., T. G. D. and J. F. C. provided significant advice; C. S. had primary responsibility for the final content.
None of the authors had a conflict of interest.











