INTRODUCTION
Stroke affects approximately 14 million people per year worldwide (Johnson et al., Reference Johnson, Nguyen, Roth, Nichols, Alam, Abate and Murray2019), many of whom will experience cognitive impairment and psychiatric symptoms after the event, resulting in a major burden on patients, families, and the society.
In total, about one-third to half of stroke survivors develop psychiatric symptoms (Ferro, Caeiro, & Figueira, Reference Ferro, Caeiro and Figueira2016). These symptoms often stay undetected (Ferro et al., Reference Ferro, Caeiro and Figueira2016) and patients’ clinical needs remain unmet (McKevitt et al., Reference McKevitt, Fudge, Redfern, Sheldenkar, Crichton, Rudd and Wolfe2011). If untreated, psychiatric symptoms are likely to have a limiting effect on rehabilitation and lead to higher rates of disability and mortality (Williams, Ghose, & Swindle, Reference Williams, Ghose and Swindle2004). Among psychiatric post-stroke sequelae, symptoms like anxiety, depression, and apathy are particularly common (Hackett, Kohler, O’Brien, & Mead, Reference Hackett, Kohler, O’Brien and Mead2014). Such sequelae may be caused by brain damage: Frontal/anterior and basal ganglia strokes, and both large and multiple strokes have been related to depression in the post-acute phase (Medeiros, Roy, Kontos, & Beach, Reference Medeiros, Roy, Kontos and Beach2020). Several pathophysiological processes have been described, including abnormal neurotrophic activity, and decreased monoamine levels, where ischemic strokes are thought to disturb neural pathways from the brainstem to the cerebral cortex leading to low monoamine levels in limbic structures of frontal-temporal regions and basal ganglia (Loubinoux et al., Reference Loubinoux, Kronenberg, Endres, Schumann-Bard, Freret, Filipkowski and Popa-Wagner2012). Additionally, life changes post-stroke, such as the loss of autonomy and disability may contribute to psychiatric symptoms (Medeiros et al., Reference Medeiros, Roy, Kontos and Beach2020). Even after mild stroke, patients still experience a negative long-term effect on their lives, which is associated with elevated fatigue and emotional symptoms (Carlsson, Moller, & Blomstrand, Reference Carlsson, Moller and Blomstrand2003; Terrill, Schwartz, & Belagaje, Reference Terrill, Schwartz and Belagaje2018). Approximately one-third of stroke patients experience depression after 2–3 years despite excellent physical recoveries (Kapoor et al., Reference Kapoor, Lanctot, Bayley, Kiss, Herrmann, Murray and Swartz2017).
Besides psychiatric symptoms, cognitive impairment is common post-stroke (Sun, Tan, & Yu, Reference Sun, Tan and Yu2014). Impaired cognition has been found to be an important predictor of stroke recovery, leading to substantial functional difficulties (Zinn et al., Reference Zinn, Dudley, Bosworth, Hoenig, Duncan and Horner2004) and hindering successful rehabilitation (Skidmore et al., Reference Skidmore, Whyte, Holm, Becker, Butters, Dew and Lenze2010). Even in cases with excellent physical recovery after stroke, more than half of the patients may still experience cognitive impairment 1 year (Ihle-Hansen et al., Reference Ihle-Hansen, Ihle-Hansen, Thommessen, Bruun Wyller, Engedal, Øksengård and Fure2011) and several years after stroke (Kapoor et al., Reference Kapoor, Lanctot, Bayley, Kiss, Herrmann, Murray and Swartz2017).
Cognitive function can be evaluated using both performance-based and self-report measures. Studies using performance-based measures have shown that different cognitive domains are affected depending on both the location and the size of the stroke. Even though there is no distinct performance-based cognitive profile post-stroke, deficits in processing speed and executive function seem to occur most frequently, and across different types of stroke pathology and location (Cumming, Marshall, & Lazar, Reference Cumming, Marshall and Lazar2012). Less details are known about subjective cognitive function post-stroke, despite 28.6 %–92.0 % of patients reporting cognitive difficulties (van Rijsbergen, Mark, de Kort, & Sitskoorn, Reference van Rijsbergen, Mark, de Kort and Sitskoorn2014). Cognitive difficulties are often reported by patients early after stroke (Nijsse et al., Reference Nijsse, van Heugten, van Mierlo, Post, de Kort and Visser-Meily2017) and remain relatively stable from 3 to 12 months post-stroke (van Rijsbergen, Mark, Kop, de Kort, & Sitskoorn, Reference van Rijsbergen, Mark, Kop, de Kort and Sitskoorn2020). Of importance, poorer self-reported cognitive function is associated with lower quality of life post-stroke, and may predict future performance-based cognitive difficulties (van Rijsbergen et al., Reference van Rijsbergen, Mark, de Kort and Sitskoorn2014).
Traditionally, research has focused on the relation of depression and anxiety with cognitive function. Here, depression has typically been associated with poorer performance-based cognitive function post-stroke (Hackett et al., Reference Hackett, Kohler, O’Brien and Mead2014; Robinson & Jorge, Reference Robinson and Jorge2016). This relationship between depressive symptoms and poorer cognitive function was also found in patients with minor stroke who have relatively good general outcomes and a promising long-term prognosis (Morsund et al., Reference Morsund, Ellekjaer, Gramstad, Reiestad, Midgard, Sando and Naess2019). The association between anxiety and performance-based cognitive function seems to be less clear, and contradictory findings exist (Grosdemange et al., Reference Grosdemange, Monfort, Richard, Toniolo, Ducrocq and Bolmont2015; Morsund et al., Reference Morsund, Ellekjaer, Gramstad, Reiestad, Midgard, Sando and Naess2019). In studies using self-report measures of cognitive function, higher levels of self-reported cognitive difficulties are commonly related to higher levels of depression post-stroke (van Rijsbergen et al., Reference van Rijsbergen, Mark, de Kort and Sitskoorn2014). Additionally, anxiety, fatigue, and general psychological distress seem to be associated with reports of poorer cognition (van Rijsbergen et al., Reference van Rijsbergen, Mark, de Kort and Sitskoorn2014).
With most studies focusing on the role of depression and anxiety, little is known about a broader spectrum of psychiatric symptoms (e.g., obsessive-compulsive symptoms or somatization) and their relation to cognitive function. For performance-based cognitive function, preliminary evidence suggests that general psychiatric distress, anxiety, depression, and insufficiency of thinking and acting predict cognitive decline 1–6 months post-stroke (Rasquin, Lodder, & Verhey, Reference Rasquin, Lodder and Verhey2005), but research in this area is sparse.
In patients with mild stroke and relatively good outcome post-stroke, psychiatric and cognitive difficulties may remain undetected in general clinical practice, but still hinder full participation in rehabilitation services and impact quality of life. Hence, understanding the association between these post-stroke sequelae is crucial in order to determine patients in need of further treatment. The aim of this study was, therefore, twofold (Figure 1). First, we studied the relationship between performance-based cognitive function and symptoms of depression and anxiety in relatively well-functioning stroke patients 3 months post-stroke (n = 86). We hypothesized that higher levels of anxiety and depression would be associated with poorer attention/executive function and memory. Second, we evaluated a broader spectrum of psychiatric symptoms and their association to self-reported and performance-based cognitive function in a subsample (n = 41).

Figure 1. Overview of included variables and analyses
METHOD
Participants
This study used data from a project conducted between April 2007 and April 2010 at the Stroke Unit, Department of Neurology, Akershus University Hospital (Ahus), Norway. Participants were consecutively recruited from the Stroke Unit at Ahus, and patients with supratentorial stroke, aged between 40 and 79 years, Mini-Mental State Examination (MMSE) (Folstein et al., Reference Folstein, Folstein and McHugh1975) score ≥23, and no significant symptoms of visual and/or auditory neglect that would have influenced test results were included. Patients with previous hospitalizations due to stroke or with a history of other neurologic and/or psychiatric disorders (self-reported or documented in patients’ journals), as well as patients with speech/language difficulties as indicated during the MMSE and/or clinical interview were excluded. Diagnosis and type of stroke were determined by neurologists, based on clinical symptoms and supported by imaging data [computed tomography for all patients, and magnetic resonance imaging (MRI) when available].
Initially, 97 stroke patients who agreed to neuropsychological examination, MRI, and cerebrospinal fluid examination were included. Eighty-six participants remained in the study after 3 months and were included in the primary analyses. Dropout was mainly due to illness, and patients who experienced new strokes between the testing phases were excluded. In subsample analyses for secondary aims, only those patients (n = 41) who completed a questionnaire assessing psychiatric symptoms (the Symptom-Checklist-90 – Revised; SCL-90-R) at the 3-month follow-up were included. The full sample is also described in a previous study (Selnes et al., Reference Selnes, Grambaite, Rincon, Bjornerud, Gjerstad, Hessen and Fladby2015).
Participants’ consent was obtained according to the Declaration of Helsinki. The study was approved by the Regional Committees for Medical and Health Research Ethics in Norway and carried out in accordance with the Norwegian Health and Research Act.
Measures
Data included in the analyses were collected while the participants were monitored or received follow-up at the Department of Neurology. All tests were performed in Norwegian by the same neuropsychologist (R.G.).
Functional Measures
The National Institutes of Health Stroke Scale (NIHSS) was used to quantify neurologic impairment 1 day and 3 months post-stroke. The NIHSS is composed of 11 items, each scoring a specific ability, e.g., language or motoric function. Scores range from 0 (=normal function) to 2–4 (=different levels of impairment). The highest possible score for non-comatose patients is 42.
The Barthel Index (Mahoney & Barthel, Reference Mahoney and Barthel1965) was used to measure performance in activities of daily living (ADL) 3–7 days, and 3 months post-stroke. Ten items describing basic ADL function, e.g., toilet use or dressing, assess the degree of assistance required. Higher scores indicate more independence. The total score ranges from 0 to 100.
Psychiatric Symptoms
The SCL-90-R (Derogatis, Reference Derogatis1992) measured overall psychopathological distress and nine psychiatric dimensions 3 months post-stroke: Depression, anxiety, phobic anxiety, somatization, interpersonal sensitivity, obsessive-compulsive symptoms, hostility, paranoid ideation, and psychoticism. Items are rated on a 5-point scale from “not at all” (0) to “extremely” (4). Subscale scores are obtained by summing up raw scores of all items belonging to a given subscale and dividing this value by the number of items. For the obsessive-compulsive-subscale, the two items measuring subjective cognitive difficulties were removed as we assumed that subjective cognitive difficulties may, independently from other obsessive-compulsive symptoms, influence performance-based cognitive function. Raw scores were converted into T scores by using age- and sex-keyed norms from the manual to identify the prevalence of clinical symptoms (T score ≥ 63) (Derogatis, Reference Derogatis1992). The SCL-90-R was found to be a valid measure for general psychopathology in a Norwegian sample (Carrozzino et al., Reference Carrozzino, Vassend, Bjorndal, Pignolo, Olsen and Bech2016).
We also administered the Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, Reference Zigmond and Snaith1983), which consists of two subscales evaluating depression and anxiety. HADS has been validated in stroke patients (Aben, Verhey, Lousberg, Lodder, & Honig, Reference Aben, Verhey, Lousberg, Lodder and Honig2002), and the Norwegian version has shown to be a valid screening tool in a Norwegian stroke population (Fure, Wyller, Engedal, & Thommessen, Reference Fure, Wyller, Engedal and Thommessen2006). Items are rated on a 4-point scale from “not at all” (0) to “most of the time” (3). A score of ≥ 8 on either subscale indicates a possible clinical problem (Leiknes, Dalsbø, & Siqveland, Reference Leiknes, Dalsbø and Siqveland2016).
Performance-Based Cognitive Function
All neuropsychological tests were performed 3 months post-stroke, except MMSE, which was assessed 3–7 days post-stroke. General cognitive ability was tested using the Vocabulary and Matrix Reasoning subtests from the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, Reference Wechsler1999).
Selected neuropsychological tests were used to form two composite scores: One for memory and one for attention, executive function, and processing speed. To enhance readability, the term attention/executive function was used for the latter. Composite variables were calculated based on z scores (Mean = 0; SD = 1), following a common procedure when developing composite scores of neuropsychological tests (Boone, Miller, Swartz, Lu, & Lee, Reference Boone, Miller, Swartz, Lu and Lee2003; Gibbons et al., Reference Gibbons, Carle, Mackin, Harvey, Mukherjee and Insel2012). Z scores were internally standardized by first subtracting the mean test score of all participants from the raw score in a given test, and then dividing this value by the sample SD (Song et al., 2013). When lower scores indicate better performance (TMT-A/B, CWIT-3/4), raw scores were multiplied by −1, so that higher scores represented better performance in all tests. To get the final composite scores, individual z scores were summed and divided by the number of included tests. Higher composite scores indicate better performance.
The following tests were included in the memory composite score:
-
1. The immediate and delayed recall trial of the Rey Auditory Verbal Learning Test (“RAVLT-1”, “RAVLT-2”) (Schmidt, 1996) were used to assess verbal memory. The immediate recall trial measures free recall ability of a 15-item wordlist immediately after five learning trials and an interference list, whereas the delayed trial measures recall ability 30 min after the last learning trial. The score is the number of correctly recalled words. Higher scores indicate better performance.
-
2. The immediate and delayed recall trial (“REY-1”, “REY-2”) of the Rey Complex Figure Test (Meyers & Meyers, Reference Meyers and Meyers1995) were used to assess visuospatial memory. The immediate recall trial is administered 3 min after a copy trial, and delayed recall is administered after 30 min. The score is calculated based on the accuracy of reproducing the figure. Higher scores indicate better performance.
The following tests were included in the attention/executive function composite score:
-
1. Trail Making Tests A and B (“TMT-A”, “TMT-B”) (Reitan & Wolfson, Reference Reitan and Wolfson1985). TMT covers visual scanning, processing speed, and divided attention (Lezak, Howieson, & Loring, Reference Lezak, Howieson and Loring2004). The score is the number of seconds spent on each task. Lower scores indicate better performance.
-
2. Digit Symbol-Coding subtest (“Coding”) of WAIS-III (Wechsler, Reference Wechsler2003) is a timed measure (120 s) of processing speed, attention, learning, and information organization. The score is calculated by counting correctly drawn symbols. Higher scores indicate better performance.
-
3. Color-Word Interference Test conditions 3 and 4 (“CWIT-3”, “CWIT-4”) of the Delis–Kaplan Executive Function System (D-KEFS) battery (Delis, Kaplan, & Kramer, Reference Delis, Kaplan and Kramer2001) were used. CWIT-3 captures response inhibition and CWIT-4 measures inhibition and cognitive flexibility. The score is the number of seconds spent on each task. Lower scores indicate better performance.
Self-Reported Cognitive Difficulties
Two items of the SCL-90-R obsessive-compulsive subscale addressing memory and concentration difficulties, rated on a 5-point scale from “not at all” (0) to “extremely” (4), were used to measure self-reported cognitive difficulties 3 months post-stroke.
A composite score was formed using z scores. Raw scores were transformed into z scores using the same procedure described above (internal standardization). Z scores were summed and divided by two (i.e., the number of items included in the composite variable). Higher composite scores indicate more self-reported cognitive difficulties.
Statistics
The Statistical Package for Social Sciences, version 25 (IBM Corp, 2017), was used. To compare characteristics of patients that filled out the SCL-90-R with those who did not, the independent samples t test and the χ 2 test were used. Associations between self-reported cognitive difficulties and test performance were studied using Pearson correlation.
Relations between psychiatric symptoms and cognitive function were analyzed using multiple linear regressions. In primary analyses (n = 86), we investigated the relation between HADS and performance-based cognitive function. In subsample analyses (n = 41) for secondary aims, we explored the relations of a broader spectrum of psychiatric symptoms to performance-based and self-reported cognitive function (Figure 1). Based on literature, we included age, sex, and education as predictors into all regression models. Predictors were entered simultaneously.
An examination of the data showed no violation of assumptions for regression analysis. For some cases, bootstrapping was used due to tendencies of the skewed distribution of residuals.
For our primary analyses, we chose the significance level p ≤ .01 to adjust for multiple testing. Results of the subsample analyses for secondary aims were not adjusted for multiple testing, and the standard significance level p ≤ .05 was used. To estimate effect sizes, Cohen’s d was used for continuous variables, where d ≥ .20 indicates a small effect, d ≥ .50 a medium, and d ≥ .80 a large effect (Cohen, 1988). For categorical variables, Cramer’s phi (ϕ c ) was used with ϕ c = .10 indicating a small effect, ϕ c = .30 a medium, and ϕ c = .50 a large effect when the number of degrees of freedom = 1 (Cohen, 1988).
RESULTS
Patient Characteristics
Patient and stroke characteristics are shown in Table 1 and Supplementary Table 1, respectively. All patients were Caucasian with a Scandinavian language as their mother tongue, and all received treatment as usual despite their participation in the study. None of the patients received a specialized cognitive rehabilitation during the first 3 months post-stroke. Men and women did not significantly differ in age in the full sample (mean (M)women = 65.7 ± 8.0; M men =64.1 ± 9.9; p = .45), nor in the subsample (M women = 67.4 ±5.7; M men = 65.3 ± 8.7; p = .53). To investigate if patients included in subsample analyses significantly differed from those who did not respond to the SCL-90-R, possibly leading to a bias, we compared characteristics of these two subsamples. Patients who were included in subsample analyses significantly differed from patients that were not included only in sex, with fewer women in the sample used for subsample analyses (19.5% vs. 42.2%), and in one measure of general cognitive function (Matrix Reasoning), with patients included in subsample analyses having higher scores. Raw scores for performance-based cognitive function are displayed in Table 2.
Table 1. Patient characteristics

*Significant group difference at p ≤ .05. Raw scores are presented (if not noted otherwise). 1Excluded patients = Patients who did not answer the SCL-90-R and thus were not used in the subsample analyses. 2Higher values indicate higher impairment/higher levels of symptoms. 3 T scores presented (age- and sex-keyed norms from the manual used). 4Range: 0–30. Baseline = Within the first week after stroke.
Table 2. Raw scores for performance-based cognitive function in both samples

All attention/executive function tests are measured in time spent on the tasks (seconds). For RAVLT-1 and -2, the number of correctly recalled words is displayed. For REY-1 and -2, raw scores based on the accuracy of reproducing the figure are displayed.
Primary Analyses: Relations of Symptoms of Anxiety and Depression to Performance-Based Cognitive Function
In the full sample (n = 86), 16 patients (18.6%) had HADS-A scores above cutoff, and 11 (12.8%) had HADS-D scores above cutoff.
Results of all primary analyses are displayed in Table 3. There was a significant negative association between HADS-D and memory, but not between HADS-D and attention/executive function. Thus, stroke patients with higher levels of depressive symptoms had significantly lower scores on memory tests, but not on tests measuring attention/executive function. HADS-A was neither significantly related to memory nor to attention/executive function. Age was a significant predictor (p ≤ .01) in all analyses, with older participants having poorer results. Women achieved significantly better test results than men (p = .001) in both models with attention/executive function as a dependent variable.
Table 3. Primary analyses (n = 86): associations between anxiety and depression and performance-based cognitive function (n = 86)

For primary analyses, a significance level of p ≤ .01 was used. All analyses are controlled for age, sex, and education. B, p, CI refer only to the predictor of interest, R 2 indicates the amount of variance explained by the whole model, i.e., with all predictors included.
Subsample Analyses I: Relationship Between Psychiatric Symptoms and Performance-Based Cognitive Function
The T score distributions of psychiatric symptoms (SCL-90-R) are shown in Figure 2. Most of the means are centered around a T score of 50 (range: 47.0–55.9), with somatization having the highest mean and paranoid ideation the lowest. A maximum of 11 patients (26.8%; somatization) and a minimum of 1 (2.4%; interpersonal sensitivity) scored above the clinical cutoff. The number of patients with clinical levels of obsessive-compulsive symptoms decreased from seven (17.1%) to three (7.3%) when an abbreviated obsessive-compulsive scale without the items assessing memory and concentration difficulties was used. None of the patients scored above cutoff on the total SCL-90-R score.

Figure 2. Pirateplot showing the distribution of psychiatric symptoms
The pirateplot reports T scores of all individuals as separate data points. Noise (jitter) was added horizontally to reduce overlap among points with similar values. Anx = Anxiety subscale; Dep = Depression subscale; Hos = Hostility subscale; InterSen = Interpersonal sensibility subscale; OC = Obsessive-compulsive subscale (adjusted scores); Para = Paranoid ideation subscale; PhoAnx = Phobic anxiety subscale; Psych = Psychoticism subscale; Som = Somatization subscale. T scores were used in order to indicate clinical symptom levels. Horizontal black lines indicate means. Standard deviation of the mean of each domain is indicated as transparent boxes. The dashed horizontal line indicates the clinical cutoff (T score ≥ 63).
Table 4 displays the associations between psychiatric symptoms and performance-based cognitive function. Phobic anxiety was significantly negatively related to memory. Higher scores in the anxiety dimension (which focuses on symptoms of general anxiety like nervousness and trembling) and phobic anxiety dimension (which focuses on symptoms of agoraphobia) were related to lower attention/executive function. Thus, two psychiatric domains had a significant negative relationship with performance-based attention/executive function, and one significant relationship between psychiatric symptoms and performance-based memory function was found. The SCL-90-R total score (both with the items measuring concentration and memory difficulties, and a modified score without these items included) was neither related to performance-based memory (p = .80 and p = .83, respectively) nor to attention/executive function (p = .11 and p = .12, respectively).
Table 4. Subsample analyses I: associations between psychiatric symptoms and performance-based cognitive function (n = 41)

bBootstrapping is based on 5000 replicates, and bias-corrected-accelerated confidence intervals are shown. As the two items constituting the composite score for self-reported cognitive difficulties (item 9 and item 55 of the SCL-90-R) are a part of the obsessive-compulsive (OC) subscale, an adjusted OC score without these two items was calculated. For exploratory subsample analyses, a significance level of p ≤ .05 was applied. All analyses are controlled for age, sex, and education. B, p, CI refer only to the predictor of interest, R 2 indicates the amount of variance explained by the whole model, i.e., with all predictors included.
Subsample Analyses II: Relationship Between Psychiatric Symptoms and Self-Reported Cognitive Function
An overview of self-reported cognitive difficulties is displayed in Table 5.
Table 5. Frequencies of self-reported cognitive difficulties (n = 41)
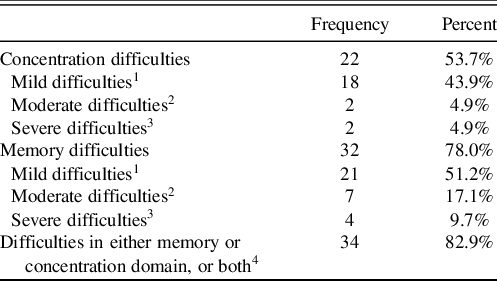
1“Mild difficulties” were defined as ratings (1) “a little bit” on the SCL-90-R. 2“Moderate difficulties” were defined as ratings (2) “moderately” on the SCL-90-R. 3“Severe difficulties” were defined as ratings (3) “quite a bit” and (4) “extremely”. 4“Difficulties in either memory or concentration domain, or both” was defined as ratings from (1) “a little bit” to (4) “extremely” in either the memory or the concentration domain, or both.
Table 6 shows the associations between psychiatric symptoms and self-reported cognitive function. More self-reported cognitive difficulties were associated with higher symptom levels in all psychiatric domains (p ≤ .05). Sex was significantly related to self-reported cognitive difficulties in the phobic anxiety (p = .05) and somatization model (p = .05). Neither age nor education was a significant predictor in any models. Both HADS-A and HADS-D were significantly positively associated with self-reported cognitive difficulties (p ≤ .05). Higher education significantly predicted lower levels of self-reported cognitive difficulties in the HADS-D-regression model (p = .05), but not in the HADS-A model. A higher SCL-90-R total score was related to more self-reported cognitive difficulties (p ≤ .001).
Table 6. Subsample analyses II: associations between psychiatric symptoms and self-reported cognitive function (n = 41)

bBootstrapping is based on 5000 replicates, and bias-corrected-accelerated confidence intervals are shown. As the two items constituting the composite score for self-reported cognitive difficulties (item 9 and item 55 of the SCL-90-R) are a part of the obsessive-compulsive subscale, an adjusted score without these two items was calculated. A significance level of p ≤ .05 was applied. All analyses are controlled for age, sex, and education. B, p, CI refer only to the predictor of interest, R 2 indicates the amount of variance explained by the whole model, i.e., with all predictors included.
Correlations Between Self-Reported and Performance-Based Cognitive Function (n = 41)
Pearson correlations showed that more self-reported cognitive difficulties were related to lower performance-based attention/executive function as measured by the composite score. Also, all individual tests measuring attention/executive function, except TMT-A, were negatively correlated with self-reported cognitive difficulties. No significant association between self-reported cognitive difficulties and performance-based memory was found (Table 7).
Table 7. Correlations between performance-based and self-reported cognitive function (n = 41)

CS = Composite score; *Significant at p ≤ .05. **Significant at p ≤ .01, two-tailed test. TMT-A/B and CWIT-3/4 scores have been multiplied with −1 so that a higher score indicates a better result.
DISCUSSION
Principal Findings
We studied the relationship of psychiatric symptoms with performance-based and self-reported cognitive function 3 months post-stroke. We found that depressive symptoms (HADS-D) were associated with performance-based memory difficulties in relatively well-functioning (based on baseline NIHSS, Barthel Index score, and MMSE) patients. Also, psychiatric symptoms were primarily related to self-reported rather than to performance-based cognitive difficulties: All nine psychiatric domains (anxiety, depression, phobic anxiety, obsessive-compulsive symptoms, somatization, hostility, interpersonal sensitivity, paranoid ideation, psychoticism) were negatively related to self-reported cognitive difficulties, whereas only two (anxiety, phobic anxiety) were associated with lower performance-based cognitive function.
Primary Analyses: Relations of Symptoms of Anxiety and Depression to Performance-Based Cognitive Function
Higher levels of depressive symptoms were associated with lower performance-based memory but not with attention/executive function. Cognitive impairments and depression are highly prevalent after minor stroke (Moran et al., Reference Moran, Fletcher, Feltham, Calvert, Sackley and Marshall2014), and several studies have found an association between symptoms of depression and cognitive domains such as verbal memory, cognitive speed (Barker-Collo, Reference Barker-Collo2007), and executive function (Morsund et al., Reference Morsund, Ellekjaer, Gramstad, Reiestad, Midgard, Sando and Naess2019). Our study extends these findings to patients with good outcomes after stroke. The patients included in our study were neither severely functionally impaired, nor had high levels of self-reported depression: With 13% having scores above cutoff on HADS-D, the prevalence of self-reported depression in our sample was substantially lower than commonly found in the post-acute phase after stroke (Hackett & Pickles, Reference Hackett and Pickles2014). This may partly be explained by the fact that our patients were assessed 3 months post-stroke, whereas the prevalence of depression seems to be higher during the first-month post-stroke compared to later stages (Kouwenhoven, Kirkevold, Engedal, & Kim, Reference Kouwenhoven, Kirkevold, Engedal and Kim2011). Also, our sample consisted of 70% men, who are known to report less depressive symptoms (Poynter et al., Reference Poynter, Shuman, Diaz-Granados, Kapral, Grace and Stewart2009). However, the fact that depressive symptoms are not reported does not necessarily mean that they do not have them. Such underlying symptoms may still negatively influence cognitive function, and may therefore explain why men had lower scores on attention/executive function tests than women.
Our results indicate that stroke patients with good outcome can have underlying cognitive and emotional difficulties that may remain undetected if not specifically assessed. Whether depressive symptoms and poorer cognitive function influence each other, or whether they occur side by side, remains unclear. Some research suggests that selective serotonin reuptake inhibitors (SSRIs) improve executive function in stroke patients (Narushima, Paradiso, Moser, Jorge, & Robinson, Reference Narushima, Paradiso, Moser, Jorge and Robinson2007), but divergent results on the effectiveness of pharmacotherapy to improve cognition post-stroke exist (Hackett, Anderson, & House, Reference Hackett, Anderson and House2005). Additionally, motivational interviewing has shown a promising effect on improving mood post-stroke (Cheng et al., Reference Cheng, Qu, Huang, Xiao, Luo and Wang2015), and future studies should explore whether psychosocial and psychological interventions may improve cognitive function post-stroke.
Interestingly, HADS-A was not associated with performance-based measures, which differs from the findings of our subsample analyses when the SCL-90-R anxiety domain was used. Different anxiety measures may, at least in part, explain divergent results on the relationship between anxiety and cognitive function post-stroke.
Subsample Analyses I: Psychiatric Symptoms and Performance-Based Cognitive Function
With 2.4%–26.6% of patients above the clinical cutoff, levels of psychiatric symptoms were lower than usually found in this population (Ferro et al., Reference Ferro, Caeiro and Figueira2016), possibly due to our patients having mild strokes without severe cognitive/language problems. The somatization subdomain had the highest percentage of clinical cases (26.8%), similar to other stroke samples (Rasquin et al., Reference Rasquin, Lodder and Verhey2005). High scores may be due to physical impairment and pain post-stroke, and thus reflect actual physical health rather than somatization of underlying psychological distress.
Moreover, clinical levels of self-reported phobic anxiety were more common than clinical levels of self-reported generalized anxiety. This aligns with research showing that phobic anxiety is the most common anxiety subtype 3 months after a minor stroke (Chun, Whiteley, Dennis, Mead, & Carson, Reference Chun, Whiteley, Dennis, Mead and Carson2018). The SCL-90-R phobic anxiety dimension measures symptoms associated with panic disorder with and without agoraphobia, which is defined as a persistent fear response to a specific person, place, object, or situation, leading to avoidance behavior (Derogatis, Reference Derogatis1992). However, avoidance behavior in stroke patients may be due to post-stroke sequelae such as motor impairment rather than symptoms of panic disorder. Thus, the phobic anxiety domain may in part capture self-reported motor symptoms instead of psychiatric distress. Nevertheless, how phobic anxiety impacts life post-stroke is an important avenue for future research, as phobic symptoms challenge the ability to live an ordinary life outside one’s home and social participation.
We also found several cases (17.1%) with obsessive-compulsive symptoms above the clinical cutoff. When using an abbreviated obsessive-compulsive-scale without the items assessing memory and concentration difficulties, the number of clinical cases decreased to three (7.3%). This indicates that self-reported cognitive difficulties are a major contributor to elevated scores on this scale. With cognitive difficulties being common after cerebrovascular accidents, the SCL-90-R obsessive-compulsive subscale should be interpreted with caution in the stroke population.
Examining the association between psychiatric symptoms and performance-based function, we found that higher levels of anxiety and phobic anxiety were associated with poorer cognitive function. This differs from earlier work, which did not find a relation between anxiety and cognition 12 months after a minor stroke (Morsund et al., Reference Morsund, Ellekjaer, Gramstad, Reiestad, Midgard, Sando and Naess2019). Inconsistent results might be due to the time of assessment, as anxiety is less prevalent 6–12 months post-stroke compared to the first 6 months (Knapp et al., Reference Knapp, Dunn-Roberts, Sahib, Cook, Astin, Kontou and Thomas2020). Furthermore, inclusion criteria led to patients in the Morsund (2019) study being younger and less functionally impaired (NIHSS score) at baseline. Future studies with robust methodology are needed to further disentangle the relationship between post-stroke anxiety and performance-based cognitive function.
There were three additional interesting findings: First, only phobic anxiety was associated with both performance-based measures. Hence, phobic anxiety may not only be particularly prevalent post-stroke, but may also be uniquely associated with poorer test performance in a broader range of cognitive function.
Second, the SCL-90-R depression/anxiety scale and HADS were differently related to performance-based cognitive function. As in the full sample, HADS-D was negatively related to memory in the subsample, although nonsignificant. Thus, it is unlikely that divergent results are solely based on sample differences. Rather, low agreement between the SCL-90-R and HADS may be due to methodological differences. For example, some items of the SCL-90-R depression subscale (e.g., “Thoughts of ending my life.”) do not have an equivalent in HADS-D, and single HADS items have shown low discrimination between anxiety and depression in stroke patients (Ayis, Ayerbe, Ashworth, & Wolfe, Reference Ayis, Ayerbe, Ashworth and Wolfe2018).
Third, the SCL-90-R total score was neither associated with performance-based attention/executive function, nor with memory. This indicates that the relationship of phobic anxiety and anxiety with cognitive function was not explained by overall psychopathological distress, but rather by specific symptom load in these domains.
Subsample Analyses II: Psychiatric Symptoms and Self-Reported Cognitive Function
Aligning with previous research, subjective cognitive difficulties were common, with around three-fourth stating memory difficulties, and about half of the patients reporting concentration difficulties. One possible explanation for the high prevalence of self-reported memory difficulties may be that patients with brain damage tend to interpret reduced processing speed, attention, and executive control as memory deficits (Lezak, Howieson, & Loring, 2012).
When looking at the association between psychiatric symptoms and self-reported cognitive function, a clear picture emerged: All nine SCL-90-R domains were positively associated with subjective cognitive difficulties. This is consistent with studies that showed a relationship between depressive symptoms and self-reported cognitive difficulties in patients with mild cognitive impairment (Grambaite et al., Reference Grambaite, Hessen, Auning, Aarsland, Selnes and Fladby2013) and in stroke patients (van Rijsbergen et al., Reference van Rijsbergen, Mark, de Kort and Sitskoorn2014). Our finding also aligns with reports of greater depression being associated with self-reported functional changes rather than performance-based cognitive changes in conditions such as HIV and hepatitis C (Bieliauskas et al., Reference Bieliauskas, Back-Madruga, Lindsay, Snow, Kronfol, Lok and Fontana2006).
While supporting previous findings, our study also provides a new insight: Our results indicate that the relationship between psychiatric symptoms and self-reported cognitive function may go far beyond depression and extend to a wide spectrum of psychiatric symptoms. Self-reported cognitive difficulties should, therefore, be in focus in clinical practice and research.
Performance-Based and Self-Reported Cognitive Function
Patients who reported more cognitive difficulties had significantly lower scores on four out of the five tests measuring attention/executive function, which may indicate that patients realistically evaluated their cognitive abilities in this domain.
However, none of the memory tests were associated with self-reported cognitive function. One possible explanation for this may be that patients face greater difficulties in their daily lives post-stroke than what memory results can indicate. Biases may also occur if patients with poorer memory simply do not remember having these difficulties and thus do not report them. Moreover, the lacking association between self-reported cognitive function and memory test results may be due to the items measuring self-rated cognitive function being rather nonspecific. For example, participants may have interpreted “Trouble remembering things” as difficulties with short-term/working memory or word-finding difficulties – domains that are not assessed with the memory tests used in this study.
Interestingly, our results differ from earlier work that has commonly found an association between self-reported cognitive difficulties and poorer performance-based memory, and not to lower function in other cognitive domains (van Rijsbergen, Mark, de Kort, & Sitskoorn, Reference van Rijsbergen, Mark, de Kort and Sitskoorn2014). However, other cognitive domains than memory were often not reported (van Rijsbergen, Mark, de Kort, & Sitskoorn, Reference van Rijsbergen, Mark, de Kort and Sitskoorn2014). Those few that reported results on the relation between subjective cognitive difficulties and performance-based attention/executive function (but were nonsignificant), had different selection criteria and tested patients in the acute phase post-stroke (Duits, Munnecom, van Heugten, & van Oostenbrugge, Reference Duits, Munnecom, van Heugten and van Oostenbrugge2008), or studied specific aspects of self-rated cognition (Winkens, Van Heugten, Fasotti, & Wade, Reference Winkens, Van Heugten, Fasotti and Wade2009).
Strengths, Limitations, and Future Directions
Our study extends and substantiates prior research by including both performance-based and self-rated cognitive function as an outcome measure. By assessing a well-functioning stroke population with an extensive neuropsychological test battery, we gained important new insights for a group of patients with relatively good outcomes post-stroke, which are relevant for research and clinical practice.
We aimed to explore a broad spectrum of psychiatric symptoms and their relation to performance-based and self-reported cognitive function, which led to numerous regression models. This comes along with a greater risk for significant results accumulated by chance due to multiple testings. As little is known about the relation between psychiatric symptoms and cognitive function in high-functioning post-stroke populations, our subsample analyses were explorative, and we did not statistically adjust for multiple testing. The results of our subsample analyses should, therefore, be interpreted with caution and further studied with a larger sample size.
The sample size of our subsample analyses was small, limiting statistical power. However, the subsample for secondary aims was highly similar to those who were not included in subsample analyses on key demographic and clinical variables. We hope that our results, though preliminary, stimulate larger studies.
Furthermore, our patients were rather well-functioning, and generalizability of our findings to patients with lower functioning after moderate or severe stroke is therefore limited.
Processing speed, which we have not controlled for, is a major component in all neuropsychological tests used to measure attention/executive function in this study. Thus, results may be influenced by stroke sequelae such as mild motor impairment. Including tests into the composite that are not majorly dependent on processing speed would be an interesting enhancement of this study.
Also, using a summarized score for cognitive function makes it impossible to predict difficulties or abilities in a specific domain of performance-based cognitive function (e.g., delayed verbal recall). However, composite scores minimize possible ceiling effects, and we see it as the strength that a substantial number of neuropsychological tests were included in each composite.
Furthermore, no performance validity tests were included. However, all patients were encouraged to put forth their best efforts to perform at their best level of ability and informed that the results of the neuropsychological testing may be used to evaluate their driving abilities. There were also no apparent gains that would be contingent on lowered performance.
Moreover, there is no “gold standard” to define self-reported cognitive function post-stroke (van Rijsbergen, Mark, de Kort, & Sitskoorn, Reference van Rijsbergen, Mark, de Kort and Sitskoorn2014). We measured subjective cognitive difficulties with two items. Although this may not suffice to capture a differentiated picture, we assessed subjective memory and concentration difficulties, which are among the most commonly reported difficulties post-stroke (Lamb, Anderson, Saling, & Dewey, Reference Lamb, Anderson, Saling and Dewey2013).
Future studies should take patients’ premorbid cognitive function and comorbidities into account, which may influence test results independently from a stroke. Finally, including a control group without stroke to compare our results to those of a healthy, age-matched population, would be an important next step.
Clinical Implications
This study provides new insights that may help to improve diagnosis and treatment for well-functioning stroke patients. First, stroke patients with self-reported cognitive difficulties in the post-acute period may be at a higher risk of psychiatric symptoms. Subjective cognitive difficulties post-stroke should be taken seriously, and clinicians should be made aware that they may not necessarily reflect actual cognitive impairment but psychiatric symptoms.
Second, an early screening of a broad spectrum of psychiatric symptoms post-stroke, and a more extensive follow-up for those at risk for higher levels of psychiatric distress, may help that patients get adequate treatment.
Third, as subjective cognitive difficulties may predict cognitive decline post-stroke (van Rijsbergen et al., Reference van Rijsbergen, Mark, de Kort and Sitskoorn2014), clinicians should be alert if their patients report cognitive difficulties because these may signal patients in need of a more thorough follow-up.
Finally, different psychiatric symptoms were associated with lower performance-based cognitive function in well-functioning stroke patients. Clinicians should be aware that patients with good outcome and prognosis may have “hidden” symptoms that are hard to detect in general clinical assessments, but may still impact patients’ life post-stroke.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617721000187.
ACKNOWLEDGMENTS
The study is supported by grants from the South-Eastern and Central Norway Regional Health Authority. We wish to thank Per Selnes and John Hald for contributions to data collection, Jūratė Šaltytė Benth for statistical advice, and all participants for their time and effort.
CONFLICTS OF INTEREST
The authors have no conflicts of interests to declare.











