INTRODUCTION
Oesophageal cancer is the eighth most common cancer worldwide with an estimated incidence of 455 784 new cases and 400 156 deaths in 2012 [Reference Anon1]. The incidence rate varies in different physiographical regions, nations and races. China has the most new cases of oesophageal cancer (more than 223 000) around world. Data has shown that the number of new oesophageal cancer cases in China will increase to 433 000 by 2035 [Reference Anon1].
The causes of oesophageal cancer have not yet been fully elucidated. Previous studies have suggested that excessive use of tobacco and alcohol [Reference Islami2], nutritional deficiencies [Reference Holmes and Vaughan3], and some chemicals [Reference Wei4], along with physical factors such as the ingestion of coarse or hot food [Reference De5] might be associated with the development of oesophageal cancer. The effect of infectious agents in oesophageal carcinogenesis has also been suggested as direct carcinogens or promoters. Infection with oncogenic human papillomavirus (HPV) types has been identified as a causal agent in a variety of human carcinomas, including those of the cervix, anogenital region and head and neck [Reference Ciapponi6–Reference Stelzer8]. HPV as a risk factor for oesophageal cancer was first suggested in 1982 [Reference Syrjanen9]. However, the frequency of HPV infection in oesophageal pre-malignant lesion or carcinomas varies between 0% and 88% in different studies [Reference Koh10, Reference Li11]. Although more than 100 different types of HPV are known, only about 15 types have been viewed as high-risk or oncogenic HPVs [Reference de Villiers12]. Two of these, HPV-16 and HPV-18, are the most frequently identified high-risk subtypes in cancers caused by HPV. A recently published meta-analysis showed that the HPV-16 prevalence in oesophageal cancer cases was 38·1% [Reference Zhang13]. However, unlike HPV-16, the role of HPV-18 in oesophageal carcinogenesis has not been clearly defined.
The prevalence of HPV in oesophageal cancers ranges widely, even within the same country [Reference Syrjanen14]. Variations in sampling methods, demographic and ethnic factors, anatomical sites and the method used for viral detection have been proposed as possible causes of differences in results. China has one of the highest rates of HPV prevalence in oesophageal squamous cell carcinoma of 41·6% by polymerase chain reaction (PCR), compared to Europe/Australia (15·6%) and North America (16·6%) [Reference Petrick15]. However, as one of the most frequently identified high-risk subtypes, the prevalence of HPV-18 in China has not been estimated so far.
We aimed to conduct a meta-analysis of all published studies in China from the English- and Chinese-language literature, to estimate HPV-18 prevalence detected in oesophageal cancer cases and the influence of different specimen types, detection methods and regions.
MATERIALS AND METHODS
Literature search strategies
We searched English- and Chinese-language databases including Medline (via PubMed), EMBASE, Chinese National Knowledge Infrastructure (CNKI) and Wanfang Data Knowledge Service Platform. Date of the literature was specified between 1 January 2005 and 12 July 2014. Combinations of key words ‘human papillomavirus’, ‘papillomavirus infections’, ‘(o)esophageal neoplasms’, ‘(o)esophageal cancer’ and ‘(o)esophageal carcinoma’ were used to screen for potentially relevant studies. Additional studies were also identified using cross-referencing.
Inclusion and exclusion criteria
All papers were reviewed by two authors independently. The inclusion criteria were (a) to inform at least 30 cases of oesophageal cancer confirmed by biopsy or histopathology, (b) to use PCR-based methods (including broad-spectrum PCR primers, type-specific PCR primers, or a combination of both kinds of primers) to amplify HPV DNA, (c) to report the type-specific HPV (type-18) prevalence in cancer tissue samples.
Studies were excluded if (a) they were not conducted in the Chinese population, (b) were animal or cellular studies, or (c) necessary data could not be extracted or calculated directly from the original article. If results based on the same study population were reported in more than one study, the one published earlier or containing more detailed information was included. Review articles and editorials were included if they contained original data. Abstracts were excluded.
Data extraction
The following information from studies included were extracted using a standardized data collection form: the name of first author, year of publication, geographical areas of the study origin, numbers of cases and HPV-positive cases, HPV detection method, types of specimen [paraffin-embedded fixed biopsies (PE), fresh or frozen biopsies (FF)].
Statistical analysis
The variance of each prevalence estimate was calculated as pq/n, where p is the prevalence, q is 1 – p, and n is the number of oesophageal cancer cases [Reference Barendregt16]. Overall pooled point estimate and 95% confidence intervals (95% CI) for HPV-18 prevalence were calculated with the method of DerSimonian & Laird [Reference DerSimonian and Laird17] using the assumptions of a random-effects model, which incorporates between-study variability. For studies with multiple HPV-type infections (including HPV-18), the multiple HPV types were separated into different types and the HPV-18 type-specific prevalence represents types for cases with either single HPV-18 infection and multiple HPV-18 infections. With respect to studies reporting HPV prevalence equal to zero, we added 0·5 to both total cases and HPV-18-positive cases to ensure that statistical analysis ran without issue.
Cochrane's Q test (P < 0·10 indicated a high level of statistical heterogeneity) and I 2 (values of 25%, 50% and 75% corresponding to low, moderate and high degrees of heterogeneity, respectively) were used to assess the heterogeneity between eligible studies, which test total variation across studies that is attributable to heterogeneity rather than to chance [Reference Higgins and Thompson18]. Subgroup analyses for HPV-18 prevalence were subsequently performed according to the geographical areas of the study origin, HPV detection method and types of specimen. In the eligible studies, two studies contained different types of specimens and two studies contained different geographical areas of study origin. We treated these as separate studies and pooled them into appropriate groups when performing stratified analysis. Sensitivity analysis was also conducted to assess the influence of each individual study on the strength and stability of the meta-analytical results. Each time, one study in the meta-analysis was excluded in order to show that study's impact on the combined effect size. Statistical tests (Begg's adjusted rank correlation test and Egger's regression asymmetry test) for funnel plot asymmetry were performed to test any existing publication bias.
In this study, meta-analyses were performed using Stata version 12 for Windows (StataCorp LP, USA). A two-tailed P < 0·05 was considered statistically significant.
RESULTS
Systematic review and study characteristics
As shown in Figure 1, the search strategy generated 417 citations, of which 156 were considered of potential value and the full text was retrieved for detailed evaluation. A total of 134 of these 156 articles were subsequently excluded from the meta-analysis for various reasons (the majority of the studies were excluded because they did not report HPV-18 prevalence or not report HPV DNA on tissue). An additional three articles were excluded because of small sample size (<30). Finally, 19 studies (11 from the English literature and eight from the Chinese literature) were eligible and included in this systematic review and meta-analysis [Reference Liu19–Reference Liu37]. Individual characteristics of the included 19 studies are summarized in Table 1. Study sample sizes ranged from 31 to 347 oesophageal cancer cases (median = 59). Summing across studies, a total of 2556 oesophageal cancer cases were identified. As reported in Table 1, 11 (57·89%) studies used PE biopsies, six (31·58) studies used FF biopsies and two (10·53%) used them both. The majority of studies were conducted in East China (n = 11, 57·89%), with the remaining studies spanning two other regions of China as follows: four (21·05%) studies in the Northeast, two (10·53%) studies in the South and two (10·53%) studies included populations from more than one region. Moreover, 12 (63·16%) studies adopted the detected gene from the L1 region of HPV, and seven (36·84%) adopted the detected gene from the E6/E7 region of HPV.
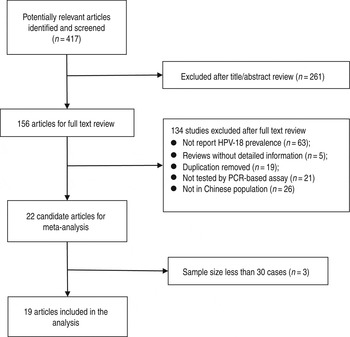
Fig. 1. Flow diagram of systematic literature search on HPV-18 infection in oesophageal cancer.
Table 1. Studies included in the meta-analysis and their characteristics

PE, Paraffin-embedded fixed biopsies; FF, fresh or frozen biopsies.
Meta-analysis of HPV-18 prevalence in oesophageal cancer cases
Individual and pooled prevalence estimates derived from a random-effects model analysis have been illustrated in a forest plot (Fig. 2). In this study, the HPV-18 prevalence ranged from 0·0% to 26·1%. The pooled prevalence rate was 4·1% (95% CI 2·7–5·5) for HPV-18 in oesophageal cancer cases in the Chinese population. Overall, there was high heterogeneity observed across the included studies (Q test P heterogeneity < 0·0001, I 2 = 87·1%).
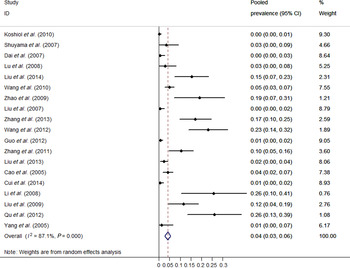
Fig. 2. Forest plot of meta-analysis on HPV-18 prevalence in oesophageal cancer tissue.
As shown in Table 2, the pooled HPV-18 prevalence was 6·7% (95% CI 4·5–9·0) in East China, 5·0% (95% CI 0·0–16·2) in South China, and 1·0% (95% CI 0·3–1·8) in Northwest China.
Table 2. HPV-18 prevalence in oesophageal cancer lesion across specimen, region and detection method

CI, Confidence interval; PE, paraffin-embedded fixed biopsies; FF, fresh or frozen biopsies.
Stratified analysis by specimen types showed that oesophageal cancer in FF tissue has the higher HPV-18 prevalence (6·1%, 95% CI 2·9–9·3) compared to PE tissue (4·0%, 95% CI 2·3–5·8). With respect to HPV-18 detection methods, the prevalence of oesophageal cancer by the E6/E7 region of the HPV gene (8·2%, 95% CI 4·6–11·7) was observed to be significantly higher than that of the L1 region of the HPV gene (2·2%, 95% CI 0·9–3·6).
Influence of analysis of individual studies
To address the potential bias due to the quality of the included studies, we performed sensitivity analysis by calculating pooled HPV-18 prevalence again while omitting one study at a time. Figure 3 shows the results of the sensitivity analysis. HPV-18 prevalence ranged from 3·5% (95% CI 2·2–4·8) to 5·1% (95% CI 3·4–6·8). The meta-analysis result of the pooled HPV-18 prevalence in oesophageal cancer cases was not significantly affected by omission of any of the 19 individual studies analysed, which indicated that each single study did not influence the stability of the overall HPV-18 prevalence estimate.

Fig. 3. Sensitive analyses for individual studies on the summary effect.
Publication bias
There was no evidence of publication bias as demonstrated by the non-significant P values for Begg's (0·276) and Egger's (0·196) tests.
DISCUSSION
China has the highest burden of oesophageal cancer globally, which was our rationale for studying the type-specific HPV (type-18) prevalence in China for this highly lethal cancer. To our knowledge, this is the first meta-analysis aimed at exploring the prevalence of HPV-18 in oesophageal cancer tissue in China. Results of this meta-analysis show that >4% of oesophageal cancer cases harboured HPV-18, which is lower than for those of cervical cancer (15·3%) [Reference Clifford38], ovarian cancer (12·2%) [Reference Rosa39], laryngeal cancer (6·2%) [Reference Li40], bladder cancer (5·91%) [Reference Li41] and lung cancer (5·6%) [Reference Srinivasan, Taioli and Ragin42].
To characterize the prevalence of HPV-18 in cases of oesophageal cancer is an important preliminary step in assessing the association between HPV-18 and cancer of the oesophagus. Estimates of HPV prevalence in cases of oesophageal cancer vary widely, even within the same country. The present meta-analysis showed that East China (6·7%) and South China (5·0%) had higher HPV-18 prevalence than Northwest China (1·0%). However, the observed differences in HPV-18 prevalence by geographical location warrant further attention because the included studies were mostly conducted in high-incidence regions and some regions only covered one or two cities. More cases involved and more cities covered would be useful for estimating the prevalence of HPV-18 in different parts of China in the future.
The detection rate of HPV-18 DNA in FF tissue was found to be somewhat higher than that in PE tissue, which could be explained as significant DNA degradation in PE tissue [Reference Srinivasan, Taioli and Ragin42]. Status of HPV infection was determined for PE tissue in the majority of included studies. The low rate of detection of HPV DNA is known to occur with the fabric of PE, especially when long DNA fragments are amplified.
A few possible reasons for HPV prevalence variation between studies include different HPV detection assays, small study sizes, inter-laboratory variability, and suboptimal sample collection and handling leading to contamination [Reference Kamangar43–Reference Hubbard45]. In this meta-analysis, HPV detection methods were limited to PCR, which eliminated the variation caused by the HPV detection method. PCR is an important tool because it allows the in vitro proliferation of unique DNA regions so that they can be detected in a large background, such as is the case with most viral infections. In addition, for better sensitivity and specificity of PCR, the time of the literature was specified from 1 January 2005 to 12 July 2014. HPV is a double-stranded circular DNA virus with a genome size of about 8000 bp that encodes early proteins (E1, E2, E5, E6, E7) and late proteins (L1, L2, E4) [Reference Garcia-Vallve, Alonso and Bravo46, Reference Munger and Howley47]. When stratified by L1 and E6/E7 gene segments based on PCR, we found that the detection rate of HPV-18 DNA in the E6/E7 gene segment (8·2%) was much higher than in the L1 gene segment (2·2%). This is mainly because of the disruption of the L1 region due to the integration of HPV into the host genome [Reference Hebner and Laimins48], which may be an important event that promotes and initiates oesophageal carcinogenesis.
Highlights from the meta-analysis include large overall sample size, inclusion of studies published in both English and Chinese languages, strict inclusion strategy, and exclusion of case reports or studies with small sample size (<30). By including studies in both English and Chinese, we avoided selection bias due to publication language. Moreover, by restricting studies to those published after 2005 and limiting detection methods to PCR, we tried to reduce HPV prevalence variation as much as possible. Finally, with the exclusion of case reports and studies with <30 cases, we tried to exclude studies that were a convenience sample or not representative of all oesophageal cancer cases.
However, the present meta-analysis has several limitations. First, the studies included in this meta-analysis are heterogeneous, which could be explained by changes in the population, the method of sample collection, and the sensitivity of different protocols of HPV primer PCR. To address this issue, random-effects model meta-analysis was reported to combine data whenever significant heterogeneity was noted. We directly examined heterogeneity by describing the prevalence of HPV in oesophageal cancer cases by geographical locations, type of specimen and DNA source. Of course, we were not fully able to explain the heterogeneity as heterogeneity still existed in most subgroups. Prevalence estimates remained heterogeneous even in stratified results (e.g. studies conducted in different regions of China). Second, bias could occur based on estimates from the study because the accuracy of these estimates depends on the detection method used and the HPV types evaluated. That is, some studies used broad primers or multiple probes to detect multiple types of HPV, while other studies only detected the HPV-18 type.
In summary, the current meta-analysis provides a quantification of the prevalence of HPV-18 in oesophageal cancer lesions in the Chinese population, although there is a variation between different variables, such as geographical areas of the study origin, HPV detection method and types of specimen. Although this study cannot give information on the aetiology of HPV and oesophageal cancer, it is an important step towards fully evaluating the relationship between HPV and oesophageal cancer in the Chinese population, and it could also give some indication of the effect of the HPV vaccine against oesophageal cancer. Further studies are needed to elucidate the role of HPV in oesophagus carcinogenesis with careful consideration of study design and laboratory detection method, which will provide more accurate assessment of HPV status in oesophageal cancer.
ACKNOWLEDGEMENTS
We are grateful to Le Wang, from China Cancer Institute and Hospital, Chinese Academy of Medical Sciences, for his support and assistance with this manuscript.
DECLARATION OF INTEREST
None.








