Pulses are defined by the FAO as legumes harvested solely for their seed which is consumed directly. The FAO list includes eleven primary pulses, including peas, and excludes the oilseed legumes and those consumed in immature form as vegetables(1). Peas, more specifically the yellow or green cotyledon varieties known as dry, smooth or field peas, are the naturally dried seeds of Pisum sativum L. and are grown around the world for human and animal consumption. World production of peas in 2009 was more than ten million tonnes, the major producers being Canada, the Russian Federation, China, the USA and India(2). Peas have long been recognised as an inexpensive, readily available source of protein, complex carbohydrates, vitamins and minerals. The high nutrient density of peas makes them a valuable food commodity, capable of meeting the dietary needs of the estimated 800–900 million undernourished individuals worldwide(3). The US Department of Agriculture My Plate Guidelines recommend consuming at least three cups of dry beans and peas per week(4). The majority of the US population consumes less than the recommended serving, with only 7·9 % of adults consuming dry beans or peas on any given day(Reference Mitchell, Lawrence and Hartman5).
In recent years, many studies have identified potential health benefits of pulses, including peas, beyond meeting basic nutrient requirements. The purpose of the present paper is to provide a comprehensive review of the demonstrated and potential health benefits associated with pea consumption. The nutrient composition (summarised in Table 1) (Reference Hood-Niefer, Warkentin and Chibbar6–Reference Dostálová, Horáček and Hasalová13) and phytochemical constituents of peas are described in order to provide context for the proposed mechanisms by which pea consumption benefits health. Limitations of current research and recommended future directions are discussed to encourage advancement in the field.
Table 1 Compositional data for peas (Pisum sativum L.)(Reference Hood-Niefer, Warkentin and Chibbar6–Reference Dostálová, Horáček and Hasalová13)
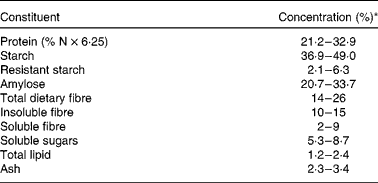
* Values are expressed on a moisture-free basis except for amylose, which is expressed on a starch basis.
Compositional information
Protein
Peas are a valuable source of protein for both man and animals. The protein content of peas may be influenced by both environmental conditions and genetic factors(Reference Hood-Niefer, Warkentin and Chibbar6, Reference Bourgeois, Jacquin and Casseculle14). Tzitzikas et al. (Reference Tzitzikas, Vincken and DeGroot15) found that the concentration of protein in fifty-nine pea lines ranged from 13·7 to 30·7 % of seed DM, with an overall average of 22·3 %. Hood-Niefer et al. (Reference Hood-Niefer, Warkentin and Chibbar6) reported an effect of environment on the concentration of protein in peas, but observed a narrow range in protein concentration (24·2–27·5 % on a moisture-free basis) in ten genotypes grown in four locations in Saskatchewan, Canada, over two growing seasons.
The chemical and physico-chemical characteristics, processing and use of proteins from pulses, including peas, were reviewed recently by Boye et al. (Reference Boye, Zare and Pletch16). The majority of pea proteins are storage proteins, or globulins, and the amino acid profile of these proteins determines their nutritional value(Reference Bourgeois, Jacquin and Casseculle14, Reference Boye, Zare and Pletch16). Tömösközi et al. (Reference Tömösközi, Lásztity and Haraszi17) compared the amino acid compositions of flour and protein concentrates and isolates from peas with corresponding products from lupin and soyabeans. The amino acid profiles of all products were similar overall, with the greatest contributions from glutamine, followed by aspartic acid, arginine and lysine, and the lowest contributions from methionine, tryptophan and cysteine. Products from peas tended to be higher in arginine, valine and methionine, and lower in glutamic acid and cysteine, than those from lupin and soyabeans. Relative to human requirements(18), the protein in peas and other pulses is rich in lysine and marginal or deficient with respect to methionine. The in vitro digestibility of raw pea protein is reduced by the presence of protease inhibitors, although the digestibility of pea protein has been reported to be higher than that of soyabean and several other pulses(Reference Boye, Zare and Pletch16, Reference Roy, Boye and Simpson19). The ability of peas to improve CVD and promote weight loss may be attributable to their high protein content(Reference Abete, Parra and Martinez20). The bioactive proteins and peptides of several pulses, including peas, were reviewed recently by Roy et al. (Reference Roy, Boye and Simpson19). In that review, the negative physiological and nutritional effects of lectins and protease inhibitors in pulses are described, as are the potential nutraceutical effects of lectins, which include anticancer and immunomodulatory properties. Hydrolysis of pea and other pulse proteins generates peptides with a variety of bioactivities in vitro, including angiotensin I-converting enzyme inhibitor activity, which has an antihypertensive effect, and antioxidant activity(Reference Roy, Boye and Simpson19).
Complex carbohydrates
Starch and fibre are major components of peas, 46 and 20 % of seed DM, respectively, on average(Reference Tzitzikas, Vincken and DeGroot15). The chemical attributes and functional characteristics of starch and fibre in pulses, including peas, were reviewed by Hoover et al. (Reference Hoover, Hughes and Chung21) and Tosh & Yada(Reference Tosh and Yada7), respectively.
Starch is composed of amylose, a linear glucan with few branches, and amylopectin, a larger and more highly branched molecule. The ratio of amylose to amylopectin influences the digestibility of starch and thus its impact on the postprandial glucose response(Reference Tahir, Ellis and Bogracheva22). Pea starch, like that of most other starchy pulses, contains an intermediate level of amylose, which is reflected in its unique functionality and its higher levels of enzyme-resistant starch and slowly digestible starch (as compared with cereal, root and tuber starches, most of which are lower in amylose)(Reference Hoover, Hughes and Chung21). The relatively low degree of digestibility of starch in pulses has also been attributed to the non-availability to amylases of starch granules enclosed in intact cell wall structures, the presence of anti-nutrients such as amylase inhibitors, phytates and phenolics, and their significant content of dietary fibre(Reference Chung, Liu and Hoover23). Perera et al. (Reference Perera, Meda and Tyler24) concluded that variety, processing method and analytical methodology all affected starch digestibility, specifically levels of resistant starch, in peas and other pulses. Flours from three pea genotypes contained 9·2–10·7, 23·3–26·5 and 10·1–14·7 % of rapidly digestible starch, slowly digestible starch and resistant starch, respectively(Reference Chung, Liu and Hoover25). Starch isolated from the same three genotypes consisted of 18·2–23·8, 53·7–59·0 and 8·1–12·6 % of rapidly digestible starch, slowly digestible starch and resistant starch, respectively(Reference Chung, Liu and Donner26). The proportion of the starch in peas that is slowly digestible is noteworthy. Annealing and heat-moisture treatment of pea starch had variable effects on the in vitro digestibility of pea starch(Reference Chung, Liu and Hoover23, Reference Chung, Liu and Hoover27). The effect of treatment on starch digestibility was variety dependent, and with all treatments, gelatinisation of starch converted essentially all of the slowly digestible starch, and in some cases most of the resistant starch, to rapidly digestible starch.
The amylose content of pea starch has been reported to vary widely among varieties and mutant lines(Reference Guillon and Champ28). Starch from wrinkled peas, which are technically not pulses since they are consumed in immature form as a vegetable, contains 60 % or more of amylose(Reference Hoover, Hughes and Chung21). Wrinkled pea starch has been reported to contain 76·8 % amylose and 4·5–17·7 % of resistant starch, as compared with 27·8 % amylose and 2·1–6·3 % resistant starch in smooth peas, with the concentration of both amylose and resistant starch dependent on variety and the growth environment(Reference Dostálová, Horáček and Hasalová13). Hood-Niefer et al. (Reference Hood-Niefer, Warkentin and Chibbar6) saw no effect of variety or environment on the amylose content of pea starch. However, this study did not include wrinkled pea varieties.
Dietary fibre in peas arises from both the seed coat (outer fibre), commonly referred to as the hull, and the cotyledon (inner fibre). The seed coat contains largely water-insoluble polysaccharides, primarily cellulose, whereas the cotyledon fibre consists of polysaccharides having various degrees of solubility, including hemicelluloses and pectins, along with cellulose(Reference Tosh and Yada7, Reference Guillon and Champ28, Reference Reichert and MacKenzie29).
The properties of both their starch and fibre constituents make peas a low-glycaemic index food, and hence beneficial in the prevention and management of type 2 diabetes(Reference Trinidad, Mallillin and Loyola30). In addition, fibre may reduce blood cholesterol by decreasing the reabsorption of bile acids(Reference Chen, Anderson and Jenkins31). Peas, like other legumes, contain significant concentrations of raffinose-family and other galactose-containing oligosaccharides(Reference Tosh and Yada7) which may exert prebiotic effects in the large intestine(Reference Fernando, Hill and Zello32).
Vitamins and minerals
Reichert & MacKenzie(Reference Reichert and MacKenzie29) determined the concentrations of the major minerals in four pea samples. Potassium (1·04 % of dry, dehulled weight) was found to be the most prominent element, followed by P (0·39 %), Mg (0·10 %) and Ca (0·08 %). The quantities of seven trace minerals also were measured. The pea samples contained an average of 97 parts per million (ppm) Fe, 42 ppm Se, 41 ppm Zn, 12 ppm Mo, 11 ppm Mn, 9 ppm Cu and 4 ppm B. Gawalko et al. (Reference Gawalko, Garrett and Warkentin33) determined that yellow peas from Canada contained higher levels of Fe, Mg and Mn, but lower levels of K, compared with green peas. In the same study, Se was found to exceed the maximum residue level established by the People's Republic of China in 56 % of the samples analysed. However, Se is considered an essential element, and this maximum residue level value is currently being re-evaluated. The authors suggested that peas produced in Canada may be beneficial for areas of the world where Se deficiency is prominent.
Despite the high mineral content of peas, bioavailability may be poor due to high phytate concentrations. Sandberg(Reference Sandberg34) reported that phytate acts as an inhibitor of Zn, Fe and Ca absorption. A study by Trinidad et al. (Reference Trinidad, Mallillin and Loyola30) found that phytate content affected Fe but did not influence Zn and Ca availability in pulses. In fact, these authors concluded that when Fe availability was low, Ca and Zn availability was high. The study also reported that peas have greater in vitro Ca bioavailability compared with other pulses. More research should be carried out to understand the effects of food processing techniques on phytate degradation. If phytate is degraded, peas could be considered a significant source of Ca, Zn and Fe(Reference Sandberg34).
Dang et al. (Reference Dang, Arcot and Shrestha35) reported that peas contained 101 μg folate per 100 g. Han & Tyler(Reference Han and Tyler36) determined that the concentration of folate in two yellow pea genotypes grown in six locations in 1 year in Saskatchewan, Canada, ranged from 23·7 to 55·6 μg/100 g DM, as determined by a microbiological assay; concentrations of folate in two green pea genotypes grown in three locations in each of two growing seasons ranged from 24·9 to 64·8 μg/100 g DM. Low dietary folate levels have been associated with anaemia and neural tube defects in humans(Reference Dang, Arcot and Shrestha35, Reference Han and Tyler36).
Phytochemicals
The concentrations of minor constituents in pulses, including peas, and their potential impacts on human health were reviewed recently by Campos-Vega et al. (Reference Campos-Vega, Loarca-Pina and Oomah37). Peas, like other pulses, contain a variety of phytochemicals, including phenolic compounds, phytates, saponins and oxalates. The major phenolic constituents in pulses are tannins, phenolic acids and flavonoids(Reference Campos-Vega, Loarca-Pina and Oomah37). Phenolic compounds have been recognised for their ability to act as antioxidants and are the best characterised phytochemicals in peas. Peas contain a variety of phenolics, with the highest concentrations of most occurring in the seed coat, particularly in dark-seeded varieties(Reference Campos-Vega, Loarca-Pina and Oomah37–Reference Xu, Yuan and Chang40). Accordingly, Xu et al. (Reference Xu, Yuan and Chang40) determined that the antioxidant activity of pea varieties was correlated significantly with seed coat colour. Examination of the seed coat and cotyledon in two dark-coloured pea varieties revealed that the seed coat contained glycosides of quercetin, luteolin and apigenin, along with a variety of simple phenolics and proanthocyanadins. The cotyledon contained mainly hydroxybenzoic and hydroxycinnamic compounds and some of the glycosides found in the seed coat(Reference Dueñas, Estrella and Hernandez39).
Peas contain other minor constituents which exhibit bioactivity and which may have positive benefits on human health, including saponins and phytates, which may exhibit hypocholesterolaemic and anticarcinogenic activities(Reference Campos-Vega, Loarca-Pina and Oomah37).
Evidence for health outcomes
Epidemiological, in vitro and interventional studies all have demonstrated the role of peas and pea constituents in maintaining metabolic, cardiovascular and gastrointestinal health in humans. Table 2 summarises the clinical evidence.
Table 2 Clinical studies related to the metabolic, cardiovascular and gastrointestinal health outcomes of peas

WPF, whole pea flour; FPF, fractionated pea flour; NCEP, American Heart Association's National Cholesterol Education Program; HOMA-IR, homeostatic model assessment of insulin resistance; IAUC, incremental area under the curve.
Glycaemic response and insulin resistance
Due to their high fibre content, peas may mediate the glycaemic response as compared with low-fibre foods with equal carbohydrate proportions. A randomised controlled study by Marinangeli et al. (Reference Marinangeli, Kassis and Jones41) investigated the use of whole yellow pea flour to create foods with a lower glycaemic index than comparable foods made from wholewheat flour. The results demonstrated that foods made with whole yellow pea flour reduced postprandial glucose responses in individuals and, thus, may have a role in the management of type 2 diabetes.
Marinangeli & Jones(Reference Marinangeli and Jones42) compared the use of whole pea flour (WPF) and fractionated pea flour (FPF; pea hulls) on insulin resistance. WPF and FPF reduced fasting insulin levels by 13·5 and 9·8 %, respectively, compared with baseline. Homeostatic model assessment of insulin resistance (HOMA-IR), a method used to quantify insulin resistance and β-cell function, revealed that insulin resistance was reduced by 25 % in both the WPF and the FPF groups compared with the control group receiving white wheat flour. HOMA-IR showed no difference in β-cell function among groups.
A study by Seewi et al. (Reference Seewi, Gnauck and Stute43) compared the use of yellow pea flour and pea starch with maize starch on glycaemic response and found a benefit with both pea flour and pea starch. Lunde et al. (Reference Lunde, Hjellset and Holmboe-Ottesen44) found that bread containing 17 % pea hull fibre significantly reduced glycaemic response; however, the fibre breads also contained higher protein.
Cardiovascular health
Fibre-rich diets have been shown to lower blood pressure, improve serum lipid levels and reduce indicators of inflammation(Reference Slavin45). Sandström et al. (Reference Sandström, Hansen and Sørensen46) investigated the effect of fibre preparations made from pea cell wall fibre on cardiovascular health. Subjects placed on the pea fibre diet showed a trend for lower postprandial TAG responses compared with subjects on a low-fibre diet matched in macronutrient content. However, no changes were seen in fasting lipid concentrations. In a randomised, cross-over intervention study, Trinidad et al. (Reference Trinidad, Mallillin and Loyola30) found no differences in serum total, LDL- or HDL-cholesterol after 2 weeks of consumption of cooked, cooled peas. The failure to affect serum cholesterol may have been due to the short length of follow-up (2 weeks). Other studies of mixed legume diets have demonstrated significant improvements in markers of cardiovascular health(Reference Abete, Parra and Martinez20, Reference Hermsdorff, Zulet and Abete47, Reference Hunninghake, Miller and LaRosa48).
Weight management
The impacts of a hypoenergetic diet rich in various legumes have been investigated(Reference Abete, Parra and Martinez20, Reference Hermsdorff, Zulet and Abete47). However, little research relating pea intake to weight control has been undertaken. Lang et al. (Reference Lang, Bellisle and Oppert49) showed no effect of pea protein on satiety, 24 h energy or macronutrient intakes, or on postprandial plasma glucose and insulin concentrations when compared with egg albumin, casein, gelatin, soya protein and wheat gluten. Lunde et al. (Reference Lunde, Hjellset and Holmboe-Ottesen44) found that pea fibre-enriched bread increased duration of satiety, when compared with intake of regular bread. Research is needed to understand how peas specifically may influence weight management, with body weight, BMI or waist circumference as primary endpoints.
Gastrointestinal function and homeostasis
The effects of peas and pea fractions on gastrointestinal function and symptoms have been investigated. Dahl et al. (Reference Dahl, Whiting and Healey50) demonstrated that the addition of 4 g pea hull fibre per d resulted in a significant increase in bowel movement frequency in residents of a long-term care facility, particularly in those with the lowest frequency. Flogan & Dahl(Reference Flogan and Dahl51) showed that the addition of pea hull fibre to snack foods, in combination with an inulin fibre supplement, provided to children with constipation significantly increased bowel movement frequency; no adverse symptoms were reported. Veenstra et al. (Reference Veenstra, Duncan and Cryne52) investigated the effect of consuming 100 g dry weight of peas per d for 4 weeks and found no differences in bowel movement frequency or perceived flatulence, bloating, cramping and intestinal discomfort compared with potatoes, chickpeas and lentils, with the exception of increased cramping in the early phase of the treatment with peas. Seewi et al. (Reference Seewi, Gnauck and Stute43) compared the use of yellow pea flour and pea starch and found that pea starch caused less flatulence in study participants and was more tolerable than pea flour.
Although peas contain potential prebiotic oligosaccharides as well as resistant starch and fermentable fibre, limited research has been carried out on the effects of consumption of peas and pea fractions on gastrointestinal microbiota and related health outcomes. Pea proteins often undergo spontaneous glycosylation during storage and processing due to the high concentration of lysine. A study by Swiatecka et al. (Reference Swiatecka, Kostyra and Swiatecki53) demonstrated that glycosylated pea proteins may escape enzymic breakdown early in the small intestine and may have an impact on the homeostasis of the large intestine by modulating the activity of the microbiota. Dominika et al. (Reference Dominika, Arjan and Karyn54) used human gastrointestinal tract simulators to predict the effects of glycosylated pea proteins on intestinal bacteria. Results of the study demonstrated a significant increase in autochthonic bacteria (Bacteroides, Lactobacillus and Bifidobacterium) and a subsequent increase in their metabolic activity and production of SCFA. Researchers concluded that pea proteins could be used to improve intestinal microbiota homeostasis. Research is needed to explore the potential impacts of consumption of peas and pea fractions on gastrointestinal microbiota and wellness.
Antioxidant activity
Phenolic compounds are considered natural antioxidants that may help protect against diseases such as cancer and various inflammatory-related diseases. Dueñas et al. (Reference Dueñas, Estrella and Hernandez39) confirmed the presence of phenolic compounds in the seed coat and cotyledon of peas. A study by Troszynska & Ciska(Reference Troszynska and Ciska38) compared the phenolic composition and antioxidant activity of white and coloured peas. Phenolic acids were found in both free and esterified form in both white and coloured peas, but higher concentrations were seen in the coloured varieties. Condensed tannins, which have been shown to have very high antioxidant activity(Reference Hagerman, Riedl and Jones55), were detected only in the coloured seed coats. The phenolic compounds were extracted with acetone and methanol, and the liposome system was used to measure antioxidant activity via the extent of peroxidation of phosphatidyl choline. The antioxidant activity in the acetone extract from the coloured seed coats was significantly higher than in the white coat extract. These properties were slightly altered by cooking the seeds for 30, 60 or 90 min. More research should be done to investigate the heat stability of polyphenols in peas.
Current research on the antioxidant activity of peas is limited to in vitro studies. Intervention studies are needed to investigate the efficiency of pea antioxidant activity in providing health benefits to humans.
Limitations of current knowledge and future directions
Current research on the health benefits of peas does not adequately address long-term consumption. Future studies should address the differences between acute and chronic consumption. There is also a lack of long-term studies with large, diverse (ethnicity, sex, age, etc.) subject populations.
Currently there is limited understanding of how food processing methods affect the physiochemical properties of peas, as well as a need for research looking at the effects of various pea fractions (i.e. fibre, protein, starch) on relevant health outcomes. In addition, further research is needed to identify whether different genotypes of peas are more effective in achieving the specific health benefits discussed in the present paper.
Summary
The present review briefly describes the nutritional characteristics of peas, along with demonstrated and potential health benefits associated with their consumption. Although some health benefits, such as improved gastrointestinal function and reduced glycaemic index, have been documented, others require further research.
Acknowledgements
The authors declare no conflicts of interest and no funding was received for the preparation of this paper. All authors contributed substantially to the preparation of the manuscript.




