Introduction
Tropical rainforests represent one of the most biologically diverse biomes but also one of the most threatened (Vancutsem et al., Reference Vancutsem, Achard, Pekel, Vieilledent, Carboni and Simonetti2021). Although people have been interacting with and modifying tropical rainforests for millennia, the intensity and scope of interaction and modification have increased substantially in recent decades (Malhi et al., Reference Malhi, Gardner, Goldsmith, Silman and Zelazowski2014). Human-induced land-use change because of agricultural expansion, logging, mining and fire, and concomitant global climate change, have resulted in substantial loss and degradation of habitat for forest-dwelling wildlife (e.g. primates; Supriatna et al., Reference Supriatna, Shekelle, Fuad, Winarni, Dwiyahreni and Farid2020).
Habitat is understood as the resources and conditions that enable organisms to survive and reproduce in a given area (Hall et al., Reference Hall, Krausman and Morrison1997). A principal strategy for protecting habitats for wildlife is to safeguard primary forests, generally as some type of designated protected area (e.g. national parks). The conservation value of primary forests is undisputed; they support high levels of biodiversity, play a critical role in storing carbon and provide numerous other ecosystem services (Gibson et al., Reference Gibson, Lee, Koh, Brook, Gardner and Barlow2011). However, given the unprecedented rate and extent of human-induced landscape change in the current era, many wildlife species occur outside protected areas in anthropogenic landscapes (Spehar & Rayadin, Reference Spehar and Rayadin2017; Santini et al., Reference Santini, González-Suárez, Russo, Gonzalez-Voyer, von Hardenberg and Ancillotto2019). This reality has led to a rethinking of the potential conservation value of anthropogenic landscapes, thereby expanding the range of forests considered to be valuable for wildlife (Chazdon et al., Reference Chazdon, Peres, Dent, Sheil, Lugo and Lamb2009; Meijaard et al., Reference Meijaard, Albar, Nardiyono, Rayadin, Ancrenaz and Spehar2010; Yabsley et al., Reference Yabsley, Meade, Martin and Welbergen2021; Nurvianto et al., Reference Nurvianto, Adriyanti, Hamdan, Triyanto and Darmonto2022). It has also bolstered efforts to assess the habitat that anthropogenically modified forests provide and to evaluate how to make them more hospitable for wildlife moving forward (Sodhi et al., Reference Sodhi, Koh, Clements, Wanger, Hill and Hamer2010; Galán-Acedo et al., Reference Galán-Acedo, Arroyo-Rodríguez, Andresen, Verde Arregoitia, Vega, Peres and Ewers2019; Tuyisingize et al., Reference Tuyisingize, Eckardt, Caillaud, Ngabikwiye and Kaplin2022).
One approach to assessing habitat quality is to measure variables associated with the species in question, such as quantifying the abundance and population density of a species in a given area or across areas (Lee et al., Reference Lee, Powell and Lindsell2015; but see Irwin, Reference Irwin, Wich and Marshall2016, for caveats with this approach) or by examining the patterns of habitat and range use of species (Gabriel, Reference Gabriel2013). Another approach is to measure attributes of the habitat itself (e.g. forest structure and composition) and to estimate potential food availability (Bryson-Morrison et al., Reference Bryson-Morrison, Matsuzawa and Humle2016; Zhang & Zang, Reference Zhang and Zang2018). The nutritional quality of available resources, which includes the balance of nutrients available, is also important to consider because animals consume foods for the nutrients within them and these nutrients could vary both within and between species in a given area, which could have consequences for animal fitness (Hanya & Chapman, Reference Hanya and Chapman2013; DeGabriel et al., Reference DeGabriel, Moore, Felton, Ganzhorn, Stolter and Wallis2014). For example, one study found that the quality of foods in different Bornean orangutan Pongo pygmaeus wurmbii habitats varied and the authors speculated that this nutritional difference affected orangutan density (Vogel et al., Reference Vogel, Harrison, Zulfa, Bransford, Alavi and Husson2015). Disturbed forests typically contain less food than intact forests, and because there are fewer food items to choose from, the overall nutritional quality of the foods available could be lower (Raubenheimer et al., Reference Raubenheimer, Simpson and Tait2012). For example, in a study of Peters’ Angolan colobus Colobus angolensis palliatus, females consumed less metabolizable energy and fewer macronutrients in disturbed forest than in intact forest (Dunham & Rodriguez-Saona, Reference Dunham and Rodriguez-Saona2018).
The moor macaque Macaca maura, which is categorized as Endangered on the IUCN Red List (Riley et al., Reference Riley, Lee, Sangermano, Cannon and Shekelle2020), is endemic to Sulawesi, Indonesia, a globally important conservation area (Supriatna et al., Reference Supriatna, Shekelle, Fuad, Winarni, Dwiyahreni and Farid2020). Moor macaques live in multimale–multifemale social groups and are primarily frugivorous, with figs comprising a large portion of their diet (Albani et al., Reference Albani, Cutini, Germani, Riley, Ngakan and Carosi2020). This primate has been observed outside protected areas including in heavily modified forests (Zak & Riley, Reference Zak and Riley2017), but little is known about the quality of these habitats for this threatened species. We aimed to address this gap in knowledge. Here, we compare aspects of the habitat quality of two areas known to be occupied by moor macaque populations (Matsumura, Reference Matsumura1998; Beltrán-Francés et al., Reference Beltrán Francés, Spaan, Amici, Maulany, Putu Oka and Majolo2022): the Karaenta Forest and the Education Forest. The two forests are affected by human modification to varying degrees and differ in their level of protection. Portions of the Karaenta Forest experienced human modification in the past, but the entire area has been formally protected and subject to minimal human disturbance since 1980. This forest is currently part of a national park. The Education Forest is a heavily modified forest dominated by non-native species and is not formally protected.
We defined habitat quality operationally in terms of forest composition attributes (richness and diversity of tree species used as food sources by the macaques), forest structure attributes (density, basal area, diameter at breast height and ecological importance of macaque food trees) and the nutritional quality of available foods. Given that anthropogenic disturbance has been shown to reduce the quality of primate habitats (Irwin et al., Reference Irwin, Raharison, Raubenheimer, Chapman and Rothman2015), we expected habitat quality to be greater in the Karaenta Forest compared to the heavily modified Education Forest. Specifically, we predicted that species richness, density, diversity, basal area and mean diameter at breast height of food trees would be higher in the Karaenta Forest compared to the Education Forest. We also predicted that concentrations of macronutrients (energy, protein, fat) in available fruits would be higher in the Karaenta Forest than in the Education Forest. We focused on fruits because they comprise the majority of the moor macaque's diet (Albani et al., Reference Albani, Cutini, Germani, Riley, Ngakan and Carosi2020). We also expected the balance of macronutrients in fruits sampled from each forest to vary. Given that both fats and sugars contribute to the non-protein energy in animal diets, we were interested in the relative balances of these nutrients within fruits, particularly because fat contains more calories per weight than sugars (National Research Council, 2003).
Study area
The two study sites are located in South Sulawesi, Indonesia (Fig. 1). The Karaenta Forest was established as a 1,000-ha strict nature reserve in 1980; it was later subsumed within the boundaries of Bantimurung Bulusaraung National Park upon the gazetting of the latter. The Karaenta Forest is dominated by primary karst forest interspersed with mature secondary karst forest (> 40 years of age). Portions of the Karaenta Forest were once used by local communities for shifting cultivation. When this area was being gazetted as a strict nature reserve, villagers were forced to abandon their agricultural plots and the area was allowed to experience natural succession. In some parts of the Karaenta Forest, native ebony trees Diospyros celebica were planted to accelerate the succession process. Sugar palm Arenga pinnata and candlenut Aleurites moluccana, which were originally planted by villagers, remain today as part of the secondary forest.
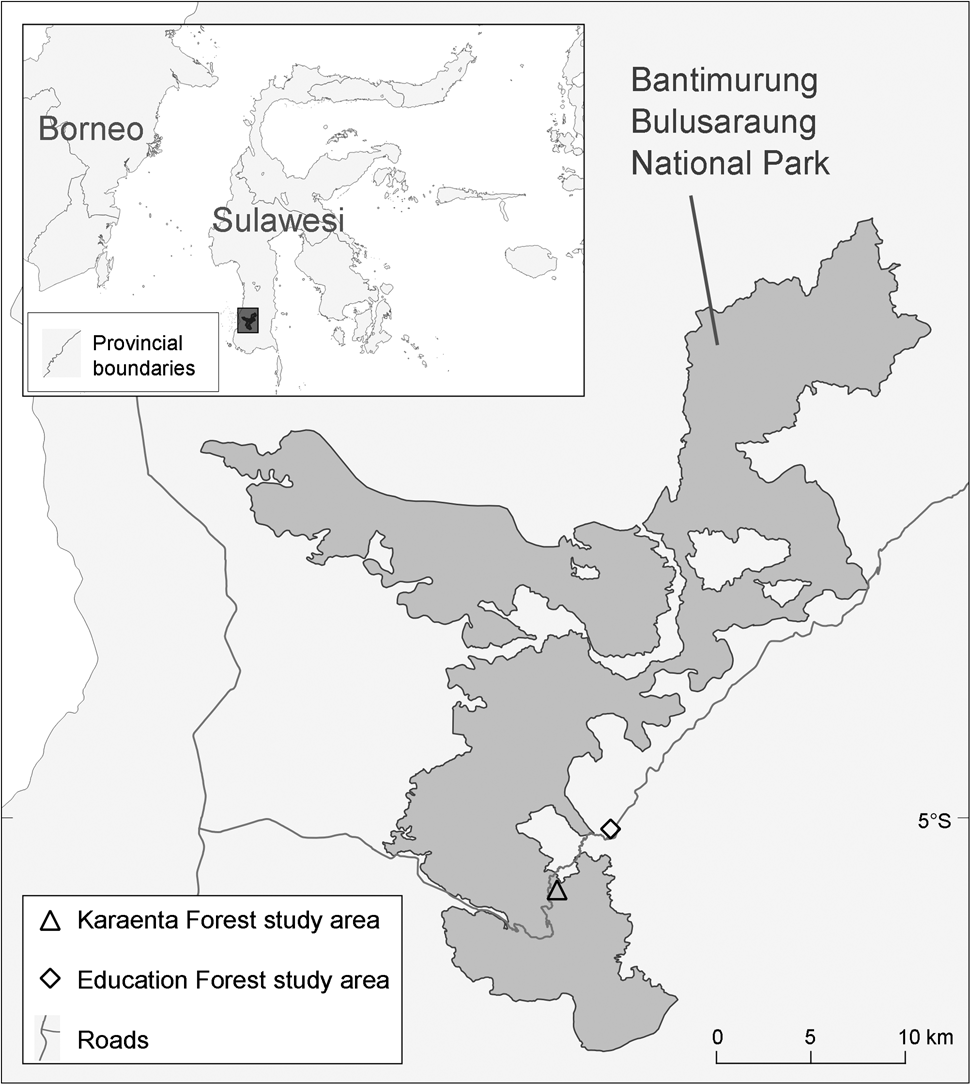
Fig. 1 Locations of the Karaenta Forest and Education Forest study areas in South Sulawesi, Indonesia. The Karaenta Forest is located in Bantimurung Bulusaraung National Park.
The Education Forest is a state forest (hutan negara), c. 1,300 ha in size, that borders the village of Bengo in the Cenrana district. This land, which was originally forested, experienced a long history of human modification, primarily forest clearing for shifting cultivation by local communities. In the late 1960s and early 1970s, the Indonesian Directorate General of Forestry initiated restoration efforts that involved the planting of non-native tree species such as Sumatran pine Pinus merkusii, which now dominates the land, acacia (mainly Acacia auriculiformis) and Honduran mahogany Swietenia macrophylla. Patches of young (broad leaf) secondary forest (< 50 years of age) also occur in this matrix landscape. In the secondary forest bordering settlements, villagers have planted candlenut, wild mangoes Mangifera lanceolate, jackfruit Artocarpus heterophyllus, ebony and sugar palm. The Education Forest is currently managed by the Faculty of Forestry of Hasanuddin University, Makassar, as a teaching resource and research site, and is hence afforded some protection from further large-scale human disturbance.
Methods
Forest composition and structure
We assessed forest composition and structure by analysing vegetation data collected from 67 vegetation plots (each measuring 10 × 20 m): 34 at the Karaenta Forest (0.68 ha) and 33 at the Education Forest (0.66 ha), which were established as part of two separate studies conducted at these sites (Zak, Reference Zak2016; Albani, Reference Albani2017). We selected plot locations using purposive sampling, ensuring they were located in areas used by resident macaque groups. In each plot we recorded the diameter at breast height (1.3 m above ground level) of all trees for which this diameter was ≥ 5 cm. We chose a threshold of 5 cm for diameter at breast height rather than the standard 10 cm because we observed reproductive parts on young trees. If a tree with a main stem of ≥ 5 cm diameter at breast height had multiple stems, we measured each additional stem regardless of its diameter at breast height. We collected, dried and sent plant samples to the herbarium at Hasanuddin University or to Herbarium Bogoriense in Bogor, Java, for species identification. We collected vegetation data during October 2014–January 2015 in the Karaenta Forest and during July–August 2014 in the Education Forest.
Sampling of macaque foods for nutritional analysis
As part of the broader studies conducted at each site, we opportunistically collected food samples from tree species known to be consumed regularly by moor macaques (Achmad, Reference Achmad2011; Sagnotti, Reference Sagnotti2013; Albani et al., Reference Albani, Cutini, Germani, Riley, Ngakan and Carosi2020). At the Karaenta Forest, we collected a total of 30 samples during September 2014–February 2015, comprising a combination of different parts of 16 plant species eaten by the macaques (Albani, Reference Albani2017). In the Education Forest, we collected 24 samples during October 2014–March 2015, comprising a combination of fruit and leaves from 18 species (Zak, Reference Zak2016). For this study, we focused on the fruit samples collected (15 samples from 17 species from each site, including ripe and unripe fruit) because fruit constitutes the primary component of the moor macaque's diet (Albani et al., Reference Albani, Cutini, Germani, Riley, Ngakan and Carosi2020). We dried all samples in an electrically powered drying box and then stored them in labelled paper bags with silica gel packets. As soon as we obtained the required dry weight (generally > 30 g per species), we sent the samples to the Laboratory of Nutrition Testing in Cibinong, Bogor, for nutritional analysis. We analysed the samples for moisture, ash and macronutrients (fibre, protein, lipids) during January–October 2015.
Data analysis
We calculated tree density (stems/ha) and size class distributions for food tree species in each habitat. We identified food trees based on existing data (Achmad, Reference Achmad2011; Sagnotti, Reference Sagnotti2013; Albani et al., Reference Albani, Cutini, Germani, Riley, Ngakan and Carosi2020). We quantified the diversity of macaque food tree species at each of the sites using the Shannon index H', which takes into account species richness and abundance and has a typical range of 1.5–3.5, with larger values denoting greater diversity. We calculated the variance of H' of each site, computed the t-value and compared it to the critical value of Student's t to determine whether the computed diversity indices were significantly different (Brower & Zar, Reference Brower and Zar1983). To further assess the importance of potential food resources in each forest, we quantified importance values of macaque food tree species and families in the Karaenta Forest and the Education Forest (Mori et al., Reference Mori, Boom, de Carvalino and Talmon1983). We calculated species importance values as relative frequency + relative density + relative dominance, where relative frequency is the frequency of the species divided by the total frequency of all species, relative density is the density of the species divided by the total density of all species and relative dominance is the basal area of the species divided by the total basal area of all species. We calculated family importance values as relative diversity + relative density + relative dominance, where relative diversity is the number of species in a family divided by the total number of species, relative density is the number of stems per family divided by the total number of stems and relative dominance is the basal area of the family divided by the total basal area of all food trees.
Given that basal area is generally a good indicator of primary net production (Sagar & Singh, Reference Sagar and Singh2006), and hence is often used as an index of food availability (Bryson-Morrison et al., Reference Bryson-Morrison, Matsuzawa and Humle2016), we calculated basal area (m2/ha) for each food tree species per site. We used non-parametric tests to analyse our data using SPSS 27 (IBM Corp., 2020). All tests were two-tailed, with the significance level (α) set at 0.05.
We analysed fruits for fibre, crude protein, lipids and ash using standard proximate analysis procedures (AOAC, Reference Helrich1990). Following Rothman et al. (Reference Rothman, Chapman and Van Soest2012), we determined crude protein using the standard formula: N (total nitrogen) × 6.25 (AOAC, Reference Helrich1990). We conducted a detergent fibre analysis (van Soest, Reference Van Soest1994), which renders the neutral detergent fibre. The neutral detergent fibre is often considered a good index of total insoluble fibre and of energy available from fibre (e.g. Conklin-Brittain et al., Reference Conklin-Brittain, Knott, Wrangham, Hohmann, Robbins and Boesch2006). We calculated the digestible carbohydrates or the total non-structural carbohydrates as: % total non-structural carbohydrates = 100 − % lipid − % crude protein − % total ash − % neutral detergent fibre. All results are presented on a dry matter basis (Rothman et al., Reference Rothman, Chapman and Van Soest2012). We also calculated the available or metabolizable energy of the foods using standard physiological fuel values for soluble carbohydrates, crude protein and lipids (4, 4 and 9 kcal/g, respectively) and a fibre digestion coefficient of 0.463 that had been determined previously for captive Japanese macaques Macaca fuscata and rhesus macaques Macaca mulatta (Sakaguchi et al., Reference Sakaguchi, Suzuki, Kotera, Ehara, Kimura, Takanaka and Iwamoto1991). We calculated the physiological fuel value of fibre as 3 × 0.463 = 1.389 kcal/g. Assuming maximal neutral detergent fibre fermentation, we calculated energy per food species as MEh kcal/g dry matter, where MEh is high-fermentation metabolizable energy (as per Conklin-Brittain et al., Reference Conklin-Brittain, Knott, Wrangham, Hohmann, Robbins and Boesch2006). We visualized the proportions of macronutrients in fruits using right angle mixture triangles constructed in SigmaPlot 14 (Raubenheimer et al., Reference Raubenheimer, Machovsky-Capuska, Chapman and Rothman2015). We compared the nutrients in the fruits between sites using Mann–Whitney U tests in SPSS 27 (IBM Corp., 2020).
Results
Food species richness and diversity
As predicted, food species richness was greater in the formally protected Karaenta Forest compared to the Education Forest. Of the 1,085 stems we enumerated in Karaenta, 84.4% were macaque food trees (n = 916), representing 71 species from 36 families. Of the 1,553 stems we enumerated in the Education Forest, 42.5% were macaque food trees (n = 660), representing 51 species from 27 families. Although both sites showed high food tree species diversity (H' > 3), the diversity of trees in the Karaenta Forest (H' = 3.64) was significantly greater than in the Education Forest (H' = 3.07; t = −8.85, df = 1,141, P < 0.05).
Density, basal area and size class distributions
As predicted, the total stem density of macaque food species was greater in the Karaenta Forest compared to the Education Forest (Table 1). However, for 24 of the food tree species shared between the sites (Table 2), there was no significant difference in stem density (Wilcoxon signed rank test, Z = −0.714, P = 0.475). In contrast to our prediction, the total basal area of macaque food trees was lower in the Karaenta Forest than in the Education Forest (Table 1). Of those food species shared between the sites (Table 2), there was no significant difference in basal area (Wilcoxon signed rank test, Z = −0.791, P = 0.429).
Table 1 Density, basal area and mean diameter at breast height (Fig. 2) of moor macaque Macaca maura food tree species in the vegetation plots sampled in the Karaenta Forest and the Education Forest, South Sulawesi, Indonesia (Fig. 1).
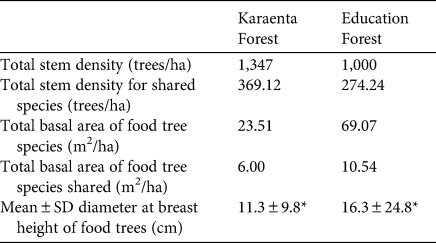
* P < 0.05.
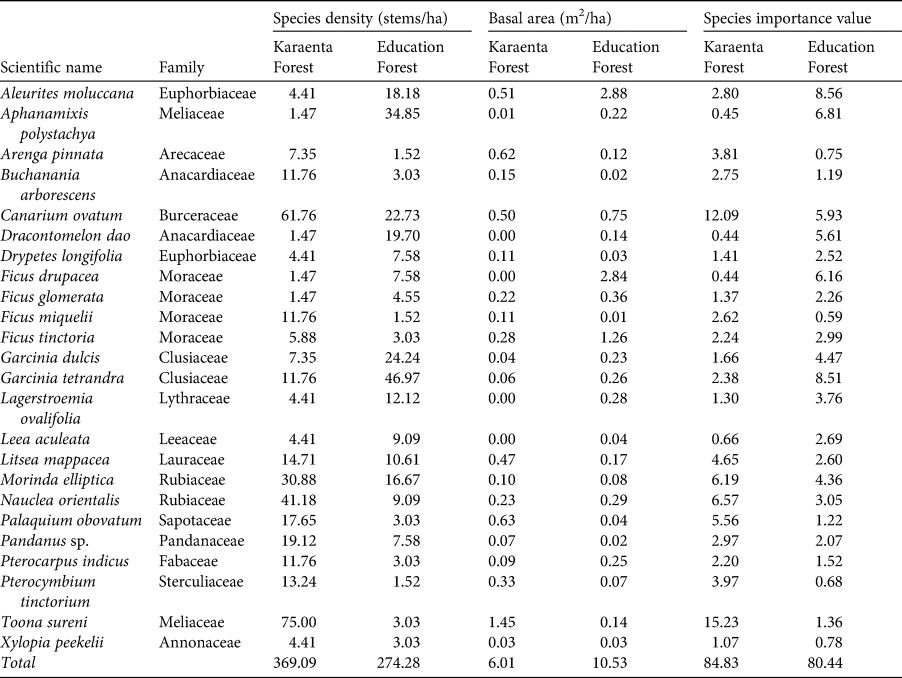
Table 2 Stem density, basal area and species importance values for moor macaque Macaca maura food tree species shared between the Karaenta Forest and the Education Forest, South Sulawesi, Indonesia (n = 24).
In contrast to our predictions, the mean diameter at breast height of macaque food trees was significantly lower in the Karaenta Forest compared to the Education Forest (Mann–Whitney, U = 274,440, P = 0.002; Table 1). At both sites, the distribution of the diameter at breast height classes conformed to an inverted ‘J’ shape curve (Fig. 2). The majority of the trees sampled represent the smaller diameter at breast height classes: 92% and 84% of the trees were < 25 cm diameter at breast height in the Karaenta Forest and the Education Forest, respectively. There was a paucity of trees in the larger diameter at breast height classes (> 35 cm) at both sites. The largest tree recorded in plots in the Karaenta Forest was Ficus congesta (family Moraceae), with 91.5 cm diameter at breast height. The largest tree recorded in plots in the Education Forest was Ficus virens (family Moraceae), with an estimated 300 cm diameter at breast height.
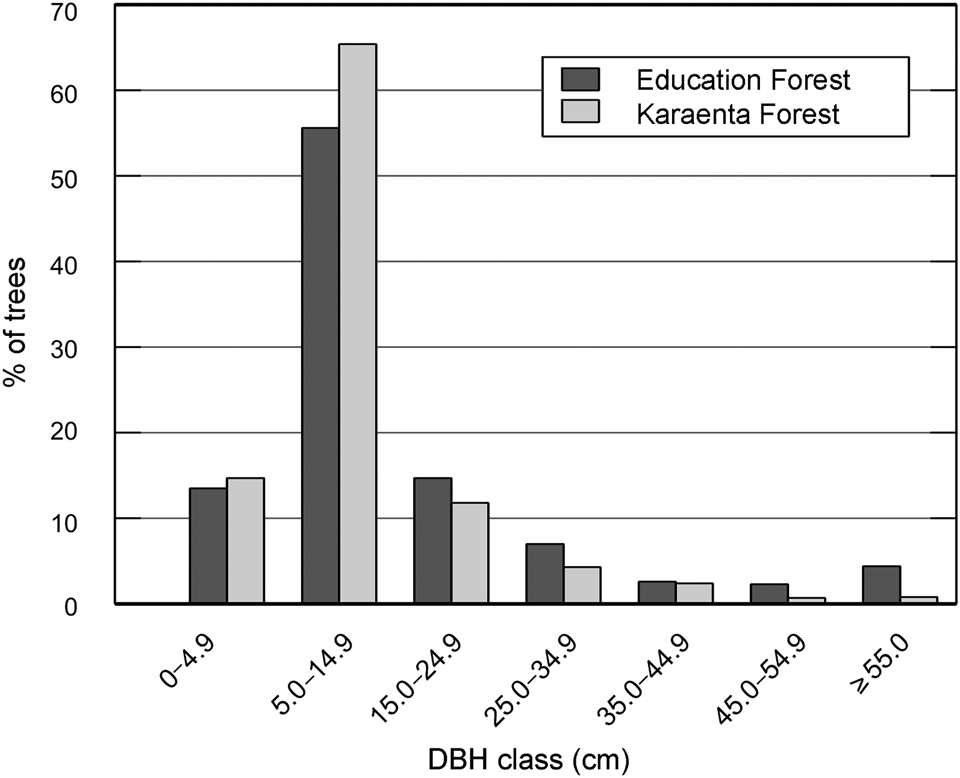
Fig. 2 Distribution of diameter at breast height (DBH) classes of moor macaque Macaca maura food tree species at the Karaenta Forest and the Education Forest, South Sulawesi, Indonesia (Table 1).
Ecological importance
At both sites, Moraceae (mulberry/fig family) was the dominant family and had the highest importance value (Supplementary Table 1). In the Karaenta Forest, the top 10 important species were all from different families; no single family was represented more than once (Supplementary Table 2). In the Education Forest, three species from the Moraceae family were amongst the top 10 most important species: F. virens, Ficus sp. and Artocarpus elasticus (Supplementary Table 3). A non-native, introduced species, P. merkusii, and a cultivated species, A. moluccana, known locally as kemiri, were also amongst the top 10 most important species.
Fruit nutrients
The mean metabolizable energy values of macaque food fruits were similar between the two sites (Karaenta Forest: 277.4 ± 59.3 kcal/100 g; Education Forest: 275.2 ± 69.0 kcal/100 g; Mann–Whitney, U = 153, P = 0.786; Supplementary Table 4). Protein (U = 154, P = 0.743), lipids (U = 119, P = 0.379), total non-structural carbohydrates (U = 178, P = 0.249) and neutral detergent fibre (U = 94, P = 0.082) were also similar in both forests (Supplementary Table 4). Fruits of three species in the Education Forest were high in lipids (Aphanamixis polystachya, Arthrophyllum diversifolium and Cinnamomum celebicum) and hence high in metabolizable energy (Supplementary Table 4).
The balance of fruit macronutrients (total non-structural carbohydrates, protein and lipids) from both study sites revealed high proportions of total non-structural carbohydrates and lipids compared to protein; fruits were also proportionally higher in total non-structural carbohydrates compared to fats (Fig. 3). The estimated energetic contribution from fibre in fruit was minor compared to total non-structural carbohydrates and lipids in both locations (Fig. 3), not exceeding 22% of the non-protein energy. Some fruits, particularly in the Education Forest, were high in lipids compared to carbohydrates and protein (Fig. 3). There were no differences in the proportions of macronutrients that contributed to metabolizable energy between the two forests (Figs 3 & 4). There were also no significant differences in the proportions of protein, lipids and total non-structural carbohydrates (Mann–Whitney: protein: U = 141.00, P = 0.0904; lipid: U = 111.5, P = 0.256, total non-structural carbohydrates: U = 171.0, P = 0.361; Fig. 4) or in the proportional contributions of digestible neutral detergent fibre (U = 115.5, P = 0.318), total non-structural carbohydrates (U = 184.0, P = 0.174) and lipid (U = 115.0, P = 0.309) to the non-protein energy between the two forests (Fig. 4).
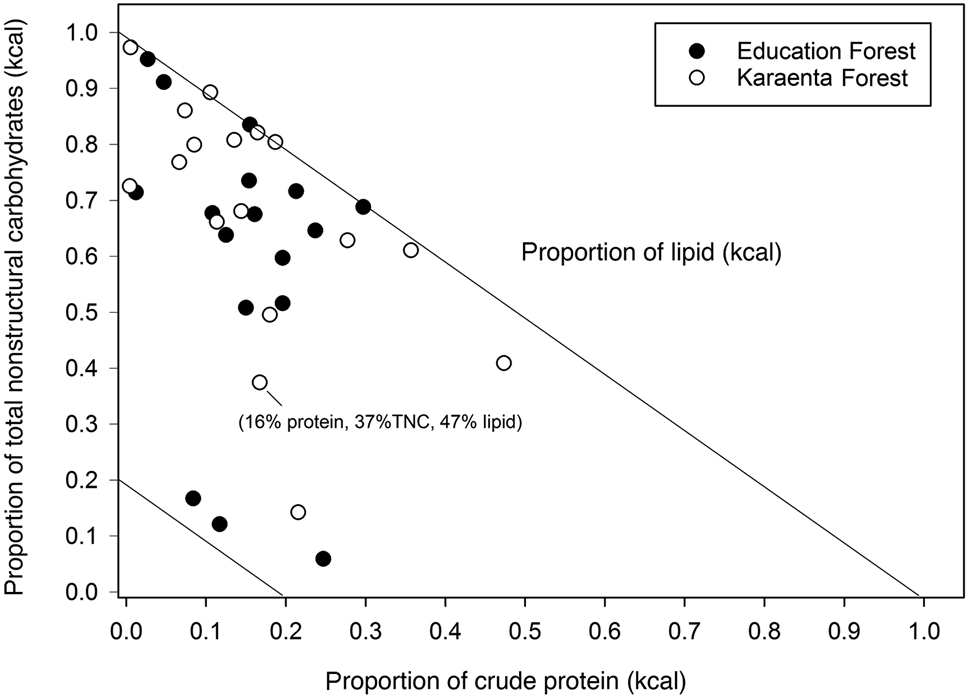
Fig. 3 Right angle mixture triangle illustrating the macronutrient balance of crude protein (x-axis), total non-structural carbohydrates (TNC; y-axis) and lipid (z-axis, implicit axis) in fruits consumed by the moor macaque in the habitats of the Karaenta Forest and the Education Forest, South Sulawesi, Indonesia. The details of one fruit species are labelled to illustrate the graph's three dimensions.
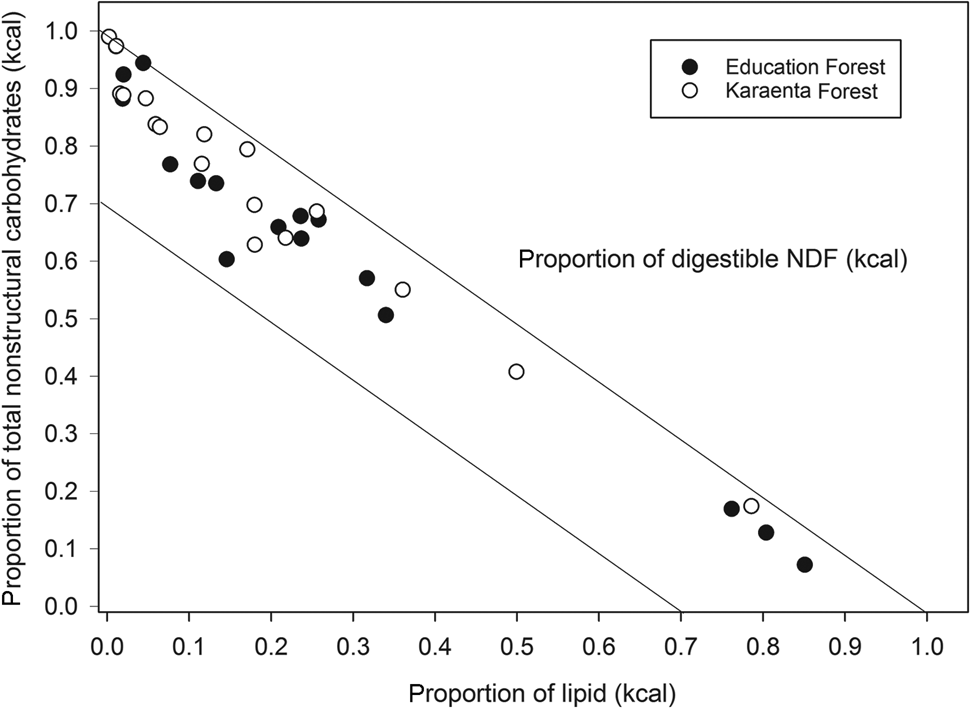
Fig. 4 Right angle mixture triangle illustrating the balance of the macronutrients contributing to non-protein energy, including lipid (x-axis), total non-structural carbohydrates (y-axis) and digestible neutral detergent fibre (NDF; z-axis, implicit axis), in fruits consumed by the moor macaque in the habitats of the Karaenta Forest and the Education Forest, South Sulawesi, Indonesia.
Discussion
Assessing the range of habitats that threatened wildlife are able to live and survive in is a critical component of wildlife conservation efforts (Sodhi et al., Reference Sodhi, Koh, Prawiradilaga, Darjono, Tinulele and Putra2005; Yabsley et al., Reference Yabsley, Meade, Martin and Welbergen2021). In this study, we assessed the habitat quality of two forests, with varying levels of human modification and protection, for the Endangered moor macaque. Our prediction that the less modified and formally protected karst forest would exhibit higher habitat quality was only partially supported. The Karaenta karst forest showed greater species richness, stem density and diversity of macaque food tree species. However, the heavily modified Education Forest had greater total basal area of food tree species and a significantly higher mean diameter at breast height of macaque food trees compared to the Karaenta Forest. In contrast to what we expected, the macronutrient compositions of the fruits sampled at the two sites were similar.
Although food species richness was higher in the protected karst forest (Karaenta Forest), the family Moraceae, which comprises figs and other species known to be important food resources for a wide array of wildlife (Kinnaird et al., Reference Kinnaird, O'Brien and Suryadi1999; Serio-Silva et al., Reference Serio-Silva, Rico-Gray, Hernandez-Salazar and Espinose-Gomez2002; Walther et al., Reference Walther, Geier, Chou and Bain2018) including moor macaques (Albani et al., Reference Albani, Cutini, Germani, Riley, Ngakan and Carosi2020), was the most important family represented at both sites. Moreover, Moraceae was the most dominant family in the Education Forest, with three fig species, F. virens, Ficus sp. and A. elasticus, being amongst the top 10 most important species (as measured by species importance values; Supplementary Table 3). Figs are important for wildlife because they tend to produce large crops, their asynchronous fruiting patterns make them a reliable food source when other preferred foods may be scarce, and they are high in calcium and digestible carbohydrates, thereby providing important nutritional benefits (Kinnaird et al., Reference Kinnaird, O'Brien and Suryadi1999; Riley et al., Reference Riley, Tolbert and Farida2013). Therefore, although the Education Forest has fewer macaque food species and hence less food species diversity, many of the species that do remain are preferred foods for the macaques. Specifically, eight tree species found in the Education Forest are key food species (Albani et al., Reference Albani, Cutini, Germani, Riley, Ngakan and Carosi2020), and one of them, A. elasticus, was amongst the top 10 most important food tree species in this forest. However, one potential concern at both sites is the high percentage of species represented by only one or two stems: 25% for the Karaenta Forest and 32% for the Education Forest. Should further disturbance and/or fragmentation occur at these sites, successful recruitment and hence the viability of these plant populations could be compromised (Sodhi et al., Reference Sodhi, Koh, Clements, Wanger, Hill and Hamer2010).
It is also important to consider the contributions that introduced and cultivated species make to habitat quality (Brockerhoff et al., Reference Brockerhoff, Jactel, Parrotta, Quine and Sayer2008; McLennan, Reference McLennan2013; Takahashi et al., Reference Takahashi, Rothman and Cords2023). In this study, both sites contain introduced species that are known to be consumed by macaques: candlenut A. moluccana, which is found at both sites, and Sumatran pine P. merkusii, which we recorded in the Education Forest only. Although P. merkusii plantations are unsuitable habitat for other primates (e.g. Hainan gibbons; Zhang & Zang, Reference Zhang and Zang2018), moor macaques consume the seeds of this species (EPR, pers. obs.). Therefore, the abundance of this introduced species, coupled with the fact that it produces seeds year-round (PON, unpubl. data, 2020), could offset the lower species richness in the Education Forest. Moor macaques that live in the Education Forest also feed on agricultural crops (e.g. maize, cacao, watermelon) that are cultivated within or on the periphery of the forest (Zak & Riley, Reference Zak and Riley2017). Access to these cultivated foods could therefore augment the quality of the habitat in the Education Forest; however, over the long term, persistent crop feeding by macaques could exacerbate human–wildlife conflict and could lead to increased rates of retaliation by affected farmers (e.g. McLennan & Hill, Reference McLennan and Hill2012).
Our findings that mean diameter at breast height and total basal area were higher in the Education Forest were in contrast to our predictions. Three large remnant fig trees (> 275 cm diameter at breast height) sampled in the Education Forest could have contributed disproportionally to these measurements. However, when we removed these trees from the analysis, mean diameter at breast height (15 ± SD 16 cm) and total basal area (38.65 m2/ha) were still higher in the Education Forest compared to the Karaenta Forest. Because tree basal area in plantation forests is comparable to primary forests and higher than in secondary forests (Forrester & Bauhus, Reference Forrester and Bauhus2016; Brown et al., Reference Brown, Berninger, Larjavaara and Appiah2020), the abundance of planted P. merkusii trees (n = 41, basal area = 7.58 m2/ha) in the Education Forest could have contributed to its higher total basal area. Structural aspects of the Karaenta Forest probably also explain our findings. The Karaenta Forest includes both karst plain forest and tower karst forest, which vary in terms of the abundance, diversity and size of macaque food trees (Albani et al., Reference Albani, Cutini, Germani, Riley, Ngakan and Carosi2020). It is possible that the lower tree diameter at breast height of the tower karst forest habitat reduced the overall mean tree diameter at breast height for the Karaenta Forest. The finding that the basal area of the food tree species shared between the sites did not differ significantly is important in terms of food availability for moor macaques as it suggests that the productivity of the Education Forest is comparable to the less modified protected area forest. Aside from differences in mean diameter at breast height, the distribution of diameter at breast height classes was similar at both sites. The inverted ‘J’ shape is common in tropical forests and implies that forest regeneration is underway (Siregar et al., Reference Siregar, Helmanto and Rakhmawait2019).
Because plant nutritional quality is linked to primate abundance (Chapman et al., Reference Chapman, Chapman, Naughton-Treves, Lawes and McDowell2004), an analysis of the nutritional composition of available foods is an important component of habitat quality assessments. A limitation of our study is that we could not collect all of the fruits comprising the macaques’ diet in both habitats because of seasonal and sampling constraints; nevertheless, our preliminary dataset shows that the macronutrient composition of the fruits we could sample was similar in both habitats. Fruits are generally higher in total non-structural carbohydrates or lipids and lower in protein than other plant parts (Lambert & Rothman, Reference Lambert and Rothman2015), and our results align with this pattern. The fruits examined here were similar in nutrient concentrations not only to each other but also to other macaque Macaca spp. habitats where fruit nutrients have been measured (Jaman et al., Reference Jaman, Huffman and Takemoto2010). Fruit protein concentrations have been suggested to be a factor influencing frugivore biomass (Donati et al., Reference Donati, Santini, Eppley, Arrigo-Nelson, Balestri and Boinski2017). Some but not all of the fruits analysed in this study (Supplementary Table 4) met the suggested adequate protein concentration of 15% noted previously (National Research Council, 2003). Our data from these two forests support the finding that Asian forests are not necessarily limited in fruit protein like those of Madagascar (Donati et al., Reference Donati, Santini, Eppley, Arrigo-Nelson, Balestri and Boinski2017), although macaques probably obtain most of their dietary protein from insects and leaves, which are typically much higher in protein. Moreover, other macaques, such as the rhesus macaque, are known to regulate their energy intake (Cui et al., Reference Cui, Wang, Shao, Raubenheimer and Lu2018); thus the amounts of lipid, total non-structural carbohydrate and fibre in their diets could be more important in limiting their biomass than protein. Nonetheless, future research should prioritize the collection and nutritional analysis of a broader sample of moor macaque foods, including additional fruit as well as leaves, as the latter can be an important energy and protein source for macaques in other habitats (Hanya et al., Reference Hanya, Ménard, Qarro, Ibn Tattou, Fuse and Vallet2011). Such work could be coupled with studies of nutrient intake to determine whether moor macaques living in habitats across a gradient of human modification show flexibility in nutrient balancing (Dunham & Rodriguez-Saona, Reference Dunham and Rodriguez-Saona2018).
Conclusions and implications for conservation
Although protecting primary old-growth forest remains a critical conservation objective, our results provide further support for the notion that heavily modified habitat should not be overlooked for its potential conservation value for primates and other wildlife. In South Sulawesi, although the Education Forest is mostly a secondary forest that is dominated by non-native species, the fact that a number of important food trees remain and that the fruits sampled provide a similar balance of macronutrients to those available in the protected forest points to the conservation value of this forest. This is assuming no new major disturbance occurs and that the remnant large food trees, such as Ficus trees that are important for many wildlife species (Kinnaird et al., Reference Kinnaird, O'Brien and Suryadi1999) and that can act as seed sources (Dent & Wright, Reference Dent and Wright2009), remain intact. It could be that heavily modified forests such as these are equally or even more important to conservation, particularly if remnant protected forests are of lower quality. For example, although karst ecosystems in South-east Asia are recognized as biodiversity reservoirs (Clements et al., Reference Clements, Sodhi, Schilthuizen and Ng2006), their quality of habitat and the conservation value for primates have been questioned (e.g. for white-headed langurs Trachypithecus leucocephalus: Li & Rogers, Reference Li and Rogers2005; Bornean orangutans Pongo pygmaeus morio: Marshall et al., Reference Marshall, Salas, Stephens, Nardiyono, Engström, Meijaard and Stanley2007). This is probably because vegetation growth is typically slow in karst forest because of their soilless limestone substrates, steep slopes, unstable soils and low water retention (Fan et al., Reference Fan, Fei, Scott, Zhang and Ma2011, p. 2251), and the concomitant variability in forest structure and food species density in karst forests (Albani et al., Reference Albani, Cutini, Germani, Riley, Ngakan and Carosi2020).
Our results have broad implications for conservation policy and action, as they suggest that safeguarding anthropogenically modified secondary forests that retain ecologically important features such as important food trees could be an effective wildlife conservation strategy. Conservation efforts should also focus on expanding the level of protection of secondary forests and augmenting their value via active forest restoration (Lamb et al., Reference Lamb, Erskine and Parrotta2005; Sodhi et al., Reference Sodhi, Koh, Clements, Wanger, Hill and Hamer2010; Chazdon, Reference Chazdon2019). Forest restoration is generally understood as a process of restoring ecosystem structure, function and species diversity, with the aim of returning an ecosystem to its original state prior to human disturbance or alteration. A strict application of these principles would involve the removal of non-native species (Mudappa & Raman, Reference Mudappa, Raman, Shahabuddin and Rangarajan2007; Chazdon, Reference Chazdon2019); however, as we found in this study, non-native species can serve as important nutritional resources for wildlife (Schlaepfer et al., Reference Schlaepfer, Sax and Olden2011; Eppley et al., Reference Eppley, Donati, Ramanamanjato, Randriatafika, Andriamandimbiarisoa and Rabehevitra2015). Forest restoration efforts should therefore rely on data from feeding ecology and nutrient balancing studies for insight, and must carefully balance the costs and benefits of removing non-native species, particularly if resident wildlife rely on those species when native foods are seasonally low (Dunham & Rodriguez-Saona, Reference Dunham and Rodriguez-Saona2018). Finally, the planting of fast-growing tree species that are important for both wildlife and people (e.g. A. pinnata for Sulawesi macaques, bats and people: Riley, Reference Riley2007; Ruslan et al., Reference Ruslan, Maulany, Nasri and Ngakan2021; Harungana madagascariensis for birds, lemurs and people; Konersmann et al., Reference Konersmann, Noromiarilanto, Ratovonamana, Brinkmann, Jensen and Kobbe2022) can assist in restoring remaining forests, addressing human well-being and facilitating the sustainable coexistence of people and wildlife.
Acknowledgements
We thank the Kementerian Negara Riset dan Teknologi Republik Indonesia (RISTEK) for permission to conduct research in Indonesia; Balai Taman Nasional Bantimurung Bulusaraung (BTNBABUL) for permission to work in the park; Universitas Hasanuddin (UNHAS) for supporting the research; Pak Haro, Pak Pado, Jack, Hendra and Amiruddin Bin Dahlan for their field research assistance; Nancy Guzman and Devon Yuwiler for assistance with data entry; and Ibu Haji and Mama Aco and her family for providing us with accommodation. This work was supported by a Henry Luce Grant from the American Institute for Indonesian Studies and funding from the University Grants Program of San Diego State University to EPR; a Safari Ravenna Zoo grant for conservation to MC and Roma Tre University doctoral funds granted to AA.
Author contributions
Concept and research design: all authors; data collection: EPR, AA, AAZ, LG, PON; data analysis: EPR, AA, JMR; writing and revision: all authors.
Conflicts of interest
None.
Ethical standards
All research and writing abided by the Oryx guidelines on ethical standards and adhered to all legal requirements for foreigners conducting research in Indonesia. Permits to conduct research in Indonesia were issued by the Ministry of Research, Technology and Higher Education of the Republic of Indonesia (issued to EPR: 170/SIP/FRP/SM/VI/2014; issued to AA: 195/SIP/FRP/SM/VII/2014; 65/SIP/FRP/E5/Dit.KI/III/2016; issued to AAZ: 172/SIP/FRP/SM/VI/2014). Permits to conduct research and to collect samples in a protected area were issued by the Indonesian Ministry of Environment and Forestry (issued to AA: SI.49/BTNBABUL-1/KEHATI/2014; SI.14/ BTNBABUL-1/2016; S.47/KKH-2/2015; SK.396/KSDAE/SET/KSA.2/10/2016).








