INTRODUCTION
Of the estimated 165 million annual episodes of shigellosis worldwide, 99% occur in low-income countries, largely in children aged <5 years, who represent 69% of the episodes and 60% of deaths [Reference Kotloff1]. A population-based study conducted in one urban slum of Bangladesh observed an incidence of shigellosis of 7·9/1000 person-years, but the rate was 60% higher (13·2/1000 person years) in children aged <5 years [Reference Von Seidlein2]. Childhood malnutrition is a major public health problem in Bangladesh, where >40% of pre-schoolchildren are malnourished [3]; these children are not only at higher risk of Shigella infections, but also experience complications and fatal outcomes more often [Reference Bennish4, Reference Bennish and Wojtyniak5]. Disease severity and outcome differ substantially for the four species of Shigella (S. dysenteriae, S. flexneri, S. boydii, S. sonnei): S. dysenteriae type 1(Sd1) outbreaks or epidemics are associated with higher rates of complications and deaths. In Bangladesh the majority of deaths due to endemic shigellosis were associated with S. flexneri [Reference Bennish and Wojtyniak5, Reference Islam and Shahid6].
Distribution of serotype differs between industrialized and developing countries. Serotype distribution changed in Thailand as the country's economy developed [Reference Chompook7]. Heterogeneous distribution of Shigella spp. and serotype have been observed in surveillance conducted in six Asian countries [Reference Von Seidlein2].
In Bangladesh, from 1968 to 1979, hospital-based studies conducted at urban Dhaka Hospital and rural Matlab Hospital of ICDDR,B observed that S. flexneri was the most predominant strain, with one Sd1 epidemic in 1973. The first documented outbreak of Sd1 in Bangladesh occurred in St Martin's Island, a coral island in the Bay of Bengal, from May to July 1973. Of 434 recognized cases 28 (6·4%) died, mostly infants and elderly people [Reference Rahaman8]. At that time, all strains of Shigella were found to be susceptible to most of the conventional antimicrobials used in Bangladesh, including tetracycline and chloramphenicol.
The prevalence of different species of Shigella and their antimicrobial susceptibility change over time, making treatment difficult [Reference Niyogi, Mitra and Dutta9]. Therefore, regular monitoring of species distribution and antimicrobial susceptibility is important, both for case management of individual patients, especially those at higher risk such as severely malnourished children aged <5 years, and for epidemic preparedness.
In this paper we aimed to describe the changing trend and antimicrobial susceptibility pattern of shigellosis by analysing the database of the Diarrhoeal Disease Surveillance system of ICDDR,B from 1980 to 2008.
METHODS
The Dhaka Hospital of ICDDR,B is located in the capital city of Bangladesh, Dhaka, and it provides care and treatment to about 100 000 patients each year, mostly residents of the city and its peri-urban areas, while a smaller proportion come from outside the city and even from distant parts of the country. The hospital has maintained a Diarrhoeal Disease Surveillance system since late 1979, enrolling a systematic 2% sample of all patients attending this facility. The system collects information on the clinical, epidemiological and demographic characteristics of every 50th patient, irrespective of age, sex or socioeconomic status. In determining this sample frame, it was assumed that the system should be able to detect a significant difference (P<0·05, two-sided) in the isolation of specific enteropathogens when cases were compared with controls (e.g. isolation rate of 5·8% in cases and 2·5% in controls), requiring an annual sample size of 660 cases and 660 controls. To facilitate subgroup analysis it was estimated that a sample size of 2000 would be adequate. A systematic sample of 2% would provide at least 2000 patients from a total of over 100 000 patients attending the hospital.
Trained research assistants interviewed patients, or the guardian of minors, using a structured questionnaire. A physician performed physical examinations at the time of hospitalization and recorded the findings. For this analysis we extracted data from January 1980 to December 2008, and reviewed patients with culture-positive Shigella infections.
For laboratory confirmation of Shigella, freshly collected stool specimens were immediately sent to the laboratory where they were inoculated onto MacConkey (MAC) and Salmonella–Shigella (SS) agar media plates. Shigella spp. were isolated and identified using standard laboratory methods [Reference Edwards and Ewing10], and the species and their serotype were confirmed using commercial antisera kits (Denka Seiken, Japan) specific for polyvalent and monovalent antigens for all serovars. The same culture method was used during the entire study period. Antimicrobial susceptibility of the isolated strains was determined by the disc diffusion method as recommended by the Clinical Laboratory and Standard Institute, CLSI [formerly the National Committee for Clinical Laboratory Standards (NCCLS)] [11]. The antibiotic discs used in this study (Oxoid, UK) included ampicillin (10 mg), mecillinam (25 mg), nalidixic acid (30 mg), trimethoprim–sulphamethoxazole (T-S) (25 mg), and ciprofloxacin (5 mg). Escherichia coli ATCC 25922 was used as the control strain for susceptibility studies [11].
χ2 was used for assessing trends, and P⩽0·05 considered as significant. For children aged <5 years, we analysed the risk of Shigella infection between malnourished and well-nourished children by calculating risk ratios (RR) and their 95% confidence intervals (CI).
Severe malnutrition was defined as (all values are z scores): height-for-age <−3; weight-for-age <−3, and weight-for-height <−3; using the World Health Organization reference value [12]. Moderate malnutrition was defined as: height-for-age −3 to >−2, weight-for-age −3 to >−2, and weight-for-height −3 to >−2. We considered children as well nourished if their weight-for-age, height-for-age and weight-for-height were −2 or more.
RESULTS
In total, 83 073 patients were enrolled in the Diarrhoeal Disease Surveillance system at the Dhaka Hospital of ICDDR,B between 1980 and 2008. The stool/rectal swab cultures of 7575 (9·1%) were positive for Shigella. Of all patients infected with Shigella, 3648 (49%) were aged <5 years, 961 (13%) were aged 5–14 years, and 2891 (39%) were aged ⩾15 years. The age distribution remained stable over time.
Of all Shigella strains, 55% were S. flexneri, 19% were S. dysenteriae, and 13% S. boydii (Table 1). The overall yearly isolation rates of Shigella ranged between 8% and 12% during 1980–1995 (Fig. 1) and then gradually decreased to 3% in 2008 (χ2 for trend P<0·001), except for a single year peak of 8% in 2000.
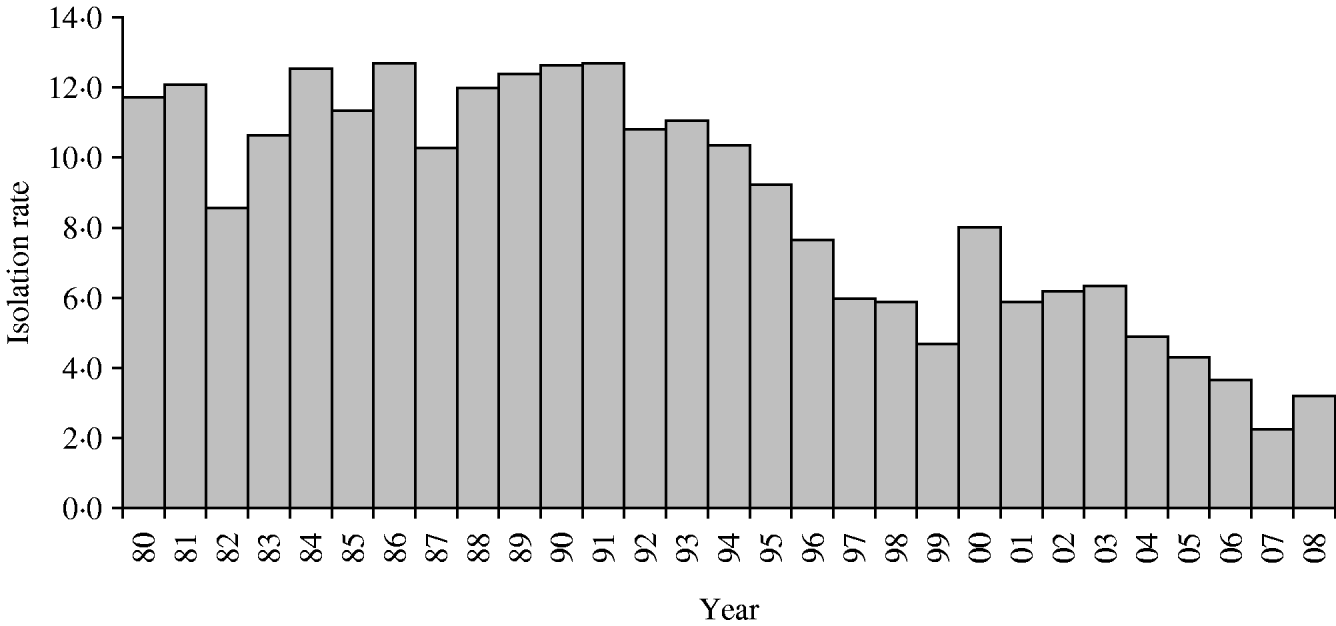
Fig. 1. Isolation rate (%) of Shigella between 1980 and 2008. Dhaka Hospital Surveillance system, ICDDR,B.
Table 1. Proportion of Shigella species distribution 1980–2008, Dhaka Hospital Surveillance system, ICDDR,B
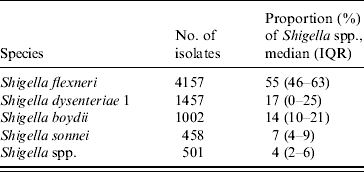
IQR, Interquartile range.
Two peaks of isolation of Sd1 occurred in 1984 and 1993 (Fig. 2), and then the isolation gradually declined. This serotype was not isolated after 2000, except for a single patient in 2004.
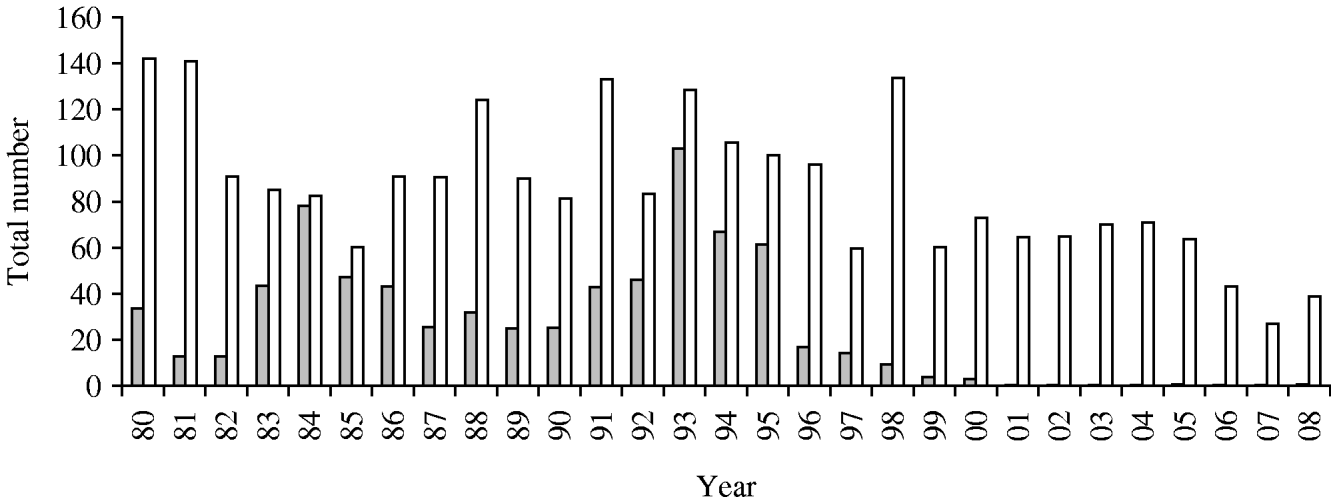
Fig. 2. Total numbers of Shigella dysenteriae type 1 (![]() ) and Shigella flexneri (□), 1980–2008. Dhaka Hospital Surveillance system, ICDDR,B.
) and Shigella flexneri (□), 1980–2008. Dhaka Hospital Surveillance system, ICDDR,B.
S. flexneri remained the most prevalent strain during the entire period of observation, except during the peak of Sd1 epidemics in 1984 and 1993 when the proportion of isolation of S. flexneri and S. dysenteriae were almost identical (Fig. 2). In both these epidemics, the strains of Sd1 had acquired resistance to newer antimicrobials, including tetracycline, T-S, and ampicillin in 1984, and nalidixic acid in 1993. In contrast, the development of resistance of S. flexneri to ampicillin, and T-S has been much slower. Similarly, resistance to nalidixic acid from 1986 to 1997 was a slow process, followed by a sharp rise from 1998 and reaching 60% in 2002. Since 2007 S. flexneri has begun to show resistance to ciprofloxacin and mecillinam and by 2008, 33% of S. flexneri was resistant to ciprofloxacin and 56% to mecillinam. Other strains of Shigella such as S. sonnei, S. boydii and Shigella spp. together had a 12% resistance to mecillinam and 9% to ciprofloxacin in 2008.
Of Shigella-positive children aged <5 years, 9% were severely underweight, 10% severely wasted and 8% severely stunted, while only 5% of well-nourished children aged <5 years were Shigella positive. Compared to well-nourished children severely malnourished children were at greater risk of developing Shigella infection: severely underweight (RR 1·8, P<0·001); severe wasting (RR 2·1, P<0·001) and severe stunting (RR 1·7, P<0·001) (Table 2). The proportion of malnourished children aged <5 years with all aetiologies of diarrhoea has decreased over time from 14% in severely underweight in 1993 to 4% in 2008. Similarly stunting was 11% in 1993 and 4% in 2008 (data not shown).
Table 2. Nutritional risk factor for shigellosis in children aged <5 years from 1993 to 2008, Dhaka Hospital Surveillance system, ICDDR,B
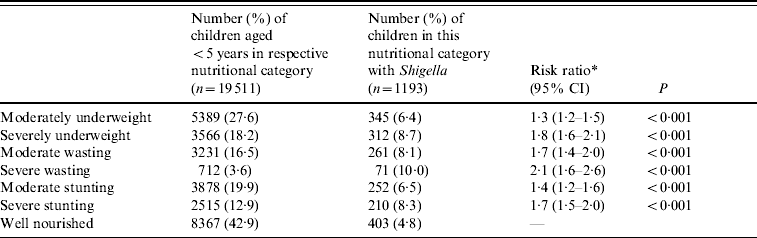
* All groups are compared to the well-nourished group.
DISCUSSION
At the Dhaka Hospital of ICDDR,B the proportions of patients admitted with shigellosis decreased over time from 12% in 1980 to 3% in 2008. A moderate reduction in the estimated number of Shigella infections and a large reduction of mortality have been reported by a literature review in Asia [Reference Sack, Bardhan and Naheed13]. Similar reductions in developed countries have been attributed to improvements in safe water supply, adequate sanitation facilities, and maintenance of good personal hygiene [Reference Cutler and Miller14]. We do not have a definitive explanation for the reduction in shigellosis in Bangladesh from our study, but higher rates of isolation of Shigella from severely malnourished children and the reductions in the proportions of severely malnourished children in Bangladesh in recent years, would suggest that improved nutrition could be a likely factor. The association between measles and shigellosis, including higher severity and deaths from these two conditions co-occurring is well documented; in Bangladesh the immunization coverage against measles was 70% in 1996 and 83% in 2007 [3, Reference Koster15]. Vitamin A is an important factor in preventing infectious illnesses. The coverage of vitamin A capsule supplementation in children increased from 40% in 1995 to around 70% in 2003 [Reference Jamil16]. An earlier study conducted at ICDDR,B demonstrated the therapeutic benefits of vitamin A supplementation when used as an adjunct to antimicrobial therapy [Reference Hossain17]. Although vitamin A was used in case management of shigellosis, not as a preventive strategy, it seems reasonable to consider improved vitamin A status as a factor that might have contributed to reducing the prevalence of shigellosis in Bangladesh.
In Bangladesh, all four species of Shigella are present. S. flexneri is the most prevalent endemic species, and Sd1 has caused two epidemics since 1980 (in 1984 and 1993) but has not been isolated since 2001 (Fig. 2). Hospital-based surveillance is not usually representative of an epidemic, yet in Bangladesh there were no population-based studies until 2001, so we considered the results of our hospital-based surveillance as a proxy indicator for these two epidemics [Reference Bennish18, Reference Ronsmans, Bennish and Wierzba19]. Both of these Sd1 epidemics were caused by strains that had acquired resistance to the antimicrobial agents in routine use for treating shigellosis. Of all the species of Shigella, the emergence of resistance and its quick progression is most notable in Sd1 strains.
After these two epidemics, separated by a decade-long interval, Shigella experts anticipated another epidemic in 2004 [Reference Legros20]. The factors underlying this trend of periodic epidemics have not been explored. Our data show that resistance to Sd1 is stable and has acquired resistance to additional antimicrobials, leading to a situation with limited options for empirical antimicrobial therapy. Despite fluctuations in the rates of isolation of Sd1, the serotype was isolated in all the years of our study period, except for 2001 to 2008, but in 2004 it was isolated from a single patient at Dhaka Hospital. The only other record was an outbreak of ciprofloxacin-resistant Sd1 in northeast Bangladesh associated with an 8% case-fatality rate in late 2003 [Reference Naheed21]. The reason behind the apparent disappearance of Sd1 from Bangladesh is not known, but Sd1 has returned after long dormant intervals, with resistance to additional antimicrobials, in both eastern India and Malaysia [Reference Lee and Puthucheary22, Reference Sarkar23].
Although not as rapid as Sd1, the growing resistance since 2007 in S. flexneri, particularly against ciprofloxacin and mecillinam (the WHO recommended first- and second-line treatment of shigellosis), is also a matter of concern. Resistance to quinolones is associated with chromosomal mutation in Shigella, or transfer of resistance factors from other co-inhibiting microbes that are resistant to the fluroquinolones [Reference Chu24, Reference Rahman25]. The development of resistance to nalidixic acid, an older quinolone, raised concerns that resistance would also emerge against ciprofloxacin. Our data demonstrated that to be the case. Second-line drugs are azithromycin and ceftriaxone. Azithromycin has been tested and proved to be effective in adults, and is less costly and easier to use compared to parenteral ceftriaxone [Reference Khan26]. Although not evaluated in the treatment of shigellosis in children, there is no reason to believe that this drug will not be effective in children.
The rate of childhood malnutrition remains high in Bangladesh, despite a reduction from 56% of children aged <5 years in 1990 to 45% in 2005 [27]. Compared to better nourished children, the prevalence of Shigella in our study was higher in underweight, stunted, and wasted children aged <5 years, which is similar to earlier observations [Reference Bennish and Wojtyniak5]. Of all diarrhoeal diseases, the negative nutritional impact is greater with shigellosis, due to presence of fever, greater anorexia, and the loss of enteric proteins, and has been associated with decreased linear growth rate in previous studies in Bangladesh [Reference Bennish4, Reference Black, Brown and Becker28].
Our dataset is hospital based, which might not represent the population at large, with a possible bias towards children with more severe disease and those who fail outpatient treatment. Thus, the prevalence of antimicrobial resistance may be less in the community. Over time, the number of hospital admissions increased because the population of Dhaka city has grown and referral mechanism towards ICDDR,B Dhaka hospital has improved. We performed systematic sampling and presented the proportion of Shigella infection, thus increased hospital admission did not affect our results.
Urban populations in Bangladesh have better access to healthcare facilities, including those services received from public and private clinics/hospitals, general practitioners, which could have been a factor for our observation of reducing prevalence of shigellosis. The availability of highly potent drugs, such as ciprofloxacin, that are available over the counter could also have contributed [Reference Hossain29]. The prevalence of shigellosis in Kamlapur, an urban field area of ICDDR,B is higher than that observed in our study with an isolation rate of 13% between 2002 and 2004 [Reference Von Seidlein2]. In addition, isolation rates of Shigella were higher in rural Matlab Hospital of ICDDR,B and another rural community-based study in Mirzapur (Dr A. S. G. Faruque, personal communication; Matlab and Mirzapur surveillance data). A nationwide Shigella surveillance could provide a more complete estimate of Shigella prevalence.
Shigellosis is one of the few diarrhoeal diseases for which the WHO recommends routine antimicrobial therapy, using an effective agent to reduce morbidity, complications and deaths. It seems that Sd1, the serotype of Shigella that causes the most severe illness with greater complications and deaths and is the most efficient in acquiring resistance to antimicrobials, has disappeared from Bangladesh, at least temporarily. However, the rapid emergence of resistance of other Shigella strains, especially S. flexneri, the most prevalent endemic species, is a matter of concern. The recent resistance to ciprofloxacin and mecillinam has left only azithromycin and ceftriaxone as drugs with proven efficacy in the management of shigellosis. However, ceftriaxone is only available in parenteral formulation that requires hospitalization in healthcare facilities. It is also expensive, making it inaccessible, particularly to families of severely malnourished children of low socioeconomic status. Severely malnourished children are not only at a greater risk of shigellosis, but are also at greater risk of related complications and deaths.
Our data highlight the importance of hospital-based surveillance in understanding the changing epidemiology of Shigella infections, including changes in antimicrobial susceptibility that would be helpful in the case management of shigellosis. Although the proportion of patients hospitalized for shigellosis has decreased over the last 28 years, the increase in antimicrobial resistance, the continued susceptibility of malnourished children to Shigella, and the history of prior Sd1 outbreaks means that Shigella represents an ongoing threat to public health in Bangladesh.
ACKNOWLEDGEMENTS
Hospital surveillance was funded by ICDDR,B and the Government of the People's Republic of Bangladesh through IHP-HNPRP. ICDDR,B acknowledges with gratitude the commitment of the Government of the People's Republic of Bangladesh to the Centre's research efforts. ICDDR,B also gratefully acknowledges the following donors which provide unrestricted support to the Centre's research efforts: Australian Agency for International Development (AusAID), Government of the People's Republic of Bangladesh, Canadian International Development Agency (CIDA), Embassy of the Kingdom of the Netherlands (EKN), Swedish International Development Cooperation Agency (Sida), Swiss Agency for Development and Cooperation (SDC), and Department for International Development, UK (DFID). We also acknowledge Mrs Dorothy Southern for her critical review. This work was done as part of a TropEd European Master of Science Programme in International Health, funded under the Erasmus Mundus programme.
Piero Olliaro is a staff member of the WHO; the authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the WHO.
DECLARATION OF INTEREST
None.






