Sindbis virus (SINV), a mosquito-borne virus, circulates in transmission cycles between pastoral birds and ornithophilic mosquitoes, and is occasionally transmitted to humans and other vertebrates. Evidence of virus activity, including seroconversion in humans and identification of infected mosquitoes, has been reported from Africa (Egypt, Cameroon, Uganda, South Africa), Eurasia (Sweden, Finland, UK, Italy, Israel, India, Saudi Arabia, China, the Russian Federation) and Oceania (Malaysia, the Philippines, Australia) [Reference Lundstrom and Pfeffer1]. Despite the wide distribution of SINV, symptomatic cases occurring as epidemics have only been reported from restricted geographical areas of Finland, Sweden, the Russian Federation and South Africa [Reference Laine, Luukkainen and Toivanen2]. This is most likely due, in part, to the underreporting of SINV and other alphavirus infections since the disease is believed to present in subclinical and self-limiting forms [Reference Laine, Luukkainen and Toivanen2]. Sindbis fever is usually mild and resolves spontaneously. Symptoms include low-grade fever, maculopapular rash, headache, arthritis, malaise, muscle pain and fatigue. Arthritis has been recorded to persist for months to years in several Sindbis fever cases [Reference Kurkela3].
The first human cases of Sindbis fever were diagnosed in South Africa in 1963 [Reference Malherbe4]. Studies conducted by McIntosh and colleagues in the 1970s [Reference McIntosh5] and by Jupp and colleagues in the 1980s [Reference Jupp6] have shown that human SINV infections occur infrequently during the summer across the central plateau of South Africa, including Gauteng, Free State and Northern Cape provinces. A large epidemic affecting an estimate of thousands of people in the Karoo and Northern Cape occurred in South Africa in 1974 [Reference McIntosh5, Reference Jupp6]. A second epidemic was recorded in the Pretoria/Witwatersrand region during 1984, affecting hundreds of people [Reference Jupp6]. The knowledge of the epidemiology and public health burden of the disease in South Africa remains largely obscure. Here we briefly report the epidemiological characteristics of SINV infection in humans in South Africa for 2006–2010, based on a retrospective study of suspected arthropod-borne virus (arbovirus) cases submitted for laboratory investigation.
The Centre for Emerging and Zoonotic Diseases (CEZD) of the National Institute for Communicable Diseases of the National Health Laboratory Service (NICD-NHLS) is the reference centre for the laboratory confirmation of human cases of arbovirus infection in South Africa. Specimens are subjected to serological screening using an in-house haemagglutination inhibition (HAI) assay followed by an in-house IgM ELISA. Reverse transcription PCR and virus isolation may also be performed in acute cases; however, due to the short period of viraemia associated with SINV infection [Reference Sane7], these assays were not included in this study. From 1 January 2006 to 31 December 2010, a total of 3631 specimens from patients with suspected arboviral infections were submitted to the CEZD for laboratory investigation. Cases were deemed positive for recent SINV infection with the detection of anti-SINV IgM antibodies. The following data-points were collected from test requisition forms and questionnaires as available: patient's date of birth, gender, geographical area, symptoms and date of specimen collection. Data analysis was performed using a Microsoft Excel database (Microsoft, USA) and statistical analysis was performed using Epi Info software v. 3·5·3 (CDC, USA).
For the period 2006–2009, a total of 87 (5·4%) specimens out of 1606 tested positive for Sindbis virus on the HAI screen. On further analysis, anti-SINV IgM antibodies were detected in only 21/87 HAI-positive cases, relating to an anti-SINV IgM detection rate of 1·3% (21/1606).
In 2010, a rise in the number of SINV cases was noted compared to the preceding period [odds ratio (OR) 8·64, 95% confidence interval (CI) 5·39–13·99, P < 0·001] (Fig. 1), with 243 HAI-positive cases out of 2025 investigated. Of the 243 HAI-positive cases, 208 were IgM positive (208/2025, anti-SINV IgM detection rate of 10%).

Fig. 1. Histogram indicating the number of specimens submitted for arbovirus testing vs. the detection of anti-SINV IgM, for 2006–2009 and for 2010. * Cumulative number. † Sindbis detection rate = (no. of Sindbis IgM positives/no. samples tested) × 100.
Follow-up specimens were not available to test for rise in IgG titre in HAI-positive cases that tested IgM negative. These cases may either present previous infection with SINV or acute cases that can only be confirmed by observing a rise in IgG titre. From previous studies, it is known that up to 60% of SINV patients do not show anti-SINV IgM antibodies within the first week of illness, therefore, a negative serology test is not uncommon during this period [Reference Kurkela3]. Further to this, the persistence of anti-SINV IgM is not known. Kurkela and colleagues showed that IgM antibodies persisted up to 6 months after the onset of disease in 36% of patients [Reference Kurkela3]. Vene and colleagues [Reference Vene, Franzen and Niklasson8] studied the IgM and IgG antibody patterns of SINV infection in 16 patients presenting with symptoms typical for SINV disease. Fifteen of these patients developed IgM antibodies against SINV within 2 weeks of the onset of symptoms. After 30 months, all of the patients tested negative for SINV IgM antibodies [Reference Vene, Franzen and Niklasson8]. Detection of virus-specific IgM together with the clinical presentation of the patient is, however, interpreted as a diagnostic result.
Of the 3631 specimens that were submitted from 2006 to 2010, almost twice the number of specimens were received from men (64%) compared to women (35%). The anti-SINV IgM antibody detection rate was higher for men (7%, 167/2334) than for women (5%, 62/1265) (OR 1·49, 95% CI 1·09–2·03, P = 0·009). This is possibly due to an increased frequency of mosquito bites in men due to employment in the farming sector and outdoor-associated labour. However, a limitation of this study is that the results obtained for gender may be biased since most of the specimens tested for SINV were originally submitted from farmers and farm workers [who are at the highest risk of Rift Valley Fever virus (RVFV) infection] for RVFV investigation. The results obtained for gender in this study differed from the results reported in studies conducted in Finland, which showed that women were more frequently infected with SINV [Reference Brummer-Korvenkontio9, Reference Kurkela10]. These authors suggested that this was because women were more likely to be exposed to mosquito bites while taking walks in the Finnish countryside to pick mushrooms and berries [Reference Brummer-Korvenkontio9].
The majority of specimens submitted for arbovirus investigation between 2006 and 2010 were received from persons aged between 20 and 49 years (2279/3537 specimens for which age data were available), while the least number of specimens were received from persons aged <10 years (176/3537) and >70 years (86/3537). Only 7% (15/229) of persons infected with SINV were aged <18 years. The risk for acquiring a SINV infection increased linearly with age [<10 years: 2% (3/176); 10–19 years: 5% (16/291); 20–29 years: 4% (32/716); 30–39 years: 4% (35/845); 40–49 years: 9% (64/718); 50–59 years: 8% (42/497); 60–69 years: 8% (16/208); >70 years: 14% (12/86); P < 0·001], while the average age of persons infected with SINV was 42 (range 7–85) years. This is comparable to results found by Kurkela and colleagues in studies conducted in Finland, which showed that the average age of persons infected with SINV was 41 years [Reference Kurkela3, Reference Kurkela10].
The months during which the majority of SINV infections were diagnosed (March and April) correspond to the period during which Culex univittatus mosquitoes are abundant in South Africa [Reference Rautenbach11]. The majority of specimens submitted and for which geographical data was available (2197/3631), originated from Gauteng province (32%, 709/2197), followed by the Free State and the Northern Cape provinces [26% (572/2197) and 11% (251/2197), respectively]. The remaining 31% of specimens were sent from the other six provinces of South Africa; the Eastern Cape (7%, 153/2197), North West (7%, 153/2197), Western Cape (9%, 208/2197), KwaZulu-Natal (3%, 75/2197), Limpopo (0%, 0/2197) and Mpumalanga (3%, 76/2197). During the study period (2006–2010), the anti-SINV IgM detection rate was higher in the Free State (18%, 102/572) and Northern Cape (14%, 36/251) provinces compared to the anti-SINV IgM detection rates of the other provinces [Eastern Cape: 3% (5/153); Gauteng: 6% (40/709); North West: 5% (8/153); Limpopo: 0% (0/16); KwaZulu-Natal: 3% (2/75); Mpumalanga: 5% (3/60); and Western Cape: 5% (11/208)] (Fig. 2). This distribution of cases corresponds with the distribution for SINV proposed by earlier studies [Reference McIntosh5].
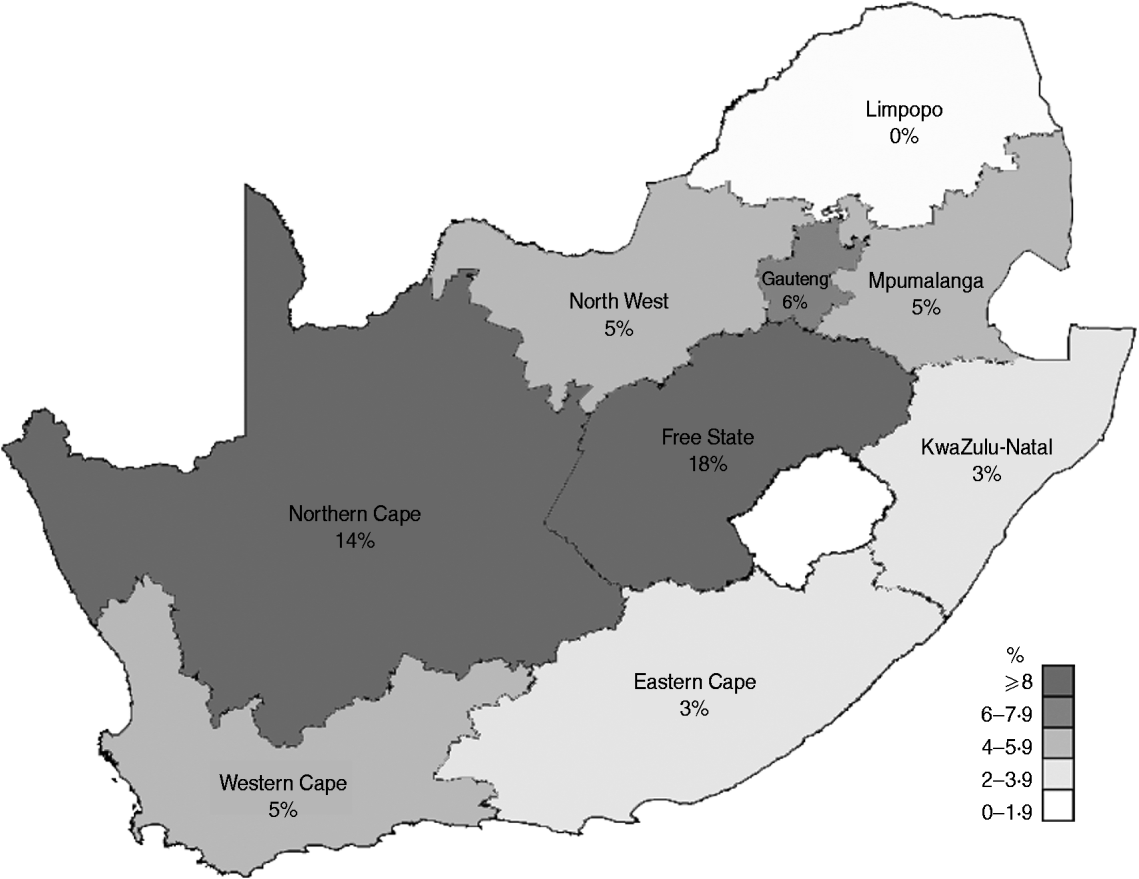
Fig. 2. Map showing the anti-SINV IgM detection rate in each province of South Africa for the period 2006–2010.
Based on an analysis of questionnaires on SINV laboratory-confirmed cases, which were sent to clinicians retrospectively, the most frequently observed symptoms for SINV infection included fever (39/58, 67%), myalgia (45/58, 78%), arthralgia (20/58, 35%), headache (40/58, 69%) and fatigue (20/58, 35%). These findings are consistent with those of other studies conducted in Finland regarding the major symptoms of SINV infection [Reference Kurkela3, Reference Turunen12].
To date, no fatal cases of Sindbis fever have been reported. Similarly, no fatalities or complicated cases were noted for this cohort of cases.
In summary, Sindbis fever appears to be a sporadic but continuous occurrence in the Northern Cape, Free State and Gauteng provinces of South Africa. An increase in the number of cases reported annually was recorded during 2010. This coincided with an outbreak of Rift Valley fever in South Africa and was ascribed to above average rainfall which in turn provided favourable breeding grounds for mosquito vectors. Sindbis fever affects mostly middle-aged men and has not been associated with severe presentations. Further investigation to clarify the burden of SINV-related disease in South Africa should address the prevalence and incidence of the disease in endemic areas. Morbidity may also be underestimated considering that patients included in this study did require medical consultation.
ACKNOWLEDGEMENTS
We thank the National Research Foundation (W.M., grant no. 66187), the Poliomyelitis Research Foundation (N.S., M.Sc. bursary 10/56), the University of Pretoria (N.S.) and the National Health Laboratory Service Research Trust (J.W., grant no.94185) for providing the financial support for this project.
DECLARATION OF INTEREST
None.




