Obesity is a growing problem on a global scale among populations in both developing and affluent countries. Pregnancy and the postpartum period is a time of increased maternal vulnerability to weight gain and body composition changes. Although many women have a desire to return to their pre-pregnancy weight after childbirth( Reference Krummel 1 ), very few achieve this goal( Reference Lovelady 2 – Reference Williamson, Madans and Pamuk 4 ). Excessive postpartum weight retention (PPWR) can contribute to maternal long-term obesity and be associated with CVD, hypertension, diabetes and degenerative joint disease( Reference Rooney and Schauberger 5 , Reference Linne and Neovius 6 ).
Although breast-feeding (BF) is associated with health benefits for both mother and baby( Reference Ip, Chung and Raman 7 , Reference Schwarz, Ray and Stuebe 8 ), its role in postpartum weight management remains unclear. Theoretically, BF should decrease PPWR during the postpartum period as it utilizes energy, but in fact some women may gain weight during lactation( Reference Butte and Hopkinson 9 ). Because of the hypothesized fat mobilization during lactation, BF is often considered as a factor that facilitates postpartum weight loss. However, given the many factors that may influence postpartum weight change, such as socio-economic status( Reference Shrewsbury, Robb and Power 10 ), ethnicity( Reference Parker and Abrams 11 , Reference Boardley, Sargent and Coker 12 ), pre-pregnancy weight( Reference Potter, Hannum and McFarlin 13 – Reference Brewer, Bates and Vannoy 15 ), parity( Reference Gunderson, Abrams and Selvin 16 ), gestational weight gain (GWG)( Reference Gunderson, Abrams and Selvin 16 ) and lifestyle( Reference Walker, Timmerman and Sterling 17 , Reference Oken, Taveras and Popoola 18 ), the weight-reducing effects of postpartum lactation remain in dispute.
Some studies have shown that BF significantly reduces PPWR( Reference Baker, Gamborg and Heitmann 19 – Reference Preer, Newby and Philipp 26 ). The energy needs of lactating women are about 2090 kJ/d greater than those of non-lactating mothers( Reference Potter, Hannum and McFarlin 13 ), which reflects the fact that producing more milk requires additional energy and, in the absence of restriction of food intake or changes in physical activity, should lead to greater weight loss. Other studies have reported no effect of BF on maternal anthropometry and body composition( Reference Dugdale and Eaton-Evans 27 – Reference Caire-Juvera, Casanueva and Bolanos-Villar 30 ). These differences may be due to the intensity and duration of BF, study population (source, size, location, loss to follow-up), how weight and weight retention were assessed, how BF was assessed and statistical methods.
To our knowledge, there have not been any quantitative attempts to further explore the possible BF–PPWR association. Given that obesity is considered to be a public health problem, a more clear understanding the role of BF in weight management is very necessary. Therefore, we carried out a systematic review and meta-analysis aiming to help clarify the association between BF and PPWR.
Methods
Search strategy and study selection
We performed a detailed search for studies that examined the association between BF and PPWR. A search of the literature was made by using Medline (PubMed, http://www.bdpubmed.com/), EMBASE (http://www.embase.com/) and Cochrane library (http://www.thecochranelibrary.com/) from their inception to October 2014 to identify relevant articles. References in key studies were reviewed to identify additional studies not indexed by Medline, EMBASE or Cochrane library.
We used the following search terms: ((‘Breastfeeding’ OR ‘formula feeding’ OR ‘bottle-feeding’ OR ‘lactation’ OR ‘non-lactation’) AND (‘weight loss’ OR ‘weight change’ OR ‘weight retention’ OR ‘body composition’) AND (‘postpartum’ OR ‘parturition’ OR ‘postnatal period’ OR ‘childbirth’) AND (‘mother’ OR ‘women’)).
Randomized controlled trials (RCT) and cohort studies were included, irrespective of sample size or follow-up duration. In addition, a hand search of reference lists of relevant and related articles was made to ensure a complete collection. The first step was a systematic review of all eligible studies on healthy women, the studies had to be published in English and report the association between BF and PPWR; in the second step a meta-analysis was conducted. Studies included in the meta-analysis had to meet the following inclusion criteria: (i) RCT or cohort study; (ii) examine infant feeding method in relation to the outcome; (iii) have data on weight change or weight retention; (iv) report both mean and standard deviation; (v) measurement of weight, rather than self-reported; and (vi) include BF (exclusive breast-feeding (EBF) or mixed breast-feeding (MF)) and formula-feeding (FF) groups. BF practices were defined as: (i) EBF, when the child received no water, tea, juice or food; (ii) MF, when the child received human milk, water, tea, juice but no food; and (iii) FF, when the child was not breast-fed.
Screening and data-extraction form
All search hits were exported to Endnote X4 (Thomson Reuters), which was used to organize the references and eliminate duplicates. Initially, two investigators (X.T. and Y. Li) independently screened the articles identified in the searches according to the predetermined criteria in order to select potentially relevant citations based on titles and abstracts; potential disagreements were resolved through consensus. For articles with relevant citations or with titles/abstracts that were not sufficient for deciding on inclusion criteria, the full-text articles were retrieved and evaluated. The following characteristics were extracted from the articles: (i) author; (ii) country; (iii) time period; (iv) sample size; (v) whether or not BF and weight retention were variables of interest; (vi) BF intensity and duration; and (vii) adjustment for potential confounding factors.
Assessment of study quality
Study quality was assessed based on: (i) follow-up rate (1 point for follow-up rate ≥75 %); (ii) clear definition of exposure and outcome about BF and PPWR (if definition or outcome was reported, then 1 point was awarded); and (iii) inclusion and exclusion criteria (if criteria were reported, then 1 point was assigned)( Reference Juni, Altman and Egger 31 ). Thus, the potential maximum score was 6 points; a high-quality study was defined as a study with ≥5 points. Two reviewers (X.T. and Y. Li) evaluated the quality of each study. A third reviewer (Q.W.) was designated to make a final decision if the initial two reviewers were unable to reach consensus.
Statistical analysis
We used the mean differences in weight loss of breast-feeders minus that of formula-feeders for meta-analysis. These differences were used to take account of the time dependency of weight change after pregnancy, which means that the effect variable is standardized mean difference (SMD). The pooled SMD and corresponding 95 % confidence intervals were calculated by using the inverse variances method( Reference DerSimonian and Laird 32 , Reference Higgins, Thompson and Deeks 33 ). We examined heterogeneity in results across studies by using the χ 2 test and I 2 statistics( Reference Higgins, Thompson and Deeks 33 ). The null hypothesis that the studies are homogeneous was rejected if the P value for heterogeneity was <0·10 or I 2 was >50 %. When substantial heterogeneity was detected, the summary estimate on the basis of the random-effects model (using the method of DerSimonian and Laird( Reference DerSimonian and Laird 32 )) was presented. Otherwise, the pooled estimate that was based on the fixed-effects model (using the inverse variance method( Reference Woolf 34 )) was presented. Subgroup analyses were carried out by study design (RCT v. cohort studies), study quality (≥5 points v. <5 points), number of confounding factors adjusted for (<6 v. ≥6) and study population (Americans v. non-Americans). We conducted a sensitivity analysis by excluding each study one by one and recalculating the combined estimates on the remaining studies to assess the effect of individual studies on the pooled result. We used Egger’s test (linear regression method)( Reference Egger, Davey Smith and Schneider 35 ) and Begg’s test (rank correlation method)( Reference Begg and Mazumdar 36 ) to evaluate potential publication bias. Meta-analysis was performed with the statistical software package Stata/SE version 9.
Results
Identification of studies
The applied search strategy yielded 349 potentially relevant publications in Medline, EMBASE and Cochrane library. No additional articles were found in the citations of the relevant studies by manual search. The evaluation of the 349 publications is shown in Fig. 1.
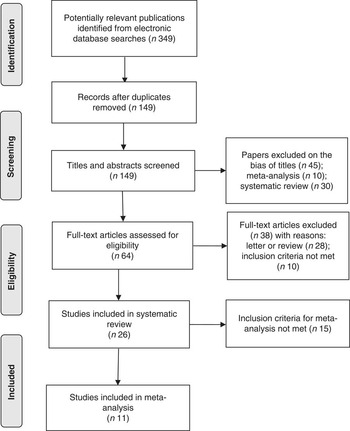
Fig. 1 Flow diagram of article selection according to PRISMA (Preferred Reporting Items for Systematic Reviews) guidelines
Results of the systematic review
In total, twenty-six studies met the inclusion criteria of the systematic review; fifteen of them were not eligible for the meta-analysis( Reference Baker, Gamborg and Heitmann 19 , Reference Mok, Multon and Piguel 20 , Reference Mulder, Johnson and Baker 22 , Reference Walker and Freeland-Graves 28 , Reference da Silva, Oliveira Assis and Pinheiro 37 – Reference Ostbye, Peterson and Krause 47 ) because they did not meet the inclusion criteria of the meta-analysis (see online supplementary material, Table S1). Most of the studies excluded from the meta-analysis had no control group( Reference Baker, Gamborg and Heitmann 19 , Reference Mulder, Johnson and Baker 22 , Reference da Silva, Oliveira Assis and Pinheiro 37 , Reference Murnane, Arpadi and Sinkala 38 , Reference AbuSabha and Greene 41 , Reference Quandt 43 , Reference Nommsen-Rivers, Chantry and Peerson 45 ). Although six of these thirteen studies had both BF and the control group, they still did not meet the inclusion criteria( Reference Mok, Multon and Piguel 20 , Reference Walker and Freeland-Graves 28 , Reference Cohen, Larson and Matthews 39 , Reference Janney, Zhang and Sowers 40 , Reference Gigante, Victora and Barros 42 , Reference Ota, Haruna and Matsuzaki 44 ). The study by Cohen et al.( Reference Cohen, Larson and Matthews 39 ) was a cross-sectional study, while the study by Mok et al.( Reference Mok, Multon and Piguel 20 ) was a case–control study. The cohort studies by Janney et al.( Reference Janney, Zhang and Sowers 40 ) and Ota et al.( Reference Ota, Haruna and Matsuzaki 44 ) did not report the data of weight change, whereas the studies of Gigante et al.( Reference Gigante, Victora and Barros 42 ) and Walker et al.( Reference Walker and Freeland-Graves 28 ) did not report the standard deviation of the data. Nine of thirteen studies showed a protective effect of BF against PPWR( Reference Baker, Gamborg and Heitmann 19 , Reference Mok, Multon and Piguel 20 , Reference Mulder, Johnson and Baker 22 , Reference da Silva, Oliveira Assis and Pinheiro 37 , Reference Cohen, Larson and Matthews 39 – Reference AbuSabha and Greene 41 , Reference Quandt 43 , Reference Nommsen-Rivers, Chantry and Peerson 45 ), whereas the other four studies failed to achieve significance( Reference Walker and Freeland-Graves 28 , Reference Murnane, Arpadi and Sinkala 38 , Reference Gigante, Victora and Barros 42 , Reference Ota, Haruna and Matsuzaki 44 ). In addition, another two studies( Reference Brandhagen, Lissner and Brantsaeter 46 , Reference Ostbye, Peterson and Krause 47 ) were excluded because weight was not measured. Reasons for exclusion from the meta-analysis are shown in Table S1.
Results of the meta-analysis
Study characteristics and quality assessment
Eleven studies were eligible for the meta-analysis comprising more than 37 000 women included in the final analysis( Reference Brewer, Bates and Vannoy 15 , Reference Butte, Hopkinson and Mehta 48 – Reference Dujmović, Kresić and Mandić 57 ). Table 1 shows characteristics of these studies and potential confounders, for which adjustment was made.
Table 1 Characteristics of the studies that met the inclusion criteria of the meta-analysis
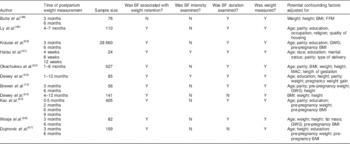
N, no; Y, yes; FFM, fat-free mass; GWG, gestational weight gain; MAC, mid-arm circumference.
Seven studies were conducted in the USA, one in Brazil, one in France, one in Georgia and one in Croatia. Most individual studies were matched or adjusted for a wide range of potential confounders, including age, pre-pregnancy weight, pre-pregnancy BMI, education and parity. The results of individual studies are presented in the online supplementary material, Table S2. For the meta-analysis, we generated four categories covering similar postpartum time periods (see online supplementary material, Table S3).
As a whole, the methodological quality of the included trials was acceptable (see online supplementary material, Table S4). Study design and inclusion and exclusion criteria were mostly well recorded. An explicit definition for PPWR was reported in eleven articles. Nine of eleven studies had follow-up rates of 75 % or more. In cohort studies and in the RCT, the percentage of women lost to follow-up ranged from 0 to 10 %. Bias in weight measurement was unlikely in eleven studies.
Overall analyses
The homogeneity hypothesis was rejected by χ 2 test (P<0·05, I 2 =96·9 %), thus we selected the random-effect model. There was non-significant effect (−0·09 kg; 95 % CI −0·76, 0·58 kg) at 1 to ≤3 months postpartum (see online supplementary material, Fig. S1). Compared with formula-feeders, breast-feeders lost 0·87 kg (95 % CI 0·57, 1·17 kg) more weight (Fig. 2). In addition, breast-feeders lost 0·37 kg more weight (95 % CI 0·14, 0·61 kg) than formula-feeders at 9 to ≤12 months postpartum (see online supplementary material, Fig. S3). This association was non-significant at 6–≤9 months postpartum (0·21 kg; 95 % CI −0·42, 0·83 kg; online supplementary material, Fig. S2). There was no indication of a publication bias from the result of either Egger’s test (P=0·635) or Begg’s test (P=0·635).
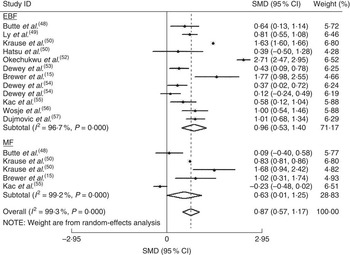
Fig. 2 Estimates for the standardized mean difference (SMD) of breast-feeding (EBF, exclusive breast-feeding; MF, mixed breast-feeding) v. formula-feeding on weight loss at 3–6 months postpartum. Study-specific SMD estimates are represented by grey squares, where the size of the square reflects the study-specific statistical weight (i.e. inverse of the variance), and their 95 % CI are represented by horizontal lines. The centre of the diamond presents the pooled SMD and its width represents the pooled 95 % CI. Dewey et al.( Reference Dewey, Cohen and Brown 54 ) provided two results, one for primiparous mothers (effect size for primiparous mothers=1) and one for mothers of low-birth-weight infants (effect size for mothers of low-birth-weight infants=2)
Subgroup and sensitivity analyses
The effects of BF on PPWR in subgroup meta-analyses are shown in Table 2. When stratified by study design, the analysis of RCT yielded an SMD of 0·57 kg (95 % CI 0·19, 0·94 kg), whereas the analysis on cohort studies yielded an SMD of 1·18 kg (95 % CI 0·74, 1·62 kg). In addition, the results did not change substantially after the analyses were stratified by some confounding factors (quality of studies, number of confounding factors adjusted for and study population).
Table 2 Sensitivity analyses of studies included in the meta-analysis of breast-feeding on postpartum weight retention
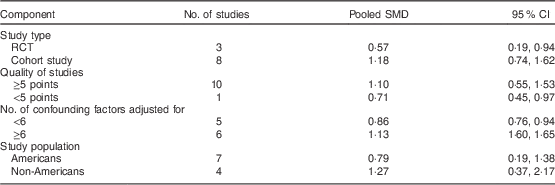
SMD, standardized mean difference; RCT, randomized controlled trial.
In sensitivity analyses, we recalculated the combined results by excluding one study per iteration. The eleven study-specific SMD ranged from a low of 0·57 kg (95 % CI 0·19, 0·94 kg) to a high of 1·27 kg (95 % CI 0·37, 2·17 kg) and were similar without great fluctuation.
Discussion
A total of twenty-six epidemiological studies that consisted of three RCT and twenty-three cohort studies were included and eleven studies met the inclusion criteria of the meta-analysis. Seven studies were conducted in the USA, one in Brazil, one in France, one in Georgia and one in Croatia. The aim of the current systematic review and meta-analysis was to examine the evidence to date regarding the role of BF in PPWR. The available evidence held belief that BF decreases PPWR. The fact that we found an association between BF and PPWR at 6 months and not at 3 months postpartum may indicate that certain BF duration is necessary for the maximal effect to be observed. Lof and Forsum( Reference Lof and Forsum 58 ) showed that expansion of plasma volume during pregnancy can persist during at least the first month postpartum. They measured body water in healthy women before, during and after pregnancy and reported an average of 2 kg of fluid remaining at 2 weeks postpartum.
In theory, weight change is supported by negative energy balance due to either increased energy expenditure or reduced energy intake, or both. Although postpartum lactation increases energy expenditure significantly due to the production of milk in the mammary glands( Reference Butte and Hopkinson 9 ), it is also accompanied by increased energy intake( Reference Dewey, Heinig and Nommsen 53 ), so PPWR cannot be explained merely by changes in energy expenditure or energy intake alone. Thus, we speculate that postpartum weight change may relate mainly to hormonal/metabolic changes induced by lactation. Indeed, after parturition, withdrawal of progesterone and the suckling of the breast by the infant facilitate the release of prolactin, thereby decreasing the level of oestrogen( Reference Butte and Hopkinson 9 ), which in turn enhances the mobilization of adipose tissue stores( Reference Pansini, Bonaccorsi and Genovesi 59 ). Furthermore, since prolactin also inhibits lipogenesis( Reference Ben-Jonathan, Hugo and Brandebourg 60 ) and suppresses glucose uptake in adipose tissue( Reference Nilsson, Roepstorff and Kiens 61 ), it is conceivable that the pregnancy-induced pattern of fat deposition may be reversed during lactation by the fluctuating hormones.
Although the majority of the studies included in the systematic review found significant associations between BF and PPWR, or significant differences in weight change between BF and FF women, it is difficult to make any firm conclusions, as many of the associations observed depended on the time at which the postpartum measurements were carried out. Among the studies that did show a positive influence of BF on weight loss, the associations tended to be relatively weak and were often confounded by other factors, such as GWG, physical activity and pre-pregnancy weight. Associations also appeared to be dependent on the duration and intensity of BF.
It appeared that for the majority of studies, BF for <3 months had little or no influence on weight change, whereas there was some evidence to suggest that BF, if continued for >6 months, may have a positive influence on weight change; but again this was not supported by all of the studies and in many of them the associations were only observed in women who continued BF until 12 months postpartum. However, our meta-analysis showed that compared with FF, BF for 3–6 months seemed to have a positive influence on weight change, and if BF continued for >6 months may have little or no influence on weight change. These differences may be due to the intensity and duration of BF, the population under study (source, size, location, loss to follow-up), how weight and weight retention were assessed, how BF was assessed and statistical methods. Nevertheless, the review provides a valuable insight into the studies to date and the findings should be useful in guiding the development of future studies.
In relation to definition of BF, it appeared that the assessment of exposure to BF differed from study to study: most of the studies compared women who breast-fed their infants with women who formula-fed infants, while a few studies compared women who have lactation with women who have non-lactation (Table 1). However, in a sensitivity analysis, homogeneity between the studies stratified by different definitions of feeding could not be rejected (Table 2).
On the other hand, there are a number of known predictors for PPWR, such as GWG( Reference Gunderson, Abrams and Selvin 16 ), pre-pregnancy weight, physical activity and other lifestyle factors( Reference Walker, Timmerman and Sterling 17 ). Because we cannot exclude residual confounding, so we could not draw any confirm conclusions regarding the role of BF in weight change. The effect of BF might not be a genuine risk factor for PPWR but rather reflect a common cause for PPWR. Irrespective of the underlying mechanisms, these data suggest that BF may help to reduce PPWR. A potentially beneficial effect of BF on PPWR needs to be balanced against other risks of PPWR. As pre-pregnancy weight and GWG were frequently cited as strong contributing factors to PPWR, observational studies should commence preconception with continued monitoring into the postpartum period, to capture the true trajectory of weight change.
Strengths and limitations
The possibility that BF may assist women in minimizing weight retention after pregnancy has long been controversial. Thus, a systematic review and meta-analysis was performed to examine the effect estimate of BF on PPWR. Broad search terms and multiple bibliographic databases were used in the searches to capture as many relevant papers as possible, and a robust systematic approach was used to select the final papers. To disentangle the effect of BF on PPWR, we studied both BF and FF postpartum. On the basis of results of our meta-analysis, we propose to use the term ‘PPWR’ only for weight retention within a limited postpartum period, for example up to 6 months or 9 months postpartum. However, because of the limitation of the data, the conclusion should be considered with caution. A large and well-designed study that addresses various patterns of BF in separate analysis by precise definitions of BF is warranted and several additional measurements, for example until 12 months or 24 months postpartum, would be necessary to provide any definitive findings.
In addition, a classical meta-analysis requires RCT. Randomization of BF on an individual level is not ethical, however. Unfortunately, there are no cluster-randomized controlled trials on BF and weight change in the literature.
Conclusions
Our meta-analysis showed that compared with FF, BF for 3 to ≤6 months seemed to have a negative influence on PPWR; however, BF continued for >6 months may have little or no influence on PPWR. As we cannot exclude residual confounding, it is difficult to draw any firm conclusions. More robust studies are needed to reliably assess the impact of patterns and duration of BF on postpartum weight retention.
Acknowledgements
Financial support: This work was supported by the National Natural Science Foundation of China (grant number 81373011). The National Natural Science Foundation of China had no role in the design, analysis or writing of this article. Conflicts of interest: None. Authorship: X.H. and M.Z. contributed equally to this work and should be considered co-first authors. X.H. and M.Z. contributed to the literature review, meta-analysis and principal authorship of the article; X.T., Y. Li and Q.W. contributed to conception of the research question, literature review, data extraction and final draft of the manuscript; C.H. contributed to the first and final drafts of the manuscript; Y. Liu reviewed the grammar of the manuscript. Ethics of human subject participation: Ethical approval was not required.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980015000828







