Dietary fibre, a complex group of substances with various physicochemical properties, has been shown to improve glucose homeostasis (Kimmel et al. Reference Kimmel, Michel, Hess and Ward2000; Chau et al. Reference Chau, Chen and Lee2004) and has consistently been linked to a reduced risk of type 2 diabetes and cardiovascular disease in population studies. In short-term intervention studies, viscous soluble dietary fibre has been shown to improve glucose handling after a meal via decreased absorption rates of carbohydrates (Tabatabai & Li, Reference Tabatabai and Li2000). Soluble fibre has also been shown to reduce postprandial insulin responses (Del Toma et al. Reference Del Toma, Lintas, Clementi and Marcelli1988). On the other hand, the consumption of highly purified insoluble dietary fibre has been shown to accelerate the acute insulin response directly after consumption, and to further enhance postprandial carbohydrate handling the following day upon the ingestion of a control meal, which has been explained by improved whole-body insulin sensitivity (Weickert et al. Reference Weickert, Mohlig, Schofl, Arafat, Otto, Viehoff, Koebnick, Kohl, Spranger and Pfeiffer2006a).
In whole foods such as cereals, other bioactive substances beyond dietary fibre might contribute to metabolic control. We recently showed that the consumption of a standardized liquid meal enriched with a polyphenol-rich insoluble dietary fibre preparation from carob pod (carob fibre) decreased serum triacylglycerols and NEFA, as well as the secretion of the gastrointestinal hormone ghrelin (Gruendel et al. Reference Gruendel, Garcia, Otto, Mueller, Steiniger, Weickert, Speth, Katz and Koebnick2006). Ghrelin is a gut hormone with multiple functions, produced primarily in the stomach to regulate energy balance. Acylated ghrelin is one of two major forms of ghrelin; it is known to exhibit most of the biological activities of ghrelin, including orexigenic effects via hypothalamic pathways (Korbonits et al. Reference Korbonits, Goldstone, Gueorguiev and Grossman2004). Ghrelin secretion has been controversially associated with insulin and glucose responses. Postprandial ghrelin secretion is affected by the caloric, macronutrient and fibre content of ingested meals (Erdmann et al. Reference Erdmann, Topsch, Lippl, Gussmann and Schusdziarra2004; Weickert et al. Reference Weickert, Spranger, Holst, Otto, Koebnick, Mohlig and Pfeiffer2006b), but preprandial secretion is not modified.
Our recent findings indicated that carob fibre rich in polyphenols decreases ghrelin secretion after a liquid meal challenge test containing 34 % energy as fat. The effects of carob fibre within a differing food matrix are, however, unknown. Additionally, polyphenols have been shown to have effects on glucose handling (Thompson et al. Reference Thompson, Yoon, Jenkins, Wolever and Jenkins1984; Kamei et al. Reference Kamei, Kitagawa, Kadokura, Hattori, Hazeki, Ebina, Nishihara and Oikawa2002).
Therefore, the aim of the present study was to investigate the dose-dependent effects of carob fibre on postprandial glucose handling and ghrelin response when administered in combination with a glucose load.
Subjects and methods
Subjects
Twenty healthy adults (twelve women and eight men) participated in the study. Inclusion criterion was a BMI ( = body weight (kg) / height (m)2) within the normal range (18·5–25 kg/m2). Exclusion criteria were a history of chronic diseases, dyslipidaemia (fasting triglycerides >2·3 mmol/l, total cholesterol >5·2 mmol/l, LDL-cholesterol >4·0 mmol/l, HDL-cholesterol < 0·9 mmol/l), impaired glucose tolerance (fasting glucose >6·1 mmol/l), intentional weight loss within 3 months prior to the study, extreme sports and any medication influencing glucose or lipid metabolism. The ethics committee of the University of Potsdam approved the study protocol. All participants gave written consent before starting the study.
Research design
The study was conducted as a randomized cross-over trial consisting of four sessions lasting 180 min each, separated by intervals of at least 1 week. Subjects entered our facilities between 07.30 and 08.00 hours after an overnight fast. Subjects consumed 200 ml water with 50 g glucose (Dextropur; Maizena Diät GmbH, Heilbronn, Germany) and 0, 5, 10 or 20 g carob fibre provided in randomized order. The energy content of the glucose preparation was 857 kJ. The carob fibre contained 5·8 g simple carbohydrates (glucose, fructose and sucrose), 5·2 g protein and 0·2 g fat per 100 g. The total dietary fibre content was 74·6 g per 100 g carob pulp preparation, corresponding to 68·4 g insoluble and 6·2 g soluble fibre. The total polyphenol content of the preparation was 2·8 g per 100 g. The major polyphenol compounds of carob fibre are gallic acid, gallotannins and flavonol glycosides. The energy contribution of the carob fibre enrichment was negligible with respect to the total energy intake of the glucose load (1·2 % for 5 g, 2·3 % for 10 g, and 4·7 % for 20 g carob fibre). Participants were blinded to the fibre content of the test meals, but due to taste and colour it was possible to distinguish the meals. Participants were not allowed to consume food or beverages during the examinations.
Blood sampling and biochemical analysis
An indwelling catheter was inserted into an antecubital vein of the subjects, and blood samples were collected before and 15, 30, 45, 60, 75, 90, 120 and 180 min after administration of the test meal.
For plasma separation, blood was collected in EDTA-containing tubes and immediately centrifuged. To obtain serum, blood was collected in tubes containing a serum clot activator and allowed to clot. Plasma and serum samples were centrifuged at 1500 g for 10 min at 4°C; plasma and serum supernatants were obtained and stored at − 40°C. For measurement of ghrelin, plasma was collected in tubes containing EDTA and 500 U aprotinin (Bayer, Leverkusen, Germany) per millilitre whole blood. For stabilization plasma supernatant was acidified with 1 M-HCl (Merck, Darmstadt, Germany) and stored at − 40°C.
Plasma glucose was measured by the hexokinase method using commercially available colorimetric reagents (ABX Diagnostics Glucose; ABX Diagnostics, Montpellier, France) with an intra-assay CV of 1·2 %. Serum insulin was determined by Mercodia Insulin ELISA assay (Mercodia, Uppsala, Sweden) with an intra-assay CV of 2·6 %. Homoeostasis model assessment for insulin resistance (HOMA-IR) was calculated as [fasting insulin (mU/l) × fasting glucose (mmol/l)/22·5].
Plasma total ghrelin was measured by a commercial RIA, which utilizes 125I-labelled ghrelin and a ghrelin antiserum to determine the concentration of total ghrelin in the plasma by a double-antibody/polyethyleneglycol technique (Linco Research, Missouri, MO, USA). The RIA was specific for the C-terminal portion of ghrelin (amino acids 14–28). The sensitivity of the method was 93 pg/ml. There were no cross-reactions with ghrelin 1–10, motilin-related peptide, glucagon, leptin or insulin. The intra- and inter-assay CV were 10·0 % and 14·7 %, respectively, and the percentage recovery was calculated as 96 %. Plasma acylated ghrelin determination was performed using RIA that recognized the N-terminal portion of the ghrelin molecule (amino acids 1–10). It utilizes 125I-labeled ghrelin as a tracer and an antibody (raised in guinea pigs) that is specific for the octanoyl group on serine 3 (Linco Research). The sensitivity of the method was 7·8 pg/ml. There were no cross-reactions with ghrelin 14–28, motilin-related peptide, glucagon, leptin or insulin. The intra- and inter-assay CV were 6·7 % and 9·6 %, respectively. Percentage recovery was calculated as 114 %.
Serum total cholesterol was analysed using the cholesterol oxidase phenol 4-aminoantipyrine peroxidase (CHOD/PAP) method (ABX Diagnostics Cholesterol; ABX Diagnostics) with an intra-assay CV of 0·82 %. Serum HDL-cholesterol was analysed using a colorimetric test (ABX Diagnostics HDL Cholesterol Direct; ABX Diagnostics) with an intra-assay CV of 1·29 %. LDL-cholesterol was calculated according to Friedewald (Friedewald et al. Reference Friedewald, Levy and Fredrickson1972).
Statistical analyses
All statistical procedures were made using SPSS for Windows 11·5 (SPSS Inc., Chicago, IL, USA). Baseline characteristics are shown as mean and standard deviations, whereas figures show means and their standard errors. Time series data for all parameters and for each subject were normalized to baseline values, which was defined as time = 0 min. Differences in response to the glucose load were tested by a mixed linear model with time, carob concentration and carob concentration × time as fixed factors and subject as a random factor. Post hoc comparisons of carob fibre concentrations with the control treatment were adjusted according to the Bonferroni method. Percentage changes in the postprandial response of the outcome variables were calculated based on the response to the control treatment as a reference. The mean percentage increase or decrease was calculated for the time point with the highest (for glucose, insulin and triacylglycerols) or lowest (for ghrelin and NEFA) time points of the curves. A probability P < 0·05 was considered as significant.
Results
Twenty healthy adults aged 29·4 (SD 2·6) years (range 22–62 years) and with a BMI of 23·0 (SD 0·5 kg/m2 (range 20·4–27·5 kg/m2) participated in the study. Fasting plasma glucose was 5·1 (SD 0·7) mmol/l, and fasting serum insulin was 43·7 (SD 25·8) pmol/l.
After the glucose load, the plasma glucose and insulin concentrations increased (P < 0·001). The consumption of carob fibre significantly affected the glucose and insulin responses (P < 0·001). After the consumption of test meals with 5 and 10 g carob fibre, plasma glucose increased significantly, up to 147 % and 164 %, respectively (P < 0·001), compared with the glucose control drink. The consumption of 20 g carob fibre did not result in a significant increase in plasma glucose compared with control (P = 0·976) (Fig. 1(A)).
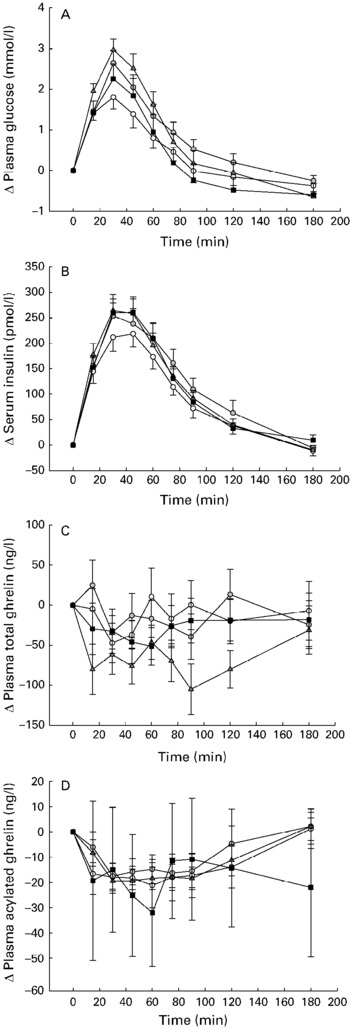
Fig. 1 Changes in plasma glucose (A), serum insulin (B), plasma total ghrelin (C), and plasma acylated ghrelin (D) as means and their standard errors relative to baseline after a test meal with or without carob fibre (200 ml water +50 g glucose +0 (–○–), 5 (–![]() –), 10 (–
–), 10 (–![]() –) or 20 (–■–) g carob) in healthy subjects (n 20).
–) or 20 (–■–) g carob) in healthy subjects (n 20).
After glucose load, acylated (P < 0·001) but not total plasma ghrelin decreased rapidly. No effects of carob fibre consumption on acylated plasma ghrelin were, however, observed (Fig. 1(D)). Total plasma ghrelin was slightly but significantly decreased compared with controls (P = 0·001) after the consumption of 10 g carob fibre. No effects were observed after 5 and 20 g carob fibre (Fig. 1(C)).
Discussion
The major finding of the present study was a significantly increased glucose and insulin response after consumption of a phenol-rich insoluble dietary fibre preparation made from carob, after a glucose load enriched with up to 10 g carob fibre, compared with a control. The increase in glucose response failed to reach statistical significance after the consumption of 20 g carob fibre, which might be explained by the higher variance in glucose response in the present experiment. In contrast to prior observations, carob fibre consumption did not alter the response of acylated ghrelin after a glucose load, with only minor changes in total ghrelin after 10 g carob fibre compared with control. Using a different food matrix (i.e. a liquid meal challenge test with 34 % of energy as fat), we recently reported that the consumption of insoluble dietary fibre from carob pulp significantly decreased postprandial acylated ghrelin and markedly improved the postprandial lipid response and fat oxidation (Gruendel et al. Reference Gruendel, Garcia, Otto, Mueller, Steiniger, Weickert, Speth, Katz and Koebnick2006).
Dietary fibre affects postprandial glucose and lipid responses by several mechanisms. Dietary fibre increases gastric distension, which has been suggested to trigger afferent vagal signals of fullness in some (Krotkiewski, Reference Krotkiewski, Bjorntorp, Vahouny and Kritchevsky1985; Saltzman & Roberts, Reference Saltzman and Roberts1997; Howarth et al. Reference Howarth, Saltzman and Roberts2001) but not all studies (Howarth et al. Reference Howarth, Saltzman, McCrory, Greenberg, Dwyer, Ausman, Kramer and Roberts2003). Some forms of soluble dietary fibre increase the viscosity of the intestinal tract contents, delay gastric emptying (Wolever & Jenkins, Reference Wolever, Jenkins and Spiller1993; Bonfield, Reference Bonfield, Kritchevsky and Bonfield1995), and decrease the absorption of macronutrients by inhibiting resorption (Slavin, Reference Slavin2005). In the present study, however, most of the effects described above are unlikely to be involved. First, effects of mastication were eliminated as a liquid solution supplemented with carob fibre powder was administered. Second, carob fibre mainly consisted of insoluble dietary fibre, which is unlikely to increase viscosity. Therefore, the effects of carob fibre could have been mediated through mechanisms related to the polyphenol content.
However, studies on the glucose- and insulin-regulating activities of polyphenols are controversial. It has been suggested that polyphenols exert both beneficial and detrimental effects on glucose metabolism. Polyphenols have been shown to lower blood glucose responses (Thompson et al. Reference Thompson, Yoon, Jenkins, Wolever and Jenkins1984) and to inhibit carbohydrate digestion by modulating the activity of α-amylase (Kandra et al. Reference Kandra, Gyemant, Zajacz and Batta2004; McDougall et al. Reference McDougall, Shapiro, Dobson, Smith, Blake and Stewart2005). Cell culture studies using 3T3-L1 adipocytes and MCF-7 breast cancer cells have shown that polyphenols inhibit the activity of phosphoinositide 3-kinase, a key regulator of insulin-induced GLUT4 translocation (Harmon & Patel, Reference Harmon and Patel2003, Reference Harmon and Patel2004). In isolated rat adipocytes, some polyphenols inhibited glucose uptake (Strobel et al. Reference Strobel, Allard, Perez-Acle, Calderon, Aldunate and Leighton2005). It has also been suggested that GLUT4 interacts directly with flavonoids and that GLUT transporters are involved in flavonoid incorporation into cells (Strobel et al. Reference Strobel, Allard, Perez-Acle, Calderon, Aldunate and Leighton2005). In contrast, other polyphenols may also stimulate glucose uptake in 3T3-L1 adipocytes via an insulin-independent tyrosine kinase pathway (Kamei et al. Reference Kamei, Kitagawa, Kadokura, Hattori, Hazeki, Ebina, Nishihara and Oikawa2002). In addition, it has been recently described that increased insulin sensitivity resulting from resistant starch consumption was not mediated by GLUT4. Therefore we cannot exclude the idea that the effects observed in the present study were mediated by the polyphenol contents rather than by dietary fibre per se.
Taken together, studies investigating the effects of polyphenols may partly explain our observations. The consumption of carob fibre appears to increase postprandial glucose and insulin responses in combination with a monosaccharide (glucose) load, an effect that is not observed when carob fibre is ingested within a liquid meal containing disaccharides, protein and fat (Gruendel et al. Reference Gruendel, Garcia, Otto, Mueller, Steiniger, Weickert, Speth, Katz and Koebnick2006). The mechanisms of action in the present study remain, however, speculative.
The lack of marked effects on total and acylated ghrelin responses observed in the present study is in contrast with a previous one (Gruendel et al. Reference Gruendel, Garcia, Otto, Mueller, Steiniger, Weickert, Speth, Katz and Koebnick2006) and may be explained by the background food matrix. It has been shown that postprandial ghrelin responses are influenced by the macronutrient content of food. Dietary carbohydrate ingestion produced more pronounced decreases in total ghrelin concentration when compared with protein- and fat-rich meals (Erdmann et al. Reference Erdmann, Lippl and Schusdziarra2003; Monteleone et al. Reference Monteleone, Bencivenga, Longobardi, Serritella and Maj2003). Recently, it has been shown that ghrelin secretion is suppressed by the presence of fat in the small intestine. Moreover, the fat-induced suppression of ghrelin is dependent on fat digestion (Feinle-Bisset et al. Reference Feinle-Bisset, Patterson, Ghatei, Bloom and Horowitz2005).
The decrease in acylated ghrelin caused by carob fibre consumption as observed after the liquid meal challenge test was not related to glucose and insulin responses and therefore, might be related to alterations in lipid metabolism (Gruendel et al. Reference Gruendel, Garcia, Otto, Mueller, Steiniger, Weickert, Speth, Katz and Koebnick2006). In the present study, carob fibre consumption after a glucose load did not result in relevant changes of plasma ghrelin concentration, a finding that we are unable to explain. In contrast, other studies have demonstrated that, after a glucose load, ghrelin levels decreased by 28 %. However, the short-term parenteral administration of glucose and insulin in physiological doses did not suppress ghrelin levels, suggesting that changes in plasma insulin and glucose were not responsible for changes in ghrelin levels after food intake.
The results of the present study must be interpreted with some caution because the water–glucose solution used here is not comparable to the food matrix of a normal mixed diet. Nevertheless, the data suggest that an excessive consumption of carob fibre may exert adverse effects on glucose handling. This might be relevant for the potential enrichment of sugar-sweetened beverages by carob fibre. Further studies within differing background meals are needed to confirm these results. Investigating the long-term effects of carob fibre on glucose handling and insulin sensitivity might be of further interest.
In conclusion, the ingestion of carob fibre rich in polyphenols increased postprandial glucose and insulin concentrations, suggesting an exacerbated glycaemic control in combination with a water–glucose solution, but did not markedly affect postprandial ghrelin concentrations. The mechanisms of this phenomenon remain to be investigated in future studies.
Acknowledgements
We thank Andreas Wagner and Minerva Petrovitsch for the excellent technical assistance.



