Disconnection syndromes are unique patterns of neurological symptoms resulting from the disruption of specific pathways interconnecting areas of the brain. The syndrome of alexia without agraphia was first described by Déjérine in the late 19th century based on observations of two patients that lost the ability to read but retained variable writing abilitiesReference Catani and ffytche1. In contrasting their individual presentations and lesions, he concluded that the dominant, typically left, angular gyrus functions in visual word image storageReference Catani and ffytche1. It is now hypothesized to facilitate visual-auditory and sensorimotor integration required for reading and writingReference Catani and ffytche1.
Alexia without agraphia is an inability to read with preserved writing capacity and classically localizes to the dominant occipital cortex and splenium of the corpus callosum (Figure 1). Left visual cortex damage compromises right visual field input, causing a right visual field deficit. Splenial involvement disconnects the right visual cortex carrying visual information from the left visual field to the angular gyrus and Wernicke’s area responsible for comprehension of spoken and written language. Therefore, written word information cannot be processed from visual to auditory for pronunciation, or even comprehended. The angular gyrus and its connections with language and motor centers are concomitantly spared so written ability is preserved, as is the remainder of language functioning including fluency, comprehension, naming, and repetition, resulting in a “pure” alexia or word blindness.
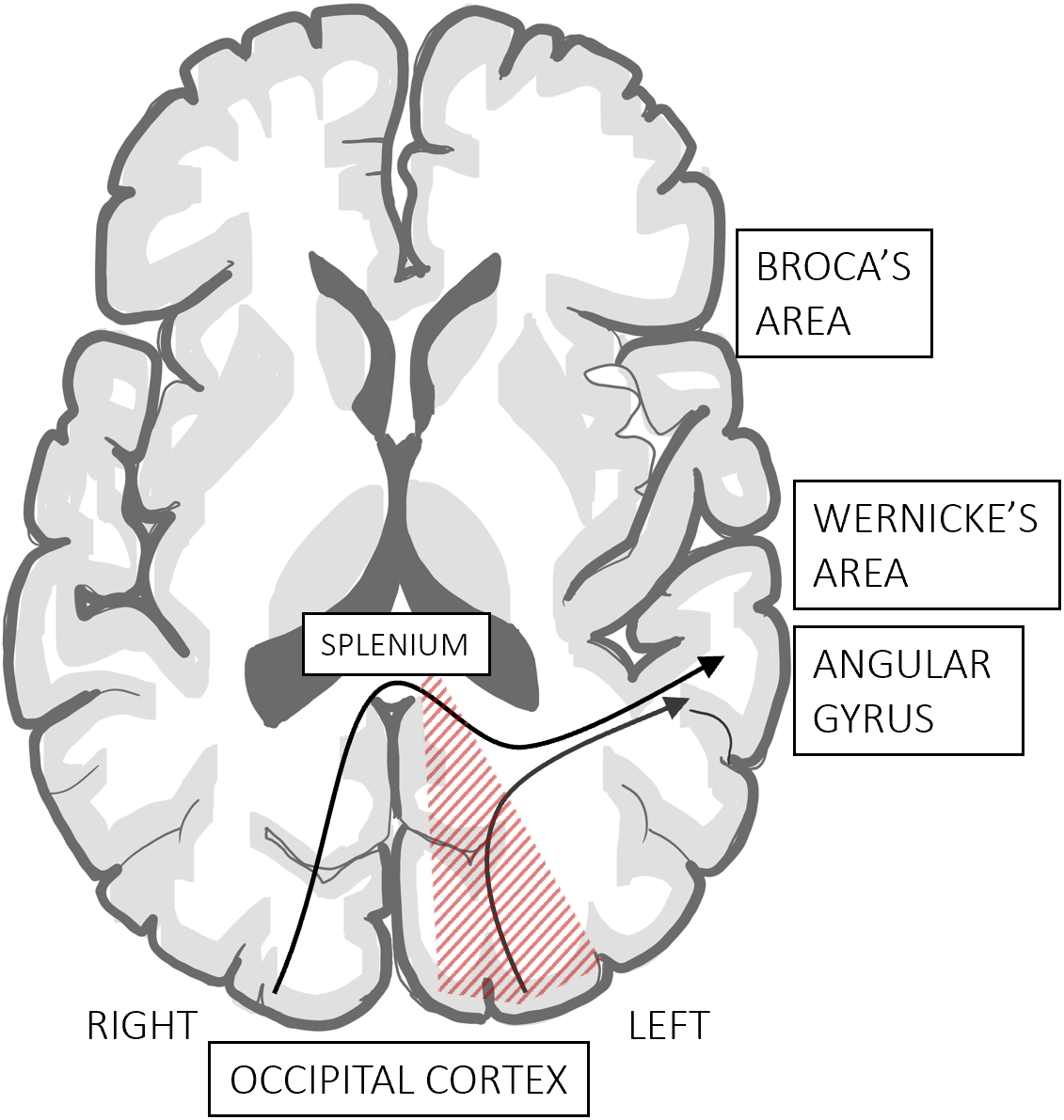
Figure 1: Alexia without agraphia localization and anatomy. There is direct damage to the left visual cortex causing a right homonymous visual field deficit. Outflow tracts from the intact right visual cortex traveling across the splenium to the angular gyrus and other language areas are interrupted disrupting the understanding and pronunciation of written language.
The most common etiology of this rare syndrome is posterior cerebral artery (PCA) stroke, although there are cases reported in acute encephalopathy, multiple sclerosis, glioblastoma, and nonconvulsive status epilepticusReference Rupareliya, Naqvi and Hejazi2-Reference Kutluay, Pakoz, Yuksel and Beydoun4. We report a case secondary to posterior reversible encephalopathy syndrome (PRES).
A 25-year-old right-handed male presented with spontaneous epistaxis and was found to have severe pancytopenia. Bone marrow biopsy revealed aplastic anemia, requiring multiple transfusions and subsequent hospital admission for immunosuppression with only anti-thymocyte globulin and cyclosporine. Four days following treatment initiation, he had gradual onset occipital headache unresponsive to analgesia and suddenly developed difficulty reading. Acute stroke activation was initiated. On assessment, he stated, “I can see and text, I just can’t read my messages.”
He was alert with no meningismus and blood pressure of 140/90 mmHg. Language assessment revealed normal fluency, comprehension, repetition, and naming. There was no finger agnosia, right–left disorientation, or acalculia. He could read individual letters, but not words. He could write sentences but could not read aloud what he had written. There was an incomplete right homonymous hemianopia. The remainder of his neurological examination was unremarkable.
Bloodwork revealed stable pancytopenia, normal electrolytes including magnesium, renal, and liver function. Urgent non-contrast CT head revealed hypoattenuation in the left subcortical occipitoparietal region and right internal capsule (Figure 2A). CT angiogram showed increased left occipitoparietal region vascularity with no stenosis or occlusion. Sparing of the cortex and paramedian occipital lobe, including the calcarine fissure, were out of keeping with PCA territory infarct as was coinciding involvement of the contralateral internal capsule. MRI brain revealed multiple areas of subcortical vasogenic edema including the left occipital lobe with extension into the left posterior splenium and a smaller area of restricted diffusion, consistent with PRES (Figure 2B–D).
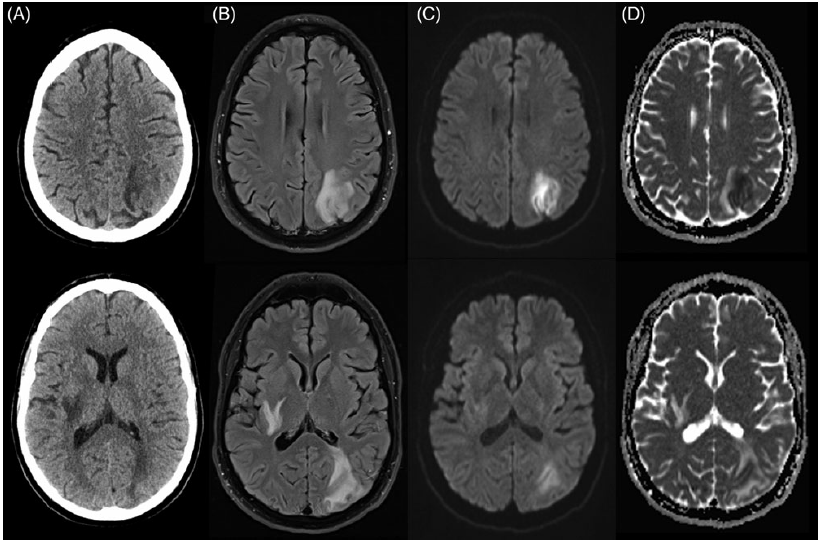
Figure 2: Non-contrast axial CT head and MRI brain at presentation portraying PRES complicated by infarction. CT head (A) revealed patchy confluent areas of hypoattenuation in the left subcortical occipitoparietal region and right internal capsule with extension into the corona radiata. MRI brain revealed multifocal areas of increased T2/FLAIR signal (B) also in the left occipitoparietal region with sulcal effacement and involvement of the left posterior splenium, the right internal capsule, basal ganglia, as well as the right occipital lobe (not pictured). In the occipitoparietal region, there was cortical restricted diffusion seen on DWI and ADC (C and D, respectively) as well as surrounding regions of vasogenic edema reflected by hyperintensity on ADC with isointense DWI. Punctate foci of microhemorrhage were present and there was no contrast enhancement (not depicted).
The patient’s cyclosporine was held while antihypertensives were used to prevent hypertension. His deficits resolved within 3 days at which point he could read and write normally. Given rapid improvement, follow-up imaging was not pursued. He was started on tacrolimus 1 month later, subsequently undergoing stem cell transplantation, with no new neurological symptoms.
PRES is a syndrome of generally reversible, posterior, and subcortical predominant vasogenic edema often presenting acutely with visual disturbances, headache, seizures, and encephalopathy in the setting of immunosuppression or blood pressure fluctuations. Additional risk factors include eclampsia, renal failure, and autoimmune disordersReference Fugate and Rabinstein5. Pathophysiology is thought to be related to autoregulation failure causing blood–brain barrier breakdown and fluid extravasation in posterior brain regions, which are more susceptible to hyperperfusion given less posterior fossa sympathetic innervationReference Fugate and Rabinstein5,Reference Tetsuka and Ogawa6 . Pronounced blood pressure fluctuations result in cytokine release, activating endothelial cells to secrete vasoactive factors increasing vascular permeabilityReference Fugate and Rabinstein5. Direct endothelial dysfunction from cytotoxic therapies is also thought to cause capillary leakage and vasogenic edemaReference Tetsuka and Ogawa6. Cyclosporine, a calcineurin inhibitor, is one of the more common culprits implicated by means of endothelin release causing vasoconstriction and magnesium loss associated with PRESReference Fugate and Rabinstein5.
PRES was diagnosed in this case given the acute onset of reversible visual symptoms with headache in a young patient recently initiated on immunosuppression with cyclosporine. His blood pressure was not particularly elevated as in 30% of PRES patientsReference Tetsuka and Ogawa6,Reference Fischer and Schmutzhard7 . Neuroimaging revealed characteristic parieto-occipital region abnormalities as well as basal ganglia involvement, which occurs in one-third of casesReference Fugate and Rabinstein5. Restricted diffusion manifests in 15–30% of cases usually within larger regions of vasogenic edemaReference Fugate and Rabinstein5,Reference Tetsuka and Ogawa6 . Lesions may also be seen in the cerebellum, brainstem, and spinal cordReference Tetsuka and Ogawa6. Although not seen here, contrast enhancement occurs in 20% of patientsReference Fugate and Rabinstein5. Intracranial hemorrhage, commonly intraparenchymal, complicates 10–25% of casesReference Fugate and Rabinstein5.
Treatment depends on precipitating causes, in this case, withdrawal of immunosuppression resulting in complete clinical resolution. Blood pressure control is important but should be lowered by no more than 25% of the initial valueReference Fugate and Rabinstein5. Seizures are treated with antiepileptics. PRES is typically considered clinically and radiologically reversible, unless complicated by extensive cerebral edema, hemorrhage, or infarctionReference Tetsuka and Ogawa6. Long-term immunosuppressive safety is unclear, with reports of successful rechallenging with the offending therapy or switching to an alternative agentReference Fischer and Schmutzhard7.
Pure alexia has previously been described in one patient with Hashimoto’s encephalopathy and PRES who presented with encephalopathy and alexia in the context of elevated antithyroid antibody titers and no other PRES risk factorsReference Yildiz, Segmen, Oztoprak, Bolayir and Topaktas3. To our knowledge, there are no previous case reports of alexia without agraphia due to isolated PRES, which might otherwise be expected given posterior predilection; however, it may remain underrecognized, especially if transient, quickly reversible, and easily missed if reading and writing were not formally tested. In summary, this case illustrates the disconnection syndrome of alexia without agraphia occurring acutely in the context of PRES and resolving with cessation of immunotherapy. Acute presentation of this rare syndrome, while most commonly seen in PCA stroke, should trigger consideration of PRES in the appropriate clinical context.
Disclosures
Authors report no disclosures.
Statement of Authorship
IC: Report conception, manuscript preparation.
TC: Report conception, manuscript preparation, and final approval.
Authors take full responsibility for the data collected, analysis, interpretation, and conduct of this report.




