Recently, researchers investigating the origins of domestication have debated the significance of resource intensification in the shift from foraging to food production (Gremillion Reference Gremillion2004; Miller Reference Miller2014, Reference Miller2018; Munro Reference Munro2004; Munro et al. Reference Munro, Bar-Oz, Meier, Sapir-Hen, Stiner and Yeshurun2018; Smith Reference Smith2011, Reference Smith2012, Reference Smith2015; Stiner et al. Reference Stiner, Munro, Surovell, Tchernov and Bar-Yosef1999, Reference Stiner, Munro and Surovell2000; Weitzel and Codding Reference Weitzel and Codding2016; Zeanah Reference Zeanah2017; Zeder Reference Zeder2012, Reference Zeder2015, Reference Zeder2016). In eastern North America, one of several independent centers of domestication across the globe (Smith Reference Smith2006), this question remains open. Some have argued that domestication was likely inspired by intensification (Weitzel and Codding Reference Weitzel and Codding2016; Zeanah Reference Zeanah2017), while others maintain that it was not (Smith Reference Smith2011, Reference Smith2012, Reference Smith2015). Here, I test the hypothesis that intensification preceded domestication by evaluating archaeofaunal assemblages from six sites in the middle Tennessee River valley.
In the strictest and most original sense, intensification is an increase in yields at the expense of efficiency (Boserup Reference Boserup1965). I follow Broughton (Reference Broughton1994a, Reference Broughton1994b) and define yield as food energy obtained per unit land area, while efficiency refers to food energy obtained per unit time. Therefore, intensification occurs when an individual obtains greater benefits from a given area of land (e.g., kilocalories per square kilometer), but doing so requires a disproportionate increase in costs relative to benefits (e.g., kilocalories per hour; Boserup Reference Boserup1965; Broughton Reference Broughton1994a, Reference Broughton1994b; Morgan Reference Morgan2015). While this was Boserup's (Reference Boserup1965) original definition of intensification, the meaning and connotations of the term have been contested over the past 50 years, with many anthropologists and archaeologists opting for a more general use of the term to refer to any increases in yields or productivity irrespective of changes in efficiency (Brookfield Reference Brookfield1972, Reference Brookfield2001; Erickson Reference Erickson, Marcus and Stanish2006; Kirch et al. Reference Kirch, Asner, Chadwick, Field, Ladefoged, Lee, Puleston, Tuljapurkar and Vitousek2012; Leach Reference Leach1999; Morrison Reference Morrison1994; Morrison et al. Reference Morrison, Feinman, Nicholas, Ladefoged, Myrdal-Runebjer, Stone and Wilk1996; Thurston and Fisher Reference Thurston, Fisher, Thurston and Fisher2007). The result is a large body of literature, predominately concerned with agricultural economies, in which the word “intensification” is often used in contradictory ways to refer to highly variable socioeconomic situations (Morgan Reference Morgan2015). For the sake of clarity, and following the recommendation of Morgan (Reference Morgan2015), I have employed the term in its original Boserupian sense to refer to increasing yields associated with declining efficiency.
Some behavioral ecologists have utilized this Boserupian concept of intensification to argue that domestication has its roots in declining foraging efficiency (energy gained by a forager relative to the time spent foraging), due to either population growth and/or packing (Munro Reference Munro2004; Munro et al. Reference Munro, Bar-Oz, Meier, Sapir-Hen, Stiner and Yeshurun2018; Stiner et al. Reference Stiner, Munro, Surovell, Tchernov and Bar-Yosef1999, Reference Stiner, Munro and Surovell2000) or environmental changes that reduce the abundance of high-return resources (Piperno Reference Piperno2006, Reference Piperno2011; Piperno et al. Reference Piperno, Ranere, Dickau and Aceituno2017). Alternatively, others have proposed that domestication arises in contexts of not only high yields but also high foraging efficiency, a scenario that rejects the role of Boserupian intensification in inspiring initial domestication (Smith Reference Smith2007, Reference Smith2011, Reference Smith2012, Reference Smith2015; Zeder Reference Zeder2012, Reference Zeder2015, Reference Zeder2016).
In eastern North America, several species of plants were domesticated by Native peoples in the Late Holocene near the confluence of the Missouri, Ohio, Cumberland, Tennessee, Arkansas, and Mississippi Rivers (Mueller et al. Reference Mueller, Fritz, Patton, Carmody and Horton2017; Smith Reference Smith2006; Smith and Yarnell Reference Smith and Yarnell2009). These plant species include squash (Cucurbita pepo, domesticated by 5025 cal BP), sunflower (Helianthus annus, 4840 cal BP), sumpweed (Iva annua, 4400 cal BP), goosefoot (Chenopodium berlandieri, 3800 cal BP), and erect knotweed (Polygonum erectum, 2000 cal BP).
Several recent studies have explored the origins of domestication in this region with reference to intensification (Miller Reference Miller2014, Reference Miller2018; Miller and Carmody Reference Miller and Carmody2016; Smith Reference Smith2015; Weitzel and Codding Reference Weitzel and Codding2016; Zeanah Reference Zeanah2017). Weitzel and Codding (Reference Weitzel and Codding2016) investigated human population change in interior eastern North America using radiocarbon date frequencies and site counts as proxies for population. These authors found evidence for a millennium of significant population growth prior to initial domestication in the region. Other researchers (Miller Reference Miller2014, Reference Miller2018; Miller and Carmody Reference Miller and Carmody2016) employed an ideal free distribution model to investigate patterns of habitat in-filling in Tennessee and detected evidence for population growth from the Late Pleistocene up to initial domestication. Such demographic patterns, in the absence of environmental deterioration, are consistent with intensification hypotheses for domestication, but they provide no direct evidence for subsistence intensification—only a potential mechanism for it.
These studies document population growth prior to initial domestication in eastern North America. This may be a key driver of intensification in some cases (Broughton Reference Broughton1994a, Reference Broughton1994b, Reference Broughton2004; Munro Reference Munro2004; Nagaoka Reference Nagaoka2002; Stiner et al. Reference Stiner, Munro, Surovell, Tchernov and Bar-Yosef1999, Reference Stiner, Munro and Surovell2000), but it is not the only pathway to intensification. Environmental change can also alter the balance between human populations and their resource base, leading to intensification and, potentially, domestication (Piperno Reference Piperno2006, Reference Piperno2011; Piperno et al. Reference Piperno, Ranere, Dickau and Aceituno2017), or to a reduction in resource extraction intensity (Byers and Broughton Reference Byers and Broughton2004; Carmody Reference Carmody2009, Reference Carmody2010; Wolverton Reference Wolverton2005). Other research in eastern North America has not supported the prediction that population growth and resource overexploitation preceded initial domestication. It suggests instead that the mitigation of risk caused by environmental variability was a more likely cause of domestication in the region (Gremillion Reference Gremillion2002, Reference Gremillion2004). Additionally, Zeanah (Reference Zeanah2017) modeled foraging decisions surrounding initial domestication in eastern North America and found that small-seed exploitation can be advantageous when high-ranking hickory nuts are not readily available, due to either poor yields resulting from natural environmental variability or restricted access to profitable but distant hickory-nut patches as a result of population packing. Zeanah provided archaeobotanical data documenting subsistence intensification prior to the widespread adoption of horticultural economies, although he did not address whether intensification preceded the earliest evidence of plant domestication in the region.
Not all researchers working on initial domestication in eastern North America have found this evidence for population growth, environmental change, or risk management compelling. Smith (Reference Smith2015) reviews the literature on domestication in this region and finds evidence for anthropogenic niche construction in the form of landscape burning, indicating that foragers managed their ecosystems prior to domestication. However, he finds no evidence in the previously published studies that he consulted that human population increase, environmental change capable of affecting resource abundance, or anthropogenic resource depression (a reduced encounter rate with prey due to the actions of a predator [Charnov et al. Reference Charnov, Orians and Hyatt1976]) occurred prior to 5000 cal BP. This lack of support for declining efficiency as a precursor to domestication casts doubt on the role of Boserupian intensification in the emergence of domestication in the region. Instead, Smith (Reference Smith2011, Reference Smith2012, Reference Smith2015) argues that domestication results not from Boserupian intensification, but from experimentation with crop management in times and places exhibiting no population resource imbalance; that is, when both yields and efficiency are high.
Smith (Reference Smith2015) not only questions results supporting the Boserupian intensification hypothesis, but he argues that the behavioral ecology approach of the preceding studies (Gremillion Reference Gremillion2002, Reference Gremillion2004; Miller Reference Miller2014, Reference Miller2018; Miller and Carmody Reference Miller and Carmody2016; Weitzel and Codding Reference Weitzel and Codding2016; Zeanah Reference Zeanah2017) is fatally flawed. He takes particular issue with their use of optimal foraging theory (OFT) to document population pressure, risk mitigation, and environmental variability. OFT is a set of behavioral ecology models that aims to predict foraging behavior given local ecological settings. Several researchers, such as Smith (Reference Smith2011, Reference Smith2012, Reference Smith2015) and Zeder (Reference Zeder2012, Reference Zeder2015, Reference Zeder2016), maintain that an OFT approach is not only unhelpful in the study of initial domestication but also detrimental. They counter these OFT-inspired hypotheses by advocating for the use of niche construction theory (NCT), a set of concepts concerning organismal modification of, and coevolution with, their environments. It is within the context of this debate regarding OFT and NCT approaches that the causes of initial domestication in eastern North America have most recently been discussed (Smith Reference Smith2015; Weitzel and Codding Reference Weitzel and Codding2016).
While the studies discussed above have debated whether Boserupian intensification precedes domestication, the question of whether foraging efficiency actually declined prior to initial domestication has not been fully evaluated in eastern North America. Such evaluation is necessary for understanding whether initial domestication resulted from subsistence intensification or not. Therefore, I used archaeofaunal data from interior eastern North America to investigate whether domestication was preceded by reduced foraging efficiency. As subsistence yields (energy output per unit of land area) are difficult to quantify archaeologically, I focused on the aspect of intensification that is more easily measured: efficiency. If demographic pressure and environmental change are absent and foraging efficiency does not decline prior to initial domestication, then domestication may not have resulted from intensification, but from experimentation with and management of crops during times and in places of resource abundance (Smith Reference Smith2012, Reference Smith2015). In contrast, if intensification characterized the context of initial domestication, foraging efficiency should decline prior to 5000 cal BP due either to the impact of environmental changes on resource abundance or to resource depression caused by human population pressure.
Materials and Methods
To evaluate patterns of intensification prior to initial domestication, I analyzed faunal data from six sites in the middle Tennessee River valley of northern Alabama and southern Tennessee (Figure 1). While much of the earliest evidence for domestication in eastern North America comes from more northern sites in the Mississippi, Illinois, and Ohio River valleys, the middle Tennessee River valley lies only about 100 km (ca. 60 mi) south of the Hayes Site, where the earliest evidence of domesticated sunflower was dated to 4840 cal BP (Crites Reference Crites1993; Smith Reference Smith2006; Smith and Yarnell Reference Smith and Yarnell2009). Furthermore, the middle Tennessee River valley lies within Weitzel and Codding's (Reference Weitzel and Codding2016) study area, which was statistically defined as a 95% confidence ellipse around the locations of the seven earliest sites of domestication in the region.

Figure 1. Map of the Middle Tennessee River Valley showing the locations of the six sites that yielded the faunal assemblages included in this study as well as the locations of nearby pollen cores that provided relevant paleoenvironmental data.
Site Descriptions
The six sites evaluated in this study include Dust Cave, Stanfield-Worley, LaGrange, Widow's Creek, Mussel Beach, and Russell Cave. These sites were occupied at various times over the last 13,000 years and have produced abundant analyzed fauna with which to test my predictions. All six sites yielded vertebrate faunal remains; however, freshwater-mussel data with the resolution required for this analysis are available for only three of them (Dust Cave, Stanfield-Worley, and Mussel Beach). The faunal remains from each site have been assigned to chronostratigraphic units by the site excavators. In most cases, these components were dated using radiocarbon methods, but some sites were relatively dated based on diagnostic artifact types. In my analyses, I have utilized the midpoint date of each component—the calendar date halfway between the earliest and latest calibrated radiocarbon date. When no absolute dates were available, I used the midpoint date of the cultural history period assigned to the component by the original excavators.
Some of these sites have poor chronological resolution and few have well-dated components that directly precede or follow initial domestication. This makes it impossible to assess changes in foraging efficiency immediately surrounding the process of domestication. Instead, the analyses herein evaluate millennial-scale trends in foraging efficiency from the terminal Pleistocene to the Late Holocene—not century- or decadal-scale changes in the years preceding initial domestication.
Dust Cave
Dust Cave is the only site in the southeastern United States outside of Florida that is radiocarbon dated to the Younger Dryas (Miller and Gingerich Reference Miller and Gingerich2013). The site is situated on the bluff line between the Tennessee River floodplain and the uplands of the Interior Plateau of northwestern Alabama (Figure 1). Excavated from 1989 through 2002, the site was occupied from the Younger Dryas through the Middle Holocene, with dates ranging from 12,700 to 5600 cal BP (Sherwood et al. Reference Sherwood, Driskell, Randall and Meeks2004). All materials were water screened through 6 mm (0.24 in) mesh (Sherwood et al. Reference Sherwood, Driskell, Randall and Meeks2004) except those from flotation samples that were screened through 1.4 mm (0.055 in) and 0.7 mm (0.003 in) mesh (Carmody Reference Carmody2009, Reference Carmody2010; Hollenbach Reference Hollenbach2005, Reference Hollenbach2009).
A random sample of faunal remains from the first five years of the excavation were analyzed by Renee Walker (Reference Walker1998). These materials originated from five 2 x 2 m units located in the entrance chamber of the cave that was excavated prior to 1994. These excavations occurred before the complexity of the cave's stratigraphy was fully understood. After 1994, the excavators developed a more complete understanding of the relationship between various stratigraphic contexts and the cave's occupation history. To ensure that the faunal remains from the site were assigned to the appropriate stratigraphic zones and chronological periods, I reevaluated the proveniences of Walker's faunal materials using original field notes and maps. I also analyzed an additional small sample of faunal remains during the spring of 2015 by randomly sampling 35 proveniences (10 cm levels from 1 x 1 m units as well as feature fill), spanning all years of excavation and all excavated units, for all recovered vertebrate remains. These data are presented here for the first time (Supplementary Table 1). My analysis contributed an additional 1,440 specimens to Walker's (Reference Walker1998) original sample. The vertebrate archaeofaunal assemblage from Dust Cave now totals 12,998 specimens (NISP), of which 46% (NISP = 6,043) were identifiable to class, and 11% (NISP = 1,412) were identifiable more specifically than class.
The Dust Cave faunal remains originate from six radiocarbon-dated cultural components (Sherwood et al. Reference Sherwood, Driskell, Randall and Meeks2004): Benton (6500–5600 cal BP; NISP = 260), Eva/Morrow Mountain (8400–6000 cal BP; NISP = 1236), Kirk (10,200–7800 cal BP; NISP = 759), Mixed Kirk (9600–9400 cal BP; NISP = 210), Early Side-Notched (12,000–11,000 cal BP; NISP = 1073), and Paleoindian (12,650–11,200 cal BP; NISP = 2505; Table 1). There are 282 identifiable specimens among shellfish remains from the Benton (NISP = 125), Eva/Morrow Mountain (NISP = 121), Kirk (NISP = 24), Early Side-Notched (NISP = 5), and Paleoindian (NISP = 7) components (Carmody Reference Carmody2009; Parmalee Reference Parmalee1994).
Table 1. Number of Identified Specimens for Relevant Taxa from Sites in the Middle Tennessee River Valley.

Russell Cave
Russell Cave is located in Doran Cove in the Sequatchie Valley, about seven miles from the Tennessee River on a smaller tributary (Figure 1). The site was occupied throughout much of the Holocene (9600 to 400 cal BP; Griffin Reference Griffin1974). Weigel et alia (Reference Weigel, Holman, Paloumpis and Griffin1974) report 30,000 vertebrate remains, comprising 66 species, although only 10% (NISP = 2,891) were identifiable to a taxonomic category. All materials from the cave were either dry screened through 6 mm (1/4 in) mesh during the initial excavation, or water screened through 6 mm (1/4 in) mesh, as soil moisture at greater depths made dry screening difficult (Griffin Reference Griffin1974:11–12). Faunal remains originated from radiocarbon-dated zones labeled G (9600–8200 cal BP; NISP = 1581), F (8700–6900 cal BP; NISP = 302), E (6300–2000 cal BP; NISP = 123), D (2150 cal BP; NISP = 173), C (1400–1100 cal BP; NISP = 347), B (400 cal BP; NISP = 291), and A (NISP = 74), although this latter zone is modern and therefore not included in this analysis (Table 1).
Stanfield-Worley
Stanfield-Worley Bluff Shelter, excavated between 1960 and 1963, is located approximately 11 km (7 mi) from the Tennessee River (Figure 1). Materials from the site were screened through 6 mm (1/4 in) mesh by hand and mechanical agitation (DeJarnette et al. Reference DeJarnette, Kurjack and Cambron1962; Hollenbach Reference Hollenbach2005:70–75). No radiocarbon dates were initially obtained, so the occupation of the site was divided into two zones based on artifact typologies. Later, Hollenbach (Reference Hollenbach2005: Table 4.2) obtained seven dates between 11,700 and 7600 cal BP from Zone D. Zone A, a later occupation of the site, spans the Late Holocene and was never radiocarbon dated, so a midpoint date for the Late Holocene is used here (Table 1). The site yielded 915 identified vertebrate faunal remains from 13 species as well as 1,222 shellfish remains. Zone D contained 297 vertebrate and no shellfish specimens, while Zone A contained 618 vertebrate and 1,222 shellfish remains (Parmalee Reference Parmalee1962).
LaGrange
LaGrange Bluff Shelter is a small rockshelter on LaGrange Mountain in northwest Alabama (Figure 1). Located several miles south of the Tennessee River, the site was excavated in 1972 and 1975. All materials were screened through 6 mm (1/4 in) mesh (DeJarnette and Knight Reference DeJarnette and Knight1976). The occupation of the site extended from the Terminal Pleistocene through the Late Holocene according to artifact typologies. Hollenbach (Reference Hollenbach2005:Table 4.5) later obtained two radiocarbon dates from hickory nutshell fragments from Zone E (11,500–11,200 cal BP) and Zone C (8300–8400 cal BP). Only 48 faunal remains could be identified from three stratigraphic zones: Zones A (Woodland to Mississippian; NISP = 28), B (Early Archaic to Late Archaic; NISP = 7), and D (Late Paleoindian to Early Archaic; NISP = 13; Curren Reference Curren1976). Because absolute dates do not exist for these three zones, midpoint dates were derived from the cultural history periods to which associated artifacts from each zone were assigned (Table 1).
Mussel Beach
Mussel Beach is located on the Tennessee River near Tennessee's border with Alabama and Georgia (Figure 1). Human occupation of the site spans much of the Late Holocene (5500–900 cal BP). The site was excavated periodically during the 1980s, in 1991, and again in 2009 and 2010. All sediments were screened through 6 mm (1/4 in) mesh, and finer mesh (2 mm) was used for flotation samples (Gregory et al. Reference Gregory, Branch-Raymer, Espenshade and Windham2011). Vertebrate (NISP = 116) and invertebrate (NISP = 2,053) faunal remains were identified from four radiocarbon-dated cultural components of the site spanning 4300 to 900 cal BP (Table 1): Late Archaic II (4300 cal BP; NISP = 2), Late Archaic III (2600 cal BP; NISP = 37), Middle Woodland (1700 cal BP; NISP = 71), and Late Woodland (1100 cal BP; NISP = 6). Approximately two-thirds of the invertebrates were bivalves (NISP = 1,407), and the remaining one-third were gastropods (Gregory et al. Reference Gregory, Branch-Raymer, Espenshade and Windham2011). Gastropods are not included in this analysis. The bivalves were identified from three cultural components: Late Archaic III (NISP = 34), Middle Woodland (NISP = 1,125), and Late Woodland (NISP = 248).
Widow's Creek
Widow's Creek is situated on the Tennessee River in northeastern Alabama (Figure 1). The site was occupied in the Late Holocene from approximately 4500 to 1000 cal BP (Morey Reference Morey1996). Excavations at Widow's Creek began in the summer of 1973 by the University of Tennessee at Chattanooga. Arbitrary 0.5 ft (0.15 m) levels were used in the excavation of 10 ft by 10 ft (3.05 by 3.05 m) units. Each unit contained a 2 ft by 2 ft (0.61 by 0.61 m) control column that was water screened through 6 mm (1/4 in) and 1.6 mm (1/16 in) mesh. All sediments from features were also water screened through 6 mm (1/4 in) and 1.6 mm (1/16 in) mesh (Olinger Reference Olinger1975; Warren Reference Warren1975). Freshwater-mussel remains were analyzed from two control columns and 26 features (Warren Reference Warren1975). However, no NISP values are given for the dated strata at the site. Thus, the mussel remains from Widow's Creek were not included in the analyses herein. Vertebrate faunal remains (NISP = 1,341) were identified from 24 features relatively dated to three cultural components, due to a lack of radiocarbon dates: Late Archaic (NISP = 60), Early Woodland (NISP = 137), and Middle/Late Woodland (NISP = 1,144; Morey Reference Morey1996). Midpoint dates from each of these cultural history periods were used (Table 1).
Prey Modeling
To test the intensification hypothesis, I have employed the prey model (also known as the prey choice, optimal diet, or diet-breadth model). This is a theoretical model of diet choice first developed in ecology (Charnov Reference Charnov1976b; Emlen Reference Emlen1966; MacArthur and Pianka Reference MacArthur and Pianka1966) and adopted by archaeologists (Bayham Reference Bayham1979; Beaton Reference Beaton1973) and ethnographers (Hawkes et al. Reference Hawkes, Hill and O'Connell1982; Hawkes and O'Connell Reference Hawkes and O'Connell1985; O'Connell and Hawkes Reference O'Connell, Hawkes, Winterhalder and Smith1981; Winterhalder Reference Winterhalder, Winterhalder and Smith1981a, Reference Winterhalder, Winterhalder and Smith1981b, Reference Winterhalder1983) soon thereafter. The prey model predicts which food resources an organism, hereafter personified as a forager, will exploit in a resource patch—a subset of the environment that hosts particular resource types. The decision to take or ignore a resource item once encountered in a patch is based on the goal of maximizing energetic intake relative to time and energy expenditures. These expenditures are divided into search costs, or those incurred while looking for the item, and handling costs, or those incurred once the item is located. Energetic intake relative to time and energy expenditures is referred to as foraging efficiency when describing a forager's overall intakes and expenditures from searching for and handling (pursuing, harvesting, processing, etc.) the item. It is called a return rate when describing the profitability of specific resource types excluding search costs. Prey items are ranked according to their post-encounter return rates, and they are sequentially included in a forager's diet set if taking the item upon encounter increases the forager's overall return rate (including search time). If taking the item does not do so, the forager ignores it and continues searching for other items. When a diet includes an abundance of high-return items, foraging efficiency is said to be high. Low foraging efficiency therefore characterizes diets in which relatively more low-ranking items are taken. More detailed discussions of this model in archaeology can be found in Bird and O'Connell (Reference Bird and O'Connell2006) and Codding and Bird (Reference Codding and Bird2015). In ecology, they can be found in Charnov (Reference Charnov1976b) and Stephens and Krebs (Reference Stephens and Krebs1986).
An important prediction of the prey model is that the highest-ranking prey item available to a forager will always be taken upon encounter. Lower-ranking items will be taken only if doing so increases the forager's overall return rate (Charnov Reference Charnov1976b; MacArthur and Pianka Reference MacArthur and Pianka1966; Stephens and Krebs Reference Stephens and Krebs1986). This means that inclusion of low-ranking items in the diet set depends on the forager's encounter rate with higher-ranking items, not on the abundance of the low-ranking items. Encounter rates may be affected by environmental changes as well as technological and social developments that make search more or less efficient, altered forager mobility that affects search potential, or resource depression. Resource depression refers to a change in prey encounter rates due to the actions of a predator. It can manifest as depletion of a prey item due to overharvesting or as prey altering their behavior or residence patterns to avoid capture (Charnov et al. Reference Charnov, Orians and Hyatt1976). Hereafter, I refer only to anthropogenic resource depression caused by human populations.
Reduced encounter rates with high-ranking prey items, therefore, inspire subsistence intensification given the nature of diet breadth expansion. Intensification, as an increase in yields and a decline in efficiency, is accomplished by widening dietary breadth. Doing so includes lower-ranking, but frequently more abundant and densely distributed, resources (Winterhalder et al. Reference Winterhalder, Baillargeon, Cappelletto, Daniel and Prescott1988; Winterhalder and Goland Reference Winterhalder and Goland1993). This reduces foraging efficiency (kilocalories per unit time), but it typically increases foraging yields (kilocalories per unit area). Intensification can therefore be understood as a behavioral process by which a forager responds to reduced encounter rates with high-return resources by investing more in the exploitation of lower-return items. It must be remembered, however, that Boserup's work (Reference Boserup1965) overemphasized population growth in explanations of intensification (Leach Reference Leach1999; Morrison Reference Morrison1994; Morrison et al. Reference Morrison, Feinman, Nicholas, Ladefoged, Myrdal-Runebjer, Stone and Wilk1996; Thurston and Fisher Reference Thurston, Fisher, Thurston and Fisher2007). As noted above, encounter rates with high-ranking prey types and, therefore, diet breadth are determined by many things, including forager population growth as well as technological, environmental, and social factors.
Available Prey and Patch Types and Their Projected Abundances
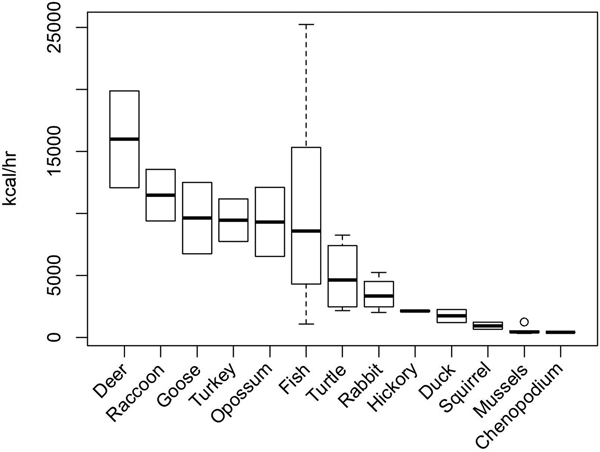
To evaluate temporal changes in the exploitation of specific animal resources using the prey model, return rates for the various prey items in the region must be estimated. Carmody (Reference Carmody2009), Hollenbach (Reference Hollenbach2005), and Thomas (Reference Thomas2008) calculated caloric return rates for most of the common prey items found in the southeastern United States, based on experimental and ethnographic data (Figure 2). Based on these calculations, as well as the infrequency of black bear and elk in faunal assemblages from this region, white-tailed deer (Odocoileus virginianus) are effectively the highest-ranked available prey item. Various species of geese are also relatively high ranking. Fish return rates are highly variable depending on harvest technique and species, but are high ranking in some instances. Lower-ranking prey types include turtles, rabbits (Sylvilagus sp.), ducks, squirrels (Sciurus sp.), and freshwater mussels.

Figure 2. Post-encounter return rates (kilocalories per hour) of select faunal and botanical taxa from the southeastern United States. Data from Carmody (Reference Carmody2009), Hollenbach (Reference Hollenbach2005), and Thomas (Reference Thomas2008).
Despite their low search costs and predictability as sessile taxa, the shellfish in this region are predominately small freshwater mussels and are therefore low-ranked prey items. The mean shell lengths of 39 species collected in a sample of freshwater mussels (n = 792) in 1971 and 1972 ranged from 47 mm to 127 mm, while the mean edible meat mass of the same species ranged from 3 g to 99 g (Parmalee and Klippel Reference Parmalee and Klippel1974). The study concluded, as others have since (Gardner Reference Gardner1992; Peacock Reference Peacock, Anderson and Mainfort2002; Steponaitis Reference Steponaitis1986:374), that freshwater mussels were a low-return prey item most useful for providing certain macro- and micronutrients rather than caloric energy (Gardner Reference Gardner1992:267). This prey type is also said to be quite susceptible to resource depression due to human exploitation (Gardner Reference Gardner1992:268; Peacock Reference Peacock, Anderson and Mainfort2002).
To best understand changes in human foraging through time, it is helpful to constrain assumptions that treat all available prey items as homogeneously distributed across the landscape. In reality, most resources are found in patches (Charnov Reference Charnov1976a; MacArthur and Pianka Reference MacArthur and Pianka1966). Keeping patch types separate in foraging analyses allows for more accurate modeling of forager decision-making. Consequently, I divided the fauna in this study into two patches, termed “wetland” and “terrestrial.” The wetland patch corresponds to aquatic, semi-aquatic, and other moisture-adapted taxa found in the Tennessee River floodplain and in or near the surrounding rivers, streams, lakes, and ponds. This patch contains all species of geese, swans, ducks, fish, and freshwater mussels. The terrestrial patch corresponds to all non-wetland habitats, including dry bottomlands and higher-elevation areas outside of the river floodplain, such as the Highland Rim and Cumberland Plateau. This patch contains white-tailed deer, squirrel, Phasianidae, prairie chicken (Tympanuchus sp.), bobwhite quail (Colinus virginianus), and wild turkey (Meleagris gallopavo).
Abundance Indexes
I measured overall foraging efficiency as well as patch-specific foraging efficiency for both the wetland and terrestrial patches using four abundance indexes. First, the Deer-Shellfish Index measures overall foraging efficiency irrespective of patch. Next, the Waterfowl Index and the Fish Index both measure foraging efficiency within the wetland patch. Finally, the Deer-Squirrel Index measures foraging efficiency within the terrestrial patch. These indexes divide the NISP of a high-ranking prey item by the sum NISP of that high-ranking item and a low-ranking item, or items (Broughton Reference Broughton1994a, Reference Broughton1994b).
To evaluate changes in overall foraging efficiency regardless of patch type, I created a Deer-Shellfish Index (Table 2). This Deer-Shellfish Index divides the NISP of white-tailed deer by the sum NISP of deer and shellfish (Table 1):
Table 2. Abundance Indexes for Sites in the Middle Tennessee River Valley.

Deer are the highest-ranking prey item in the region, while shellfish are one of the lowest (Figure 2). However, this Deer-Shellfish Index violates the fine-grained search assumption of the prey model. The model assumes that all prey items are randomly encountered in proportion to their abundance, yet white-tailed deer and shellfish are not found in the same patches and are not taken with the same technology. For this reason, I constructed additional measures of patch-specific foraging efficiency for wetland and terrestrial patches.
To assess wetland patch-foraging efficiency, I used two indexes: the Waterfowl Index and the Fish Index (Table 2). These indexes respectively compare the NISP of higher-ranking Anatidae (waterfowl) and Actinopterygii (fish) to the NISP of lower-ranking shellfish species:
Two indexes are used to assess wetland patch-foraging efficiency due to the small sample of waterfowl remains recovered from the middle Tennessee River valley and the highly variable return rates of fish. Only the three sites with shellfish remains attributed to specific chronological periods (Dust Cave, Stanfield-Worley, and Mussel Beach) could be used for this analysis. It is important to note that materials from each of these three sites were screened through 6 mm (1/4 in) mesh, but some of the faunal materials from Dust Cave and Mussel Beach were recovered from flotation samples screened through much finer mesh (1.4 mm and 0.7 mm mesh at Dust Cave, and 2 mm mesh at Mussel Beach). Stanfield-Worley may, therefore, contain fewer fish remains than the other two sites, biasing the Fish Index. Fortunately, there are more components from Dust Cave and Mussel Beach. Consequently, these sites drive the patterning in the Waterfowl and Fish Indexes.
Changes in terrestrial patch-foraging efficiency were assessed using a Deer-Squirrel Index (Table 2). This index is calculated as the NISP of white-tailed deer remains divided by the sum NISP of deer and all specimens from the genus Sciurus, including gray squirrel (Sciurus carolinensis), fox squirrel (Sciurus niger), and unidentified squirrel (Sciurus sp.; Table 1):
All six sites were used to construct this terrestrial patch index. The finer mesh used in flotation samples at Dust Cave and Mussel Beach is not expected to affect terrestrial as much as aquatic fauna due to the substantially larger sizes of squirrel and deer bones compared to fish bones.
While only plants were domesticated in eastern North America, changes in the faunal indexes utilized here track the general state of the foraging economy in the region. As stated above, decisions to exploit lower-ranking food resources such as seeds are contingent upon encounters with higher-ranking, typically faunal, resources (Charnov Reference Charnov1976b; Hawkes and O'Connell Reference Hawkes and O'Connell1992). Therefore, changes in foraging efficiency indicated by faunal data provide a reliable indication of a forager's subsistence system and the presence or absence of intensification, even when the subsistence shift in question concerns plants.
To evaluate statistical trends in these data through time, I used binomial-family generalized linear models (GLMs) with logit link functions weighted by sample size. Weighting the model by the sample size of a particular data point accounts for problems of occasional small samples by fitting the GLM to the data proportionately to the sample size of a given component. For example, a Deer-Squirrel Index value for a component with 100 deer and 100 squirrel bones would influence the GLM 100 times more than a component containing only one deer and one squirrel bone, even though the index value for both components is 0.50. This weighting process permitted me to include all available faunal assemblages without arbitrarily deciding on a cutoff point for appropriately large sample sizes. However, this also means that interpretations should be based only on the fitted models and not on individual data points. Since conventional R2 values cannot be calculated for GLMs, I employed McFadden's pseudo-R2 (denoted here as R2Mc) in these analyses to evaluate goodness of fit (McFadden Reference McFadden and Zarembka1973). McFadden's pseudo-R2 is a common goodness-of-fit statistic for logistic regression, but it is known to result in values that are smaller than true R2 values for equivalent model fits (McFadden Reference McFadden, Hensher and Stopher1978; Smith and McKenna Reference Smith and McKenna2013). It should, therefore, not be interpreted as identical to an R2 value, but as a more general goodness-of-fit statistic. All analyses were run in the R environment (R Core Team 2017), and code is available as a supplementary file (see also https://github.com/weitzele/MTRV_ForagingEfficiency).
Due to the coarse-grained nature of available paleoenvironmental data, the results herein are discussed not only in relation to the earliest dated evidence for domesticates in the region (ca. 5000 cal BP) but also in terms of broad climate periods: the Younger Dryas (12,800–11,700 cal BP), the Early Holocene (11,700–8200 cal BP), the Middle Holocene (8200–4200 cal BP), and the Late Holocene (4200–0 cal BP; Walker et al. Reference Walker, Berkelhammer, Björck, Cwynar, Fisher, Long, Lowe, Newnham, Rasmussen and Weiss2012).
Results
The GLM for the Deer-Shellfish Index (Table 2) shows that there is a strong, significant, and negative relationship between this index and time in the middle Tennessee River valley over the last 14,000 years (R2Mc = 0.708; p < 0.0001; Figure 3). This suggests that, when treating the landscape as a single homogenous patch, overall foraging efficiency declines over time. This decline includes the years prior to and following initial domestication at 5000 cal BP.

Figure 3. A generalized linear model of overall foraging efficiency through time (R2Mc = 0.708; p < 0.0001), as measured by the Deer-Shellfish Index, shows a general decline in overall foraging efficiency from the Younger Dryas through the Late Holocene, including prior to initial domestication (5000–2000 cal BP; cross-hatched).
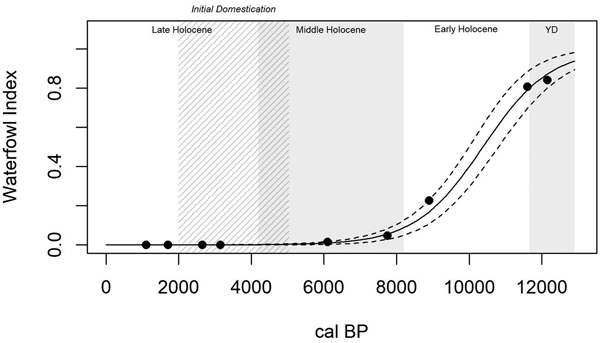
Trends in the Waterfowl Index (Table 2), measuring human foraging efficiency in only the wetland patch, are significantly explained by time (R2Mc = 0.995; p < 0.0001). The overall trend is a strong sigmoidal decline towards the present (Figure 4). The Fish Index (Table 2), a second measure of wetland patch foraging efficiency, also shows a strong and significant sigmoidal decline through time (R2Mc = 0.946; p < 0.0001; Figure 5). Wetland patch foraging efficiency, therefore, appears to have declined through time, both prior to and following initial domestication.

Figure 4. A generalized linear model of wetland patch foraging efficiency through time (R2Mc = 0.995; p < 0.0001), as measured by the Waterfowl Index, shows a general decline in wetland patch foraging efficiency from the Younger Dryas through the Late Holocene, including prior to initial domestication (5000–2000 cal BP; cross-hatched).

Figure 5. A generalized linear model of wetland patch foraging efficiency through time (R2Mc = 0.946; p < 0.0001), as measured by the Fish Index, shows a general decline in wetland patch foraging efficiency from the Younger Dryas through the Late Holocene, including prior to initial domestication (5000–2000 cal BP; cross-hatched).
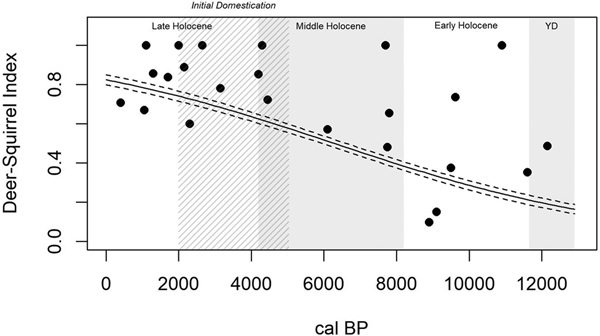
The Deer-Squirrel Index (Table 2) reveals that time significantly accounts for variation in terrestrial foraging efficiency (R2Mc = 0.477; p < 0.0001). This model suggests that terrestrial patch foraging efficiency was low in the Younger Dryas and Early Holocene, increased through the Middle Holocene, and peaked in the Late Holocene (Figure 6). Terrestrial foraging efficiency, therefore, shows the opposite pattern to that of the wetland patch: a general increase through time, both prior to and following initial domestication approximately 5,000 years ago.

Figure 6. A generalized linear model of terrestrial patch foraging efficiency through time (R2Mc = 0.477; p < 0.0001), as measured by the Deer-Squirrel Index, shows a general increase in terrestrial patch foraging efficiency from the Younger Dryas through the Late Holocene, including prior to initial domestication (5000–2000 cal BP; cross-hatched).
Discussion
These results indicate that non-patch-specific overall foraging efficiency, measured by the Deer-Shellfish Index, has gradually declined in the middle Tennessee River valley over the last 13,000 years. Therefore, overall foraging efficiency declined prior to initial domestication in eastern North America. However, at a finer-grained scale, declining foraging efficiency did not characterize all habitat types. Instead, patch-specific analyses reveal that foraging efficiency progressively declined within wetland patches prior to initial domestication, but not within terrestrial patches. These analyses nonetheless demonstrate that intensification did precede initial domestication in this region, even if only in wetland settings. The anti-intensification hypothesis proposed by Smith (Reference Smith2015) argues that no intensification occurred whatsoever. Therefore, even a patch-specific reduction in foraging efficiency fails to support this hypothesis. That foraging efficiency does not increase or decrease in tandem in each of these patches is to be expected given that environmental changes and anthropic resource exploitation would have affected each habitat type differently. Intensification is, therefore, likely to have been a motivating factor behind initial domestication in interior eastern North America, even if this intensification was patch-specific and not universal.
While the results presented here suggest that intensification preceded domestication, additional data are needed on human foraging efficiency elsewhere in interior eastern North America. The middle Tennessee River valley is not far from the Hayes Site, where the earliest evidence of domesticated sunflower has been identified (Crites Reference Crites1993; Smith Reference Smith2006; Smith and Yarnell Reference Smith and Yarnell2009), and it falls within previously defined study areas of initial domestication in eastern North America (Weitzel and Codding Reference Weitzel and Codding2016). However, it should not be assumed that the patterns of faunal exploitation in this area parallel those of the larger region. Additional work should investigate whether declines in foraging efficiency also occur in the valleys of the Mississippi, Ohio, Illinois, and Cumberland Rivers, as well as other river valleys, prior to initial domestication.
Interpreting Changes in Foraging Efficiency
Although these analyses reveal changes in foraging efficiency through time, the drivers of changes in these indexes beg further explanation. Greater wetland foraging efficiency in the Younger Dryas and Early Holocene appears to correspond to a proliferation of wetland habitats at that time: when wetlands were abundant, wetland-adapted species of high-ranking waterfowl and fish thrived, as evidenced by high Waterfowl and Fish Indexes. Paleoenvironmental data indicate that the Younger Dryas and Early Holocene in the middle Tennessee River valley were cool and wet with abundant wetland plant communities. Mean annual precipitation was high within the valley (Bryson 1999 in Homsey Reference Homsey2004) and at the pollen core site of Anderson Pond, Tennessee, from approximately 14,000 to 11,500 cal BP (Figure 1; Delcourt Reference Delcourt1979:Figure 13). Additionally, lake levels at the pollen core site at Cahaba Pond, Alabama (Figure 1) were high between 14,060 and 10,785 cal BP (Delcourt et al. Reference Delcourt, Delcourt and Spiker1983). Wetland and bottomland pollen such as beech and hophornbeam are common in Younger Dryas sediments at this pollen core site (Delcourt et al. Reference Delcourt, Delcourt and Spiker1983). This abundance of such moisture-adapted taxa may suggest proliferation of wetland plant communities. Furthermore, the Tennessee River and other rivers in the Southeast were unstable and characterized by frequent flood outbursts and channel changes during the Terminal Pleistocene, potentially contributing to the predicted proliferation of wetland plant communities outlined here. Abundant wetlands may have permitted growth of high-ranking wetland prey populations such as waterfowl and fish, increasing foraging efficiency within this patch type.
Yet while wetlands flourished, increased seasonality (Broughton et al. Reference Brougton, Byers, Bryson, Eckerle and Madsen2008) and, possibly, competition with other large-bodied herbivores (Wolverton et al. Reference Wolverton, Lyman, Kennedy and La Point2009) at the Pleistocene/Holocene transition may have reduced high-ranking white-tailed deer populations, thereby lowering terrestrial foraging efficiency as measured by the Deer-Squirrel Index. Human populations were relatively low at this time (Miller and Carmody Reference Miller and Carmody2016; Weitzel and Codding Reference Weitzel and Codding2016), making anthropogenic resource depression less likely to have had pronounced effects in any patch type.
Unlike the Younger Dryas and the Early Holocene in the Southeast, the Middle Holocene was characterized by the warm and dry Hypsithermal climate event. The Hypsithermal likely contributed to reduced wetland and elevated terrestrial foraging efficiency in the centuries prior to and concurrent with domestication in the region. Precipitation progressively declined from the Younger Dryas into the Hypsithermal in the middle Tennessee River valley (Bryson 1999 in Homsey 2004) and at Anderson Pond between 8800 and 5700 cal BP (Delcourt Reference Delcourt1979:Figure 13). Water levels were also low at Cahaba Pond from 9990 to 6440 cal BP, and the pond was desiccated afterward until 3420 cal BP (Delcourt et al. Reference Delcourt, Delcourt and Spiker1983). Additionally, the Tennessee River system stabilized during the Middle Holocene, when flood outbursts declined in frequency, the river channel became established, and water levels lowered (Peacock Reference Peacock, Anderson and Mainfort2002; Sherwood Reference Sherwood2001; Styles and Klippel Reference Styles, Klippel, Sassaman and Anderson1996). This drying may have reduced the abundance and size of wetland habitats, which negatively impacted populations of high-return waterfowl and fish around the time of initial domestication, thereby lowering foraging efficiency within this patch.
While oak and hickory began to increase in abundance in the Southeast during the Early Holocene, these taxa were most prevalent in the middle Tennessee River valley during the early millennia of the Middle Holocene from approximately 9000 to 6500 cal BP (Delcourt and Delcourt Reference Delcourt and Delcourt1987). Oak-hickory forest expansion began earlier in more southern latitudes, peaking between 11,500 and 9400 cal BP at Cahaba Pond in central Alabama, but peaking between 6900 and 4500 cal BP at Anderson Pond in central Tennessee (Figure 1; Delcourt Reference Delcourt1979; Delcourt et al. Reference Delcourt, Delcourt and Spiker1983). As the middle Tennessee River valley lies halfway between these two pollen core sites, a date range of 9000 to 6500 cal BP for the peak of the oak-hickory forest expansion is suggested by interpolated maps of the region (Delcourt and Delcourt Reference Delcourt and Delcourt1987). The expansion of oak-hickory forests during the Hypsithermal was likely to have been especially prevalent in the uplands, based on historical forest surveys documenting the abundance of oak and hickory trees at higher elevation locations in northern Alabama (Hollenbach Reference Hollenbach2005, Reference Hollenbach2009). Newly abundant mast resources and the opening of forests should have led to the growth of high-ranking deer populations in addition to lower-ranking squirrel and turkey populations, as well as other taxa that prefer such conditions (Hollenbach Reference Hollenbach2005, Reference Hollenbach2009; Walker Reference Walker1998). These taxa would have been abundant at the time of initial domestication around 5,000 years ago, and terrestrial patch foraging would have been high due to the presence of larger deer populations. The human population in the Middle Holocene was larger than in the Early Holocene, and it increased significantly in the millennium prior to initial domestication (Weitzel and Codding Reference Weitzel and Codding2016). This makes anthropogenic resource depression a strong possibility at this time.
Compared with the Younger Dryas and Middle Holocene, climatic differences between the Middle and Late Holocene were less dramatic. Moisture reached more moderate levels and temperatures were slightly lower than during the Hypsithermal. Precipitation remained relatively low in the middle Tennessee River valley (Bryson 1999 in Homsey 2004) but increased at Anderson Pond after approximately 5700 cal BP (Delcourt Reference Delcourt1979:Figure 13). Lake levels at Cahaba Pond increased to an intermediate point between those of the Middle Holocene and the Younger Dryas after 3420 cal BP and have remained that way to the present (Delcourt et al. Reference Delcourt, Delcourt and Spiker1983). More hydric species, such as tupelo and pine, dominated the pollen assemblage at Cahaba Pond from 6550 cal BP onward (tupelo pollen peaked ca. 3200 cal BP and pine pollen peaked ca. 750 cal BP; Delcourt et al. Reference Delcourt, Delcourt and Spiker1983) suggesting a return to moister conditions and potentially the expansion of moisture-adapted plant communities. This increase in moisture may have promoted the expansion of wetland plant communities, thereby increasing the abundance of relatively high-ranking waterfowl and fish, along with other aquatic and semiaquatic taxa. Yet, an increase in wetland foraging efficiency is not evident in the abundance indexes presented here. Human population size peaked in the region during the Late Holocene (Weitzel and Codding Reference Weitzel and Codding2016), potentially causing anthropogenic resource depression. This may explain continued low foraging efficiency in wetlands despite increasing moisture (Figures 4 and 5).
In Late Holocene terrestrial habitats, slight declines in oak and hickory abundance after approximately 6500 cal BP (Delcourt and Delcourt Reference Delcourt and Delcourt1987) have been argued to have reduced deer populations from a peak in the Middle Holocene (Miller Reference Miller2014, Reference Miller2018). Human population levels were also high at this time (Weitzel and Codding Reference Weitzel and Codding2016), making depression likely. Nevertheless, no declines in foraging efficiency are evident here in the terrestrial patch. The fact that terrestrial foraging efficiency remained high in the Late Holocene warrants further exploration but may relate to the curious ability of deer populations to thrive under moderate predation (Whitaker Reference Whitaker2009) and respond favorably to landscape modification—a kind of anthropogenic niche construction (Smith Reference Smith2009; Yerkes Reference Yerkes2005). Further work is needed to investigate this issue.
These paleoenvironmental and paleodemographic reconstructions suggest that, in the millennia prior to initial domestication around 5000 cal BP, shrinking wetlands and increasing human populations progressively reduced wetland foraging efficiency. Simultaneously, the patchy expansion of oak-hickory forests promoted growth in white-tailed deer populations and increased terrestrial foraging efficiency over time. Initial domestication was thus preceded by millennia of foraging intensification in wetland patches, despite gradual deintensification of terrestrial foraging. Both patterns are consistent with changes in human population size, moisture, and forest ecology. Further research investigating scheduling conflicts and foraging goals may add additional detail to these explanations concerning divergent patterns in the use of these two patch types.
As previously noted, the resolution of the available data makes finer-grained statements about shifts in efficiency impossible. Yet, the millennial-scale changes in foraging efficiency demonstrated here indicate clear differences before and after domestication, even if they cannot demonstrate abrupt, century-scale shifts associated with the appearance of food production.
Rebounding Populations of Fish, but Not Waterfowl, in the Late Holocene
As noted above, increasing moisture in the Late Holocene does not correspond to an increase in wetland foraging efficiency following the Hypsithermal. Either resources were depressed in the Late Holocene and fish and waterfowl remained scarce or shellfish were so numerous that they dampen the subtler increases in waterfowl and fish abundance in the indexes presented here. The observed patterns in wetland foraging efficiency are, indeed, largely driven by an increasing abundance of shellfish remains in faunal assemblages in the Middle and Late Holocene. This pattern of intensified shellfish exploitation has been documented elsewhere in the Southeast and has been tied to population pressure on resources (e.g., Peacock Reference Peacock, Anderson and Mainfort2002; Steponaitis Reference Steponaitis1986:374). Alternatively, other researchers have proposed that Middle and Late Holocene increases in shellfish harvesting were enabled by the stabilization of river systems, which permitted growth of shellfish populations (Dye Reference Dye, Carstens and Watson1996; Smith Reference Smith1986:22; Styles and Klippel Reference Styles, Klippel, Sassaman and Anderson1996). However, as foraging theory makes clear, low-ranking items such as shellfish are not taken according to their own abundance, but according to encounter rates with higher-ranking items in the diet. Therefore, even if shellfish had been more abundant in the Middle Holocene, their exploitation would have remained contingent upon the availability of higher-ranking waterfowl and fish.
To investigate whether possible Late Holocene increases in waterfowl and fish exploitation are masked by more dramatic increases in shellfish abundance, I calculated the proportions of waterfowl and fish in the assemblages relative to all other vertebrate taxa identified, at least to order. I modeled changes in these proportions using binomial family GLMs, as above, but permitted up to three polynomials in model fits according to the greatest rate of change in R2Mc values. These proportions show that waterfowl declined in abundance from the Younger Dryas though the Late Holocene and never recovered (Figure 7a). However, the proportions of fish in the assemblages declined from the Younger Dryas to the Middle Holocene but then increased in the Late Holocene (Figure 7b). Therefore, fish abundance was likely more closely linked to moisture—increasing moisture in the Late Holocene resulted in higher abundances of fish. Unlike fish abundance, however, waterfowl abundance did not increase in the Late Holocene. This may indicate anthropogenic depression of waterfowl, since the expansion of wetland habitats suggested by the paleoenvironmental data predicts an expansion of waterfowl populations to mirror that observed for fish. These indices clarify that while abundant shellfish (Figure 7c) clearly impacted the Waterfowl and Fish Indexes during the Late Holocene, waterfowl and fish exploitation still declined prior to initial domestication. Resource depression may have reduced Late Holocene waterfowl abundance, whereas elevated moisture increased fish abundance. Middle Holocene declines in waterfowl and fish prior to initial domestication are thus consistent with both environmental change (i.e., warming and drying during the Hypsithermal) and anthropogenic resource depression resulting from growing human populations in the region (Weitzel and Codding Reference Weitzel and Codding2016).

Figure 7. Generalized linear models of taxonomic proportions in each assemblage for taxa used to construct the abundance indexes employed herein: (a) the proportion of waterfowl (R2Mc = 0.726; p < 0.0001), (b) fish (R2Mc = .422; p < 0.0001), (c) shellfish (R2Mc = 0.986; p < 0.0001), (d) white-tailed deer (R2Mc = 0.314; p < 0.0001), and (e) squirrels (R2Mc = 0.721; p < 0.0001).
The declines in wetland foraging efficiency documented here could also result from differential bone preservation between sites. For example, waterfowl and fish remains are abundant at Dust Cave, which was occupied from the Younger Dryas though the Middle Holocene, but less so at Mussel Beach, which was occupied during the Late Holocene. This pattern may accurately reflect changing foraging efficiency through time, but it could also result from taphonomic processes. Materials from both sites were similarly screened through 6 mm (1/4 in) or finer mesh, but the protective setting of Dust Cave may have preserved delicate fauna such as birds and fish better than the open-air conditions at Mussel Beach. This taphonomic explanation is supported by the increased abundance of fish remains in more northern sites in interior eastern North America during the Late Holocene, as opposed to the Early and Middle Holocene (Styles and Klippel Reference Styles, Klippel, Sassaman and Anderson1996). Further evaluation of these Late Holocene patterns in wetland foraging efficiency is therefore warranted. Nevertheless, declining wetland foraging efficiency prior to initial domestication remains well supported by these data.
The Likelihood of Resource Depression of Waterfowl and Fish
While both environmental shifts and resource depression may be drivers of change in these assemblages, concerns have been raised about the viability of resource depression as an explanation for declining prehistoric abundances of various eastern North American taxa. In particular, Smith (Reference Smith2009) reasons that it probably was difficult to depress populations of migratory waterfowl and fish. He argues that historical and contemporary records of migratory waterfowl abundance along the Mississippi Flyway suggest that prehistoric populations of such taxa were likely too large to be substantially impacted by human hunting. He similarly argues that fish depression was unlikely given that breeding populations of fish probably clustered in deeper channels where human fishers were unable to effectively reach them due to the constraints of prehistoric fishing technology.
However, these arguments rest on several problematic assumptions: first, that colonial and historical records reflect prehistoric animal abundances; second, that prey population size is the variable controlling its susceptibility to depression; and third, that human fishers could not, or did not, target breeding populations of fish. Contrary to these assumptions, historic accounts of prey abundance are very likely misleading due to the effects of prey population rebound following Native depopulation in the colonial period (Fisher Reference Fisher2018; Jones Reference Jones, Herhahn and Ramenofsky2016). Modeling has also demonstrated that a prey population's growth rate and its ranking among other available resources determine susceptibility to resource depression, not the prey population's size (Winterhalder et al. Reference Winterhalder, Baillargeon, Cappelletto, Daniel and Prescott1988; Winterhalder and Goland Reference Winterhalder and Goland1993).
If one were to uncritically accept these claims of resilience to depression, one could infer that environmental changes were a more likely cause of the prey population fluctuations documented here than anthropogenic resource depression. However, as discussed above, declines in wetland foraging efficiency in the Middle Holocene coincided with a period of elevated human population in the region, making anthropogenic resource depression a possible cause of intensification. Additionally, while wetland foraging efficiency remained low in the Late Holocene despite increases in moisture, this appears to have been caused by depression of waterfowl, not fish or shellfish. This result indicates that depression of waterfowl was indeed possible in interior eastern North America in precolonial times. However, further investigation of the susceptibility of these taxa and others to resource depression would be very useful for testing these arguments further.
The Compatibility of Niche Construction Theory and Optimal Foraging Theory
As noted above, the question of whether intensification preceded initial domestication has been recently framed as a debate between two competing bodies of theory: one derived from optimal foraging theory (OFT) and the other from niche construction theory (NCT; Smith Reference Smith2015; Weitzel and Codding Reference Weitzel and Codding2016; Zeder Reference Zeder2016). Common hypotheses derived from OFT emphasize population resource imbalance driven by human population growth, environmental change, risk mitigation, or some other mechanism resulting in foraging intensification prior to domestication (Gremillion Reference Gremillion2004; Hawkes and O'Connell Reference Hawkes and O'Connell1992; Piperno et al. Reference Piperno, Ranere, Dickau and Aceituno2017; Weitzel and Codding Reference Weitzel and Codding2016; Winterhalder and Goland Reference Winterhalder, Goland and Gremillion1997). The specific hypothesis commonly advanced by NCT practitioners entails an absence of population resource imbalance and intensification, wherein domestication arises from experimentation with crop management and the formation of coevolutionary relationships in times and places of resource abundance (Smith Reference Smith2011, Reference Smith2015; Zeder Reference Zeder2012, Reference Zeder2015, Reference Zeder2016).
This juxtaposition of OFT and NCT is misleading, however, and perhaps does more harm than good. While Smith (Reference Smith2011, Reference Smith2012, Reference Smith2015) and Zeder (Reference Zeder2012, Reference Zeder2014, Reference Zeder2015, Reference Zeder2016) contend that NCT and OFT are mutually exclusive approaches—that the latter perspective is fatally flawed and should be abandoned—this view is not shared by OFT users (Bird et al. Reference Bird, Taylor, Codding and Bird2013; Bird et al Reference Bird, Bird, Codding and Taylor2016; Broughton et al. Reference Broughton, Cannon and Bartelink2010; Gremillion et al. Reference Gremillion, Barton and Piperno2014; Mohlenhoff and Codding Reference Mohlenhoff and Codding2017; Piperno et al. Reference Piperno, Ranere, Dickau and Aceituno2017; Stiner and Kuhn Reference Stiner and Kuhn2016; Weitzel and Codding Reference Weitzel and Codding2016; Zeanah Reference Zeanah2017) or even other niche construction theory advocates (O'Brien and Laland Reference O'Brien and Laland2012:448; Odling-Smee et al. Reference Odling-Smee, Laland and Feldman2003:294–295). Indeed, NCT has provided useful concepts, insights, and criticisms for anthropologists and archaeologists (Bird et al. Reference Bird, Taylor, Codding and Bird2013; Bird et al. Reference Bird, Bird, Codding and Taylor2016; Broughton et al. Reference Broughton, Cannon and Bartelink2010; Laland and O'Brien Reference Laland and O'Brien2010, Reference Laland and O'Brien2012; O'Brien and Laland Reference O'Brien and Laland2012). Many also recognize its importance in understanding domestication and promote its use in conjunction with OFT models (Broughton et al. Reference Broughton, Cannon and Bartelink2010; Gremillion et al. Reference Gremillion, Barton and Piperno2014; Mohlenhoff and Codding Reference Mohlenhoff and Codding2017; Stiner and Kuhn Reference Stiner and Kuhn2016; Zeanah Reference Zeanah2017).
As many OFT users have said, OFT and NCT can and should be used together to construct and test hypotheses concerning initial domestication (Gremillion et al. Reference Gremillion, Barton and Piperno2014; Mohlenhoff et al. Reference Mohlenhoff, Coltrain and Codding2015; Mohlenhoff and Codding Reference Mohlenhoff and Codding2017; Piperno et al. Reference Piperno, Ranere, Dickau and Aceituno2017; Stiner and Kuhn Reference Stiner and Kuhn2016; Zeanah Reference Zeanah2017). As a simple example, if declining foraging efficiency prior to domestication had been brought about by resource depression, this would constitute niche construction (Broughton et al. Reference Broughton, Cannon and Bartelink2010), as resource depression is environmental modification caused by a predator's activity (Charnov et al. Reference Charnov, Orians and Hyatt1976). It is, therefore, a type of inadvertent perturbational niche construction (Odling-Smee et al. Reference Odling-Smee, Laland and Feldman2003), given that niche construction need not be deliberate environmental modification but can include by-products of other behaviors (Laland et al. Reference Laland, Matthews and Feldman2016:193). Resource depression alters a forager's local ecology, affects payoffs and decision-making, can be passed on intergenerationally via “ecological inheritance,” and can structure present and future natural selection (Laland et al. Reference Laland, Matthews and Feldman2016; Odling-Smee et al. Reference Odling-Smee, Laland and Feldman2003). A hypothesis that predicts resource depression prior to domestication could therefore be derived from both OFT and NCT.
Similarly, if initial domestication in eastern North America was indeed preceded by declining foraging efficiency as argued here—a more classically OFT explanation, although also a perspective shared by other NCT users (O'Brien and Laland Reference O'Brien and Laland2012:448)—such a result does not disavow the role of transgenerational plant management systems and the inheritance of modified landscapes and traditional ecological knowledge, as Smith (Reference Smith2012, Reference Smith2015) argues. Many concepts and predictions from these two bodies of theory can be easily combined to create a broader and more detailed understanding of domestication.
Furthermore, many shortcomings of each body of theory can be addressed by the other. OFT hypotheses concerning domestication have long focused on the nature of the foraging economy prior to domestication and not on the way the process of domestication itself occurs. NCT approaches have attempted to more specifically address the coevolutionary relationship between domesticates and humans as well as the actual process of domestication (Smith Reference Smith2015; Zeder Reference Zeder2016). On the other hand, NCT hypotheses of domestication have been lacking in detailed explanations of motivation: Why would people modify their landscapes? What motivates niche construction? This issue has recently been addressed from within OFT by the development of a model of optimal niche construction to predict contexts in which environmental modification may occur (Mohlenhoff and Codding Reference Mohlenhoff and Codding2017). Integrating these two perspectives clearly contributes to a more thorough treatment of domestication in archaeological research.
The overlap between and complementarity of NCT and OFT highlight the fundamental compatibility of these two perspectives (Mohlenhoff et al. Reference Mohlenhoff, Coltrain and Codding2015; Piperno et al. Reference Piperno, Ranere, Dickau and Aceituno2017; Stiner and Kuhn Reference Stiner and Kuhn2016; Weitzel and Codding Reference Weitzel and Codding2016). While clear differences exist between NCT and the standard, neo-Darwinian evolutionary theory from which OFT originates (Laland et al. Reference Laland, Uller, Feldman, Sterelny, Müller, Moczek, Jablonka, Odling-Smee, Wray, Hoekstra, Futuyma, Lenski, Mackay, Schluter and Strassmann2014, Reference Laland, Matthews and Feldman2016; Scott-Phillips et al. Reference Scott-Phillips, Laland, Shuker, Dickins and West2014; Wray et al. Reference Wray, Hoekstra, Futuyma, Lenski, Mackay, Schluter and Strassmann2014), a concern with understanding the interactions between humans and their environments unites NCT and OFT (Gremillion et al. Reference Gremillion, Barton and Piperno2014). NCT tends to emphasize long-term coevolutionary processes as opposed to the short-term decisions modeled by OFT (Stiner and Kuhn Reference Stiner and Kuhn2016), yet the breadth and generality of both approaches provide much room for cooperation. Further collaboration between NCT and OFT will certainly lead to progress in understanding not only initial domestication but human behavior in general.
Conclusions
Analysis of faunal data from the middle Tennessee River valley indicates that gradual intensification over several millennia, evidenced by declining overall foraging efficiency, preceded initial domestication in eastern North America. However, declines in foraging efficiency were not uniform across patches: wetland foraging efficiency progressively declined, while terrestrial foraging efficiency increased. Declining wetland foraging efficiency from the Terminal Pleistocene into the Late Holocene, prior to initial domestication, is consistent with both environmental and human population changes, whereas steadily increasing terrestrial foraging efficiency prior to initial domestication 5,000 years ago is consistent with Middle Holocene changes in forest ecology. It also appears that anthropogenic resource depression affected waterfowl in the Late Holocene, after domestication was initiated, but not fish. The declines in overall and wetland foraging efficiency demonstrated herein present a serious challenge to hypotheses arguing against any sort of Boserupian intensification as a driver of domestication. It is now important to determine whether low foraging efficiency precedes initial domestication elsewhere in interior eastern North America to ensure that this is a regional trend, not a pattern unique to the middle Tennessee River valley. Finally, while the discussion of whether intensification inspired domestication has been framed in the context of a larger theoretical debate between niche construction theory and optimal foraging theory, these bodies of theory are compatible and should be integrated to better understand the context of domestication in eastern North America and around the world.
Acknowledgments
Thank you to Renee Walker for her work with the Dust Cave faunal remains and for graciously sharing her data, to Kandi Hollenbach for assisting me with obtaining a sample of the Dust Cave fauna for my own analysis, and to Sarah Sherwood for her hospitality and assistance with checking the proveniences of faunal materials from Dust Cave. Additionally, thank you to the Smithsonian Institution for their loans of comparative osteological specimens and to Allison Wolfe for translating the abstract into Spanish. Finally, thank you to Stephen Carmody, Rich Sosis, Steve Wolverton, Brian Codding, Jack Broughton, Natalie Munro, and three anonymous reviewers for providing valuable feedback on this research.
Data Availability Statement
The data generated for this analysis are available in the text and as supplemental materials.
Supplemental Materials
For supplementary material accompanying this paper, visit https://doi.org/10.1017/aaq.2018.86.
Supplemental Table 1. Complete archaeofaunal assemblage from Dust Cave, Alabama.
Supplemental Table 2. Archaeofaunal data from Dust Cave, Alabama analyzed as part of this study.
Supplemental Table 3. Return rates for common prey items in southeastern North America from Carmody (Reference Carmody2009), Hollenbach (Reference Hollenbach2005), and Thomas (Reference Thomas2008).
Supplemental Text. R script used to conduct all analyses and create all figures.