Despite the widespread use of potent antiretroviral therapies (ARTs), neurocognitive impairment (NCI) has remained a serious clinical challenge for patients infected by the human immunodeficiency virus (HIV) but NCI is especially common among persons with the acquired immunodeficiency syndrome (AIDS).Reference Chan and Brew 1 - Reference Heaton, Clifford and Franklin 3 The consequences of neurocognitive impairment are multiple including worsened adherence to therapy, obstacles in daily living and worse survival, depending on the severity of the disability. The causes of NCI in HIV/AIDS are multifactorial and encompass the direct neuropathogenic effects of the virus, resulting in HIV-associated neurocognitive disorders (HAND), as well as past or present substance abuse, traumatic brain injury (TBI), opportunistic central nervous system (CNS) infections, polypharmacy (the amount and interactions of prescribed non-HIV medications), cerebrovascular disease, hepatitis C virus (HCV) co-infection and possibly neurotoxic effects of some ARTs.Reference McCombe, Vivithanaporn, Gill and Power 2 , Reference Devlin, Gongvatana and Clark 4 The prevalence of HAND varies (20-50%), depending on the study, clinical setting and assessment protocols.Reference Heaton, Clifford and Franklin 3 , Reference Hasbun, Eraso and Ramireddy 5 - Reference Woods, Moore, Weber and Grant 7 While HIV-associated dementia (the most severe form of HAND) is typically not observed during successful viral suppression, the milder forms of HAND such as minor neurocognitive disorder (MND) and asymptomatic neurocognitive impairment (ANI), often defined by disturbances in psychomotor speed, executive functions and memory, are increasingly reported as people with HIV/AIDS live longer.Reference Woods, Moore, Weber and Grant 7 The underlying neuropathological features of HAND include chronic neuroinflammation together with synaptic loss and neuronal death.Reference Chan and Brew 1 The impact of chronic exposure to different antiretroviral drugs has come under close scrutiny in recent years in part because of efforts to select drugs with higher brain penetration, assessed by the CNS penetration efficacy (CPE) score as well as the reports of the neurotoxic potential of some ART agents.Reference Vassallo, Durant and Biscay 8 , Reference Cross, Combrinck and Joska 9
When screening for HAND an appropriate tool should be validated for both the detection of neurocognitive cognitive impairment and the principal predictors of HAND. The Montreal Cognitive Assessment (MoCA) is a popular screening tool for neurocognitive disorders and reliably differentiates between cognitively intact individuals and those with neurocognitive impairment in Alzheimer’s disease, Parkinson’s disease and vascular dementia.Reference Kandiah, Zhang, Cenina, Au, Nadkarni and Tan 10 - Reference Koski, Brouillette and Lalonde 14 Both its brevity and its broad applicability make the MoCA an appealing test for identifying patients with NCI. The present study objectives were twofold; the first was to determine the relationships between the MoCA score and individual demographic, biological comorbid factors within an HIV/AIDS cohort receiving active care and the second was to examine the sensitivity and specificity of the MoCA for symptomatic HAND in the same cohort of HIV-infected person(s).
Methods and Materials
Study population
The protocol including the informed consent was approved by the University of Calgary’s Human Ethics Committee. Patients with cognitive complaints reported by the patient (or by his/her caregiver) from June 29, 2009 to January 06, 2014 were asked to complete the English version of the MoCA as a part of a neurocognitive assessment for determining symptomatic HAND.Reference McCombe, Vivithanaporn, Gill and Power 2 , Reference Kim, Jewison, Milner, Rourke, Gill and Power 15 , Reference Vivithanaporn, Heo and Gamble 16 Montreal Cognitive Assessment scores and clinical data were evaluated in HIV-1 seropositive persons receiving ongoing care at the Southern Alberta Clinic (SAC) in Calgary, Alberta, Canada. Southern Alberta Clinic (SAC) provides free multidisciplinary care, including ART, for all HIV patients residing in Southern Alberta. In order to determine the predominant biological predictors of sHAND and MoCA performance, patients with NCI related to clinically proven pre-existing conditions (TBI, substance abuse, etc.) were excluded (<5.0%). Additionally, nine patients were excluded from the initial selection, eight patients were excluded due to low educational levels (<Grade 7), one patient’s test results was removed due to incomplete testing and serial test entries from five patients were removed and only the initial score was included in the present analyses.
HAND diagnosis
Based on the Frascati criteria there are three distinguishable categories for HAND differing in measures of dysfunction including ANI, MND and HIV-associated dementia (HAD).Reference Antinori, Arendt and Becker 17 Given the small subset of patients with MND and HAD in our sample (n=24) these patients were placed in a group designated as symptomatic HAND (sHAND). The diagnosis of sHAND was based on a multi-step clinical assessment, including neuropsychiatric symptoms (impairment in memory, concentration, motor functions and gait) conveyed through self-report or by the patient’s caregiver/family member and verified by neuropsychological testing, neurological and medical assessments and neuroimaging.Reference McCombe, Vivithanaporn, Gill and Power 2 , Reference Antinori, Arendt and Becker 17 Neuropsychiatric symptoms were evaluated during clinical visits. If neurocognitive impairment was suspected, a brief neuropsychological test battery was assessed including Symbol-Digit Modalities test, Grooved Pegboard, Trails A and Trails B together with the MoCA. In addition, the patient’s complete medical and social history was reviewed, which involved screening for prior neurological disorders (e.g., traumatic head injury, substance abuse) together with a physical examination, neuroimaging, and cerebrospinal fluid analyses to exclude nonHAND causes of neurocognitive impairment. Other relevant variables including education, ethnicity, substance abuse history, Hepatitis C Virus serostatus, CD4 T cell levels, plasma HIV-1 viral level, antiretroviral regimen, veterans aging cohort study (VACS) index and quality of life measurement Reference Crane, Van Rompaey and Dillingham 22 at the time of testing were analysed. The diagnosis of sHAND was determined based on the collective assessments by the SAC clinical team.
MoCA description
The MoCA is a well-known neuropsychological screening tool available in the public domain (www.MoCAtest.org).Reference Nasreddine, Phillips and Bedirian 18 It consists of cognitive subtests in areas of; executive functioning (assessed by alternating trail making test, phonemic fluency and a two–item abstraction task); visuospatial ability (evaluated by a clock-drawing task and three-dimensional cube copy); language (assessed by sentence repetition, phonemic fluency and a naming task); orientation (assessed through correct identification of the current date, location, and city; short term memory (assessed through a five minute delayed recall); four subtests are used to assess attention/working memory: digit spans forward and backward, tapping test and a serial seven subtraction task. The MoCA was implemented by a trained health care provider within the clinic.
Statistical analyses
All statistical analyses were performed using SPSS (v. 21). Univariate statistics were carried out using the Student t and Mann-Whitney U tests to test differences between individuals with and without sHAND (2-tailed). Montreal Cognitive Assessment subtest differences between sHAND and nonHAND patients were assessed with a repeated measures analysis of variance (ANOVA). In addition, Pearson/Spearman correlations were used to test relationships between patient characteristics and MOCA scores. Point-biserial correlations were conducted between MOCA total score and dichotomous variables (e.g., gender, ethnicity). To assess the variables which contributed most to prediction of the MoCA score, multivariate linear regression was applied using only significant predictors of the MoCA previously identified in the univariate analyses. Dichotomous variables included in the model were dummy-coded. Finally, a receiver operating characteristic (ROC) curve was calculated using the MoCA-score to predict sHAND status.
Results
MoCA score and clinical-demographic factors
Neurocognitive performance represents a complex set of abilities that is influenced by multiple factors. To determine which variables were associated with MoCA scores, correlational analyses were made between the individual’s MoCA and routine clinical and demographic data collected as part of clinical care. Among all subjects in the present study (n=125), the mean MoCA score was 24.8±2.97. Subsequent analyses revealed that ethnicity, education and employment status were positively (and significantly) associated with total MoCA score (Table 1). However, polypharmacy, nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) side-effects, total CPE score and ART counts at the time of testing were negatively (and significantly) associated with MoCA performance. Of note, many variables previously reported to be predictive of neurocognitive impairment including immune and viral burden status, age, substance abuse, concurrent psychiatric diagnosis and VACS Index did not show a relationship with the MoCA score. To explore this issue further, a multivariate regression (automatic linear model) was performed on the total MoCA score. Predictors were restricted to those previously associated with the total MoCA score in the univariate correlations. The regression model revealed that the MoCA score was best predicted by a combination of employment (dummy-coded: unemployed=0, employed=1; β=21.8, SE=5.96, p<0.000), ethnicity (dummy-coded: Caucasian=0, non-Caucasian=1; β=23.1, SE=7.89, p<0.004), and CPE value (β=−2.85, SE=1.67, p<0.009) (Table 2). In our cohort, individuals with the highest MoCA scores had a Caucasian ethnic background, were employed, and had a low CPE score. These findings highlighted the overall low MoCA scores in this population and the susceptibility of an individual’s performance on the MoCA to the effects of a wide range of clinical and demographic factors.
Table 1 Sociodemographic and clinical features of HIV/AIDS cohort

♢Pearson r for continuous variables, Spearman rho for non-normally distributed variables, or point-biserial r for dichotomous variables; *p<0.05 level (2 tailed ); **p<0.01 (2-tailed); ns=not significant
+Student t test, Mann Whitney U test and Chi-Squared test were used to evaluate comparisons of normally distributed, non-normally distributed and proportional data respectively. HIV=human immunodeficiency virus; SD=standard deviation, HCV=hepatitis C virus, CPE=central nervous system penetration-effectiveness, ART=antiretroviral therapies, NRTI=Nucleoside reverse transcriptase inhibitor; VACS=Veterans Aging Cohort Study; NonHAND=non HIV-associated neurocognitive disorder; sHAND-symptomatic HIV-associated neurocognitive disorder
Table 2 Multivariate regression of MoCA score showing significant predictors
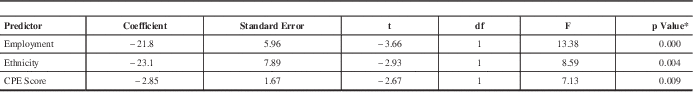
Adjusted r2=0.268
MoCA identification of sHAND
Among subjects diagnosed with or without sHAND, differences in gender, education, employment status, the presence of psychiatric disorder(s), and mean CD4 T cell nadir levels were noted (Table 1). The mean MoCA score was significantly lower in subjects with sHAND compared to those without sHAND (sHAND, 22.8±3.51; nonHAND 25.2±2.64) (p<0.05) (Table 1). Significantly different mean scores for several MoCA subtests including those in the domains of naming, orientation and delayed recall with consistently worsened performance were seen in the sHAND group versus non sHAND patients (Figure 1A). Stratification of all individual subtests also revealed additional between-group differences in language fluency (sHAND, 0.5±0.51; nonHAND 0.72±0.47) (p<0.05) and attention (“tap at A task” sHAND, 0.96±0.2; nonHAND 0.76±0.43 (p<0.05); “serial subtraction” sHAND, 1.92±0.97; nonHAND 2.41±0.86) (p<0.05) (Supplementary Table 1). The ROC curve on MoCA scores differentiating subjects with the diagnoses of sHAND from nonHAND subjects displayed an area under the curve of 0.71 (Figure 1B). The optimized MoCA cut-off score for distinguishing sHAND from nonHAND in the present cohort was 23.5 with corresponding sensitivity (75.2%), specificity (62.5%), positive predictive (37.5%) and negative predictive (89.4%) values. These findings emphasize the limits of the MoCA in identifying subjects at risk for sHAND.

Figure 1 Analyses of MoCA performance. (A) Comparision of sHAND and nonHAND mean scores in each of the cognitive domains represented in the Montreal Cognitive Assessment. (B) Receiver operating characteristic (ROC) curve for the Montreal Cognitive Assessment. Area under the curve was noted at 0.71; 95% CI (0.592-0.930). Cut off score of 23.5 (sensitivity=0.752; 1-specificity=0.375)
Conclusions
Although the MoCA is widely used as a screening tool for different neurocognitive disorders, the present study represents one of a few evaluations of its utility in HIV infection. Ethnicity, employment status and total CPE score were strongly predictive of MoCA performance but other factors including polypharmacy, ART drug numbers and NRTI side effects were significant, albeit negative contributors. Earlier studies have assessed the MoCA’s efficacy in identifying patients at risk for HAND;Reference Milanini, Wendelken, Esmaeili-Firidouni, Chartier, Crouch and Valcour 12 , Reference Ku, Lee and Ahn 19 we have examined in the context of patients with and without the diagnosis of sHAND, which disclosed a low area-under-the-curve value with matching low sensitivity and specificity and an optimal cut off score of 23.5. These observations underline the restrictions of using the MoCA for identifying patients with sHAND despite its robust associations with several host variables.
Despite the limits raised above, these findings raise important issues as the MoCA score was dependent on three major factors including employment, ethnicity and total CPE. A positive association between employment or ethnicity and MoCA score are plausible given the composition of SAC’s clientele, ~65% of whom are Caucasian homosexual males and ~30% are from sub-Saharan countries, often with limited education. Nevertheless, the total CPE score contributed negatively to the MoCA performance raising the intriguing possibility that the MoCA might identify persons at greater risk for ART-induced neurotoxicity due to high CNS penetration properties of select ARTs. Antiretroviral therapy-induced neurotoxicity is an increasingly recognized issue but poorly defined clinical entity.Reference Robertson, Liner and Meeker 20 Indeed, the data in this area are conflicting with reports of substantial improvement or worsening in NCI depending on the ART regimens and individual cohorts. Like previous studies, we found the MoCA displayed limited capacity to detect sHAND, likely because few neurocognitive domains were assessed by the MoCA, of which only three (delayed recall, naming and orientation) distinguished sHAND from non-sHAND subjects.Reference Milanini, Wendelken, Esmaeili-Firidouni, Chartier, Crouch and Valcour 12 , Reference Ku, Lee and Ahn 19 Indeed, the MoCA has been applied to HIV/AIDS populations in eight separate studies with variable outcomes but in general it showed low efficacies in detecting neurocognitive impairments, especially HAND (sHAND in this study). Previous studies have not examined systemic variables to the same extent as performed in the present study.Reference Milanini, Wendelken, Esmaeili-Firidouni, Chartier, Crouch and Valcour 12 , Reference Overton, Azad and Parker 13 Of note, the mean MoCA score for the present cohort was 24.8, which is lower than the normative MoCA cut off value, 26.0, for impairment; this latter finding speaks to the high prevalence of neurocognitive impairment in this population but also underscores the MoCA’s susceptibility to the effects of subject-specific factors.
Our study has several limitations. The small sample numbers of the clinical groups forced us to combine the MND and HAD groups and might have resulted in under-powering the predictive capacity of the MoCA as well as introducing unrecognized biases. A larger study population might also have yielded MoCA associations with previously reported risk factors for HAND including age, CD4 T cell nadir, and peak viral loads, etc. Although all of the present subjects had a brief neuropsychological assessment, a more comprehensive assessment is more desirable; despite this shortcoming, our observations are similar to previous studies with in-depth neuropsychological assessments. Another challenge in the present study is the extent to which the nonHAND group contained subjects with asymptomatic neurocognitive impairment, especially as recent studies have implied that there is a risk of progression from asymptomatic to symptomatic phases;Reference Grant, Franklin and Deutsch 21 again only rigorous neuropsychological assessments would delineate this number but previous studies report prevalence as high as 30-50%, depending on the individual population and test battery. Moreover, the present study did not explicitly address memory functions or attention in its standard neuropsychological battery, which may have resulted in an underestimation of the extent of NCI in this cohort. Future studies might include the MoCA as a measure of ART neurotoxicity given the present study’s findings although its utility for identifying persons at risk for HAND is not compelling at present.
Disclosures
Noshin Koenig, Esther Fujiwara, M. John Gill, and Christopher Power do not have anything to disclose.
Acknowledgements
The authors thank the staff and clients at SAC for their assistance and participation in the present study. Special thanks to Soheil Ramazani and Brenda Beckthold for their assistance in collecting clinical data. This study was supported the Canadian Institutes of Health Research Emerging Team grant (CP, MJG). CP holds a Canada Research Chair (T1) in Neurological infection and Immunity.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/cjn.2015.306





