VIROLOGY
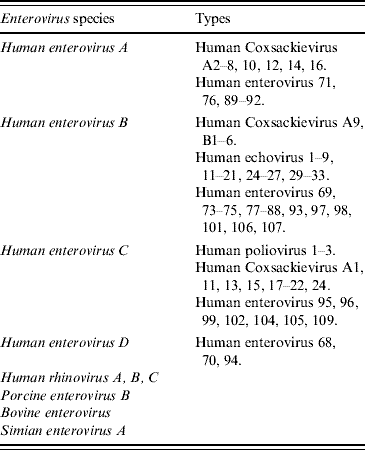
The family Picornaviridae contains 12 genera of non-enveloped, linear positive-sense, single-stranded RNA viruses which include Aphthovirus, Cardiovirus, Enterovirus, Erbovirus, Hepatovirus, Kobuvirus, Parechovirus, Teschovirus, Tremovirus, Avihepatovirus, Senecavirus, and Sapelovirus [1]. The last five genera have not so far been associated with human infections to date. The currently recognized species and types of Enterovirus are listed in Table 1 [1, Reference Brown2]. In addition to the classical communicable disease syndromes such as poliomyelitis, herpangina, hepatitis A, and common cold, several new picornaviruses are found in humans. These newly described viruses include human parechoviruses, Saffold Cardiovirus, Klassevirus, Salivirus and other yet unclassified viruses, but their association with clinical disease is still unclear [Reference Wolthers3–Reference Li8].
Table 1. Enteroviral species and types currently recognized
The genome of human enterovirus 71 (HEV-71) is about 7·4 kb in size, which is flanked by 5′ and 3′ untranslated regions. The protein-coding region can be divided into three regions: P1 encodes for the structural proteins VP4, VP2, VP3, and VP1; P2 and P3 for the non-structural proteins 2A, 2B, 2C and 3A, 3B, 3C, 3D, respectively [Reference Muir9, Reference McMinn10]. A single, long polyprotein is translated from the viral RNA which is then cleaved to form the individual proteins. VP1 carries most of the type-specific neutralizing antibody epitopes. Mutations in VP1 have been associated with increased virulence in animal models [Reference Chua11].
HEV-71 has three genotypes (A, B, and C) based on VP1 and VP4 gene sequences. Genotypes B and C are each further divided into five subtypes B1–B5 and C1–C5, respectively. More recently, analysis of complete genome sequences of HEV-71 (including non-structural protein genes) suggested that subgenotype C4 should be classified as a new genotype D (Fig. 1) [Reference Chan, Sam and AbuBakar12]. Retrospective analysis of strains of HEV-71 isolated in The Netherlands from 1963 to 1967 suggested that there is a new subgenotype B0 [Reference Van der13].

Fig. 1. Phylogenetic analysis of all genotypes of EV-71 represented by their most recent isolates based on alignment of VP1 gene sequence available in GenBank (891 nucleotide positions in each VP1 region were included in the analysis). The scale bar indicates the estimated number of substitutions per 100 bases. Phylogenetic tree construction was performed using neighbour-joining method with GrowTree using Kimura's two-parameter correction, with bootstrap values calculated from 1000 trees (Genetics Computer Group Inc., USA)
Genetic recombination among RNA viruses was first noted in poliovirus [Reference Cuervo14]. Recombination occurs through template switch and other mechanisms mainly in the non-structural protein region of the genome [Reference Cuervo14, Reference Lukashev15]. Intra-typic recombinations are commoner than inter-typic ones owing to the higher degree of sequence homology between the gene segments. Recombination is also frequent and a major source of genetic variation in other non-polio enteroviruses [Reference Santti16–Reference Simmonds and Welch20]. Most recombination occurs within the same species of virus; natural inter-species recombination is uncommon. Intra-typic and inter-typic HEV-71 recombinants have been detected in outbreaks in the Asia Pacific region. In Malaysia, inter-typic recombinants of HEV-71 have been described with the P3 region being derived from CV-A16 [Reference Chan and AbuBaker21]. In China, intra-typic recombinants were found to be circulating in the 2008 outbreak of HEV-71 [Reference Ding22]. Although recombinants can be a driving force in the genesis of new epidemics, other factors such as cross-protection with other genotypes, virulence, and transmissibility may also play a role in determining the size and outcome of epidemics. Examples of both intra-typic and inter-typic recombinations are shown in Figure 2. Bootscan analysis of a recent EV-71 strain identified in Guangzhou suggested that intra-typic recombination occurs between EV-71 genotypes B and C at junctions 2A–2B and 3C; however, the analysis of a recent HEV-71 strain identified in Shanghai suggested inter-typic recombination between HEV-71 genotype C and CA16 G-10.

Fig. 2. (a) A recent example of intra-typic recombination reviewed by bootscan analysis [bootscanning was conducted with Simplot version 3.5.1 (Kimura distance model: window size 500 bp, step 20 bp) on a gapless nucleotide alignment, generated with ClustalX, with the genome sequence of the EV-71 strain (01/Guangzhou/China/2008) as the query sequence]. (b) A recent example of inter-typic recombination reviewed by bootscan analysis [bootscanning was conducted with Simplot version 3.5.1 (Kimura distance model: window size 500 bp, step 20 bp) on a gapless nucleotide alignment, generated with ClustalX, with the genome sequence of the EV-71 strain (036/Shanghai/China/2009) as the query sequence].
PATHOGENESIS AND PATHOLOGY
At least three human cellular receptors of HEV-71 have recently been identified [Reference Lin23–Reference Yamayoshi25]. The relative importance of these receptors in different tissues or in different phases of the infection awaits clarification. Human dendritic cells are susceptible to infection by HEV-71 through DC-SIGN [dendritic cell-specific intracellular adhesion molecules (ICAM)-3 grabbing non-integrin, also known as CD209] [Reference Lin23]. Infected dendritic cells serve as antigen-presenting cells with the ability to prime T cells to generate protective immune responses. DC-SIGN is unlikely to be the sole receptor for HEV-71 since cell types not expressing DC-SIGN can also be infected by the virus.
Another HEV-71 cellular receptor is the human P-selectin glycoprotein ligand-1 (CD162) [Reference Nishimura24]. CD162 is a cell surface glycoprotein which plays an important role in the binding of leukocytes to endothelial cells and platelets. It is expressed on the surface of cells of haematopoietic origin but not on parenchymal cells of most tissues. Cells expressing the ligand include circulating leukocytes, dendritic cells, tissue macrophages (such as those in liver, lung, bowel, and Langerhans cells in the skin) and myeloid progenitor cells [Reference Moore26]. Its presence on the macrophages in the mucosa-associated lymphoid tissues of the alimentary tract has been postulated to represent the primary site of viral multiplication after infection and the infection and activation of Langerhans cells in the skin reflects the genesis of the skin lesions typical of hand, foot and mouth disease (HFMD) [Reference Nishimura24].
A third receptor is the scavenger receptor BII (SR-BII) which has the physiological function of mediating high-density lipoprotein uptake into and cholesterol efflux from cells [Reference Yamayoshi25, Reference Mulcahy, Riddell and Owen27, Reference Webb28]. It is expressed in significant amount in various organs and cells, including the liver, spleen, testes, retinal pigment epithelial cells, osteoblasts, macrophages, and importantly, the brain [Reference Thilakawardhana29–Reference Brodeur31]. The scavenger receptor class B is also involved in the uptake of the hepatitis C viruses [Reference Grove32, Reference Barth33].
The ubiquity of HEV-71 receptors in different organs may account for the systemic nature of HEV-71 infection in severe cases and the predilection for involvement of the central nervous system (CNS). In poliovirus infection, the poliovirus receptor CD155 is present in a large number of organs and tissues, yet not all these organs are sites of viral replication or exhibit the pathology of infection [Reference Mueller, Wimmer and Cello34, Reference Racaniello35]. Hence, the presence of receptors per se may be a necessary but not sufficient prerequisite for the pathogenesis of the infection. Both polioviruses and HEV-71 are neurotropic, thus explaining their propensity to cause neurological complications such as acute flaccid paralysis. Neuropathological examination of fatal human encephalomyelitis patients showed that inflammation involved the whole grey matter of the spinal cord, tegmentum of the midbrain, hypothalamus, and subthalamic and dentate nuclei, and more focally and less intensely in the cerebral cortex, especially the motor cortex. The virus may enter the CNS via the motor pathway of the peripheral nervous system, possibly through retrograde axonal transport [Reference Wong36, Reference Chen37]. This is supported by the in vitro finding that infection of the human neuronal cell line (SK-N-SH) by HEV-71 resulted in the highest level of viral replication when compared to human laryngeal (HEp-2), human glial (U373MG), and African green monkey kidney (Vero) cell lines [Reference Wen38]. Experimental evidence suggested that HEV-71-infected cells (including neurons and Vero cells) undergo apoptosis through a variety of pathways [Reference Kuo39–Reference Chen43]. In addition, neuronal damage can also be caused by HEV-71-induced cellular autophagy [Reference Huang44]. Finally, HEV-71 infection of endothelial cells can lead to activation and apoptosis; this may serve as an alternative mechanism of end organ damage in systemic infections [Reference Chen43].
The brains of patients who developed HEV-71 encephalitis generally showed oedema, vascular congestion, and typical pathological features of encephalitis [Reference Chan45–Reference Yang47]. There is a predominant involvement of the grey matter especially in the brainstem; inflammation in cerebellum, spinal cord, and the meninges is often present. Involvement of the cerebrum is generally less intense. Neuronal degeneration and necrosis are common. The inflammatory response is characterized by perivascular mononuclear cell infiltration. Micro-abscess formation and glial nodules may also be seen. The lung of a patient with cardiopulmonary failure is characterized pathologically by oedema, diffuse alveolar damage and the presence of inflammatory infiltrates [Reference Chan45]. Viral myocarditis is not a feature of HEV-71-associated cardiopulmonary failure in patients who developed brainstem encephalitis. The myocardium showed coagulative myocytolysis and myofibrillar degeneration, which suggested that the pathogenesis is one of neurogenic cardiac damage rather than direct involvement by infection [Reference Fu48, Reference Shieh49].
PROTECTIVE IMMUNITY
There is clinical and experimental evidence on the roles of different arms of immune responses in the protective immunity against HEV-71 infection. The cellular and humoral immune responses are both essential for decreasing the viral load and mortality in mice [Reference Lin50]. In humans, cellular immunity is important in preventing the development of serious complications after HEV-71 infection [Reference Yang51, Reference Chang52]. On the other hand, the neutralizing antibodies from the humoral response appear to be crucial in the protective immunity against infection [Reference Yu53–Reference Wu55]. During the 1998 epidemic in Taiwan, attack and case-fatality rates were lowest in seropositive infants aged <6 months, suggesting a protective role of maternal antibodies [Reference Chang56, Reference Ho57]. In humans, presence of maternal anti-HEV-71 antibodies has also been demonstrated in neonates, the prevalence and titre of which correlate with those levels in the mothers [Reference Luo58]. In mice, transplancental transfer of antibodies following maternal immunization against EV-71 protects against lethal infection of newborn mice [Reference Chiu59]. Thus, it appears that the seroprevalence of neutralizing antibodies in women of childbearing age is important in protecting infants aged <6 months. The protective role of breastfeeding also needs to be studied: breast milk contains lactoferrin which inhibits binding of HEV-71 to host cells. Whether secretory IgA in maternal milk contributes to the mucosal immunity against HEV-71 (as has been proven in the case of poliovirus) is not known.
CLINICAL DISEASE
Many picornaviral infections occur mainly in childhood. The propensity to cause outbreaks is an important feature of some of these viruses, most notably poliomyelitis in the pre-vaccination era and now, enteroviruses. Enterovirus outbreaks range from small community clusters of acute haemorrhagic conjunctivitis due to Coxsackieviruses to large nationwide HEV-71 epidemics. Most enteroviral infections are asymptomatic which adds to the difficulty in controlling spread in the community.
Clinical syndromes typically associated with enteroviral infections include undifferentiated fever; neurological manifestations (acute flaccid paralysis, aseptic meningitis, meningoencephalitis); respiratory infections with exanthems and/or enathems (HFMD, herpangina); eye infections (acute haemorrhagic conjunctivitis); cardiovascular infections (pericarditis, myocarditis); muscle diseases (pleurodynia and Bornholm disease); and systemic infections.
HFMD is a common illness in children aged <10 years. The infection typically has an incubation period of 3–7 days. The main manifestations are fever, lymphadenopathy, followed in 1–2 days' time by the appearance of vesicles on the palmar and plantar skin, buccal mucosa, and tongue. Papular and vesicular lesions can also occur on other parts of the body. The oral enanthem helps to distinguish HFMD from other causes of childhood exanthems, although cases without oral lesions have been described. Uncomplicated HFMD usually resolves in 5–7 days. CV-A16 and HEV-71 are the commonest causes of HFMD, the latter is especially common in the Asia Pacific region. Other enteroviruses causing HFMD include CV-A4 to A7, A9, A10, A24, B2–B5, echoviruses 1, 4, 11, 18, and HEV-18. Clinical features of HFMD caused by these viruses are indistinguishable. In contrast to CV-A16, HFMD caused by HEV-71 is more likely to cause a high fever (⩾39°C) and fever for >3 days, more severe illness, and a higher risk of developing complications and fatalities [Reference Chang60, Reference Chong61]. HFMD is rare in adults but cases due to HEV-71 have been reported. Adults can also develop severe HEV-71 infections such as encephalitis as a result of intra-familial transmission [Reference Tai, Hsieh and Wu62, Reference Hamaguchi63].
HEV-71 infection commonly manifests as HFMD or herpangina. In a few patients, neurological and cardiopulmonary complications with substantial mortality may occur. No specific genotype is associated with more severe disease [Reference Cardosa64]. Children aged <5 years have the highest incidence of severe complications [65–Reference Yang67]. Fatal cases of HFMD due to HEV-71 were more often associated with vomiting and a lower incidence of mouth ulcers [Reference Chong61]. The predominant forms of neurological syndrome include aseptic meningitis, acute flaccid paralysis, brainstem encephalitis, or cerebellitis and vary in different epidemics. These complications often appear early, at 2–5 days after the onset of illness [Reference Huang68]. Over an 8-year period from 1998 to 2005, the case-fatality rate of complicated enteroviral infections – most of which were caused by HEV-71 – ranged from 10·0% to 25·7% [65].
Long-term neurological sequelae are common in survivors who had more serious CNS disease and cardiopulmonary failure. Late complications include limb weakness, dysphagia requiring tube-feeding, cerebellar dysfunction, delayed neurodevelopment, and impaired cognitive functions [Reference Chang69, Reference Huang70].
Acute cardiopulmonary failure as a complication of systemic HEV-71 infection has a high mortality. Pulmonary oedema is related to cerebral compression or increased intracranial pressure which leads to sympathetic hyperactivity [Reference Kao71]. Pulmonary oedema and the associated hypoxaemia and acute respiratory distress syndrome are the commonest causes of death in severe HEV-71 infections. Similarly, brainstem encephalitis leads to acute heart failure in 19% of patients and this complication has a high mortality rate of 77% [Reference Fu48]. Again, hyperactivity of sympathetic stimulation to the heart leading to a ‘catecholamine storm’ and neurogenic cardiac damage is believed to be the mechanism of cardiac damage in this infection.
Risk factors associated with the progression to CNS involvement without pulmonary oedema in HEV-71 infection included fever for ⩾3 days, peak body temperature of ⩾39°C, the presence of headache, lethargy, vomiting, seizure, and hyperglycaemia. Hyperglycaemia, leucocytosis, and limb weakness were found to be risk factors for pulmonary oedema [Reference Chang72]. In some series, a higher level of leucocytosis was found in patients with CNS involvement [Reference Li73]. In a series of 333 patients with CNS involvement due to non-poliovirus in Taiwan, severe CNS disease was associated with age <4 years, leucocytosis (over 13×109/l), seizure, myoclonic jerks, and a higher incidence of skin rash and oral ulcers [Reference Yang74].
In those patients who developed serious CNS disease due to non-poliovirus infection, poor prognostic factors included age <2 years, higher peak leucocyte counts (over 17×109/l), a higher incidence of skin rash, and a lower yield of virus from the cerebrospinal fluid [Reference Yang74]. For HEV-71 cardiopulmonary failure, poorer clinical outcomes were associated with higher troponin I level, lower initial systolic blood pressure, longer duration of hypotension, greater requirements for inotropic support, lower PaO2:FiO2 ratio, higher white blood cell counts in the cerebrospinal fluid, and the lowest Glasgow coma score. Fatality correlated best with the troponin I level [Reference Hsia75].
LABORATORY DIAGNOSIS
Aetiological diagnosis of HFMD can be achieved by examining the vesicular fluid aspirated from the skin lesion and naso-/oro-pharyngeal swabs. In complicated cases with brainstem encephalitis and cardiopulmonary failure with sparse skin lesions HEV-71 may still be detectable in the cerebrospinal fluid, naso-/oro-pharyngeal secretions, or faeces. The commonest rapid diagnostic test is by reverse transcription–polymerase chain reaction (RT–PCR) of the RNA extracted from these specimens, targeting towards the 5′ untranslated or VP1 region of the viral genome [Reference Iturriza-Gómara, Megson and Gray76–Reference Nix, Oberste and Pallansch78]. Isolation of virus from clinical specimens is possible using conventional cell culture or rapid shell viral culture with rhabdomyosarcoma (RD), HEp-2, colonic carcinoma (CaCo-2), or Vero cell lines and cytopathic effects can be seen in 3–7 days [Reference Terletskaia-Ladwig79]. Cell lines infected by HEV-71 or CV-A16 can be differentiated by immunostaining with specific monoclonal antibodies against their VP1 proteins. The isolates can be genotyped by PCR sequencing of the VP1 and/or VP4 genes. IgM antibody to HEV-71 has been detected as early as 2 days after onset of illness but, as the test is not yet widely available, serological diagnosis generally requires demonstration of a fourfold rise in neutralizing antibody titre taken 10–14 days after the onset of illness [Reference Tsao80].
EPIDEMIOLOGY
HEV-71 was first detected in 1969 in California in an infant suffering from encephalitis. Initial isolations of HEV-71 were made in the USA and in Australia in the early 1970s, and outbreaks of HFMD occurred in Sweden and Japan [Reference Blomberg81, Reference Hagiwara, Tagaya and Yoneyama82]. In the late 1970s, Bulgaria (1975) and Hungary (1978) witnessed large epidemics of HEV-71 infection with prominent neurological manifestations (aseptic meningitis, encephalitis, acute flaccid paralysis) [Reference Chumakov83, Reference Nagy84]. Since the late 1990s the densely populated Asia Pacific region has been the hotspot for epidemics: Taiwan, Singapore, Malaysia, China, Vietnam, and Australia have experienced recurrent epidemics of various sizes. The reason for this geographical distribution is uncertain, but the association between HLA-A33 (which is common in some Asian populations) and susceptibility to HEV-71 infection has been suggested as a possible explanation [Reference Chang85]. Other factors such as genetic predisposition (glucose-6-phosphate dehydrogenase deficiency, polymorphisms in cytotoxic T lymphocyte antigen-4 and scavenger receptor BII), food and water hygiene, and micronutrient deficiencies require further studies to confirm their significance [Reference Yang51, Reference Ho86–Reference Cermelli91].
Recent epidemics of HFMD disease in the Asia Pacific region were mainly caused by HEV-71 (Table 2). However, more than one subgenotype of HEV-71 can be found co-circulating in the same epidemic, as well as other non-HEV-71 enteroviruses, such as CV-A16 (Table 3). Co-infection is possible in enteroviral HFMD [Reference Chan92, Reference Ooi93]. In Sarawak, Malaysia, co-infection of HEV-71 with other viruses occurred in 10% of patients [Reference Ooi93]. Co-infection does not appear to result in more severe clinical disease [Reference Chan92].
Table 2. Recent outbreaks of HFMD due to HEV-71 in the Asia Pacific region

n.a., Not available; CV-A, Coxsackievirus A; HEV, human enterovirus; EV, echovirus. Viruses or genotypes in parentheses are not the predominant strains.
* Actual number of cases estimated to be ten times higher.
† Compared to 0·7–0·8% in epidemics due to CV-A16.
Table 3. Summary of predominant EV-71 genotypes in the Asia Pacific region

Boldface indicates major outbreaks as indicated by the reports showing a significant increase in incidence above their local baseline.
Data from references [Reference Ding22, Reference Cardosa64, Reference Podin124, Reference Chua171, Reference Sanders175, Reference Yang179, Reference Li181–Reference Ang186].
The Taiwan epidemic of HEV-71 HFMD in 1998 was the largest outbreak in the Asia Pacific region until 2008, when 488955 cases of HFMD were reported in China (Table 2), certainly an underestimation of the actual burden of disease. More than 600 000 cases were reported from March to June 2009, but this could be because HFMD became a notifiable disease in China from May 2008. The epidemic centre in China in 2008 was the southeastern provinces of Guangdong, Zhejiang, and Anhui. The overall case-fatality rate reported from China in 2008 and 2009 was about 0·03%, but in certain local outbreaks, such as the 2008 outbreak in Fuyang City of Anhui Province, the case-fatality rate was as high as 0·3% [94].
Humans are the only known natural hosts of HEV-71. Intra-familial transmission of HEV-71 occurs commonly. In a prospective cohort in Taiwan, transmission rates from infected children to siblings were as high as 84% [Reference Chang66]. The rate of symptomatic infection after household transmission is higher than that in other community settings (94% vs. 29%) which is attributed to more prolonged contact with the cases and possibly a larger infective dose. Faeces and oropharyngeal secretions are likely to be important in the transmission of the virus. Shedding of non-polio enteroviruses in the stool can persist for 3–11 weeks after the onset of illness, while the duration of shedding from oral secretion is shorter [Reference Chung95, Reference Tosato, Rocchi and Archetti96]. Enteroviruses are also detectable in throat swabs. During the 1998 Taiwan HEV-71 epidemic, detection of the virus from the throat swabs was more frequent than from stool specimens; the time to positivity by viral culture was also shorter [Reference Wang97]. The superior recovery rate of enteroviruses, mainly HEV-71 and CV-A16, from throat swabs over stool samples (with or without the testing of vesicle swabs) in HFMD was confirmed in another study in Sarawak, Malaysia [Reference Ooi98]. A recent study from Mongolia showed that hand washing after defecation was associated with a lower risk of infection by non-polioviruses in households, whilst having a bathroom in the house was a risk factor for infection [Reference Kuramitsu99]. These findings suggested that the faeco-oral route is probably important. However, other hygiene measures did not affect the incidence of virus isolation. Hence, modes of transmission other than faeco-oral could also play an important role in the households. The relative importance or infectivity of oropharyngeal secretions vs. faeces in real-life situations have not been determined. We postulate that in developing countries, sanitation plays a more important role. In developed countries, although personal hygiene and sanitation facilities are much better, there is also a much larger number of facilities where great numbers of susceptible children congregate. Under such circumstances, respiratory transmission may become more significant. Faeco-oral transmission may contribute more to an endemic disease in the community, whereas the respiratory tract, whose secretions contain a higher viral load but have a shorter duration of shedding than faeces, may contribute more to the epidemic spread of the viruses during outbreak situations. In the 1998 Taiwan epidemic, in addition to intrafamilial contact with cases, attendance at a kindergarten or childcare centre and residence in a rural area were significant risk factors associated with HEV-71 infection. This suggests that close contacts at schools are important in the epidemiology of the disease by acting as sources of spread to the community [Reference Chang56]. Contact transmission from blister fluid may have a minor role.
Enteroviraemia has been demonstrated in healthy Scottish blood donors at a predicted prevalence of 0·023% [Reference Welch100]. The viruses being detected were primarily Coxsackieviruses and human echoviruses. Similarly, enterovirus, as well as cytomegalovirus, parvovirus B9, and adenovirus genomes have been detected in explanted heart myocardial tissues from heart transplant donors and recipients in Germany. The prevalence of enterovirus as detected by RT–PCR reaction ranged from 21% to 60% of the heart samples [Reference Donoso Mantke101]. During the HEV-71 epidemic in Taiwan in 1998, 20% of patients in one study had viraemia as detected by RT–PCR [Reference Li73]. Although these findings suggested that transfusion of blood products and transplantation could be potential routes of transmission, such cases have not yet been reported. One case of intrauterine infection by HEV-71 was reported and the pregnancy was complicated by stillbirth with virological evidence of brain and liver involvement [Reference Chow102]. Another baby who developed perinatal HEV-71 infection with HFMD and aseptic meningitis had a benign course and recovered without long-term neurological sequelae [Reference Nishikii103].
The contribution of environmental factors in the ecology and transmission of HEV-71 is not well understood. Few environmental studies specifically addressed HEV-71, although one may draw inferences from studies involving other better studied picornaviruses such as poliovirus and Coxsackieviruses. Enteroviruses have a higher level of resistance to biocides compared to other enveloped viruses. They are fairly tolerant to temperature, salinity, pH, and sewage treatment procedures. Different types of enteroviruses – including HEV-71 – can readily be detected in sewage and other environmental waters [Reference Hsu, Chen and Wan104, Reference Chen, Hsu and Wan105]. The finding of enteroviruses in surface waters has no correlation with the level of other indicator organisms of water pollution. Outbreaks of picornavirus meningitis have occurred with the sources traced to potable or recreational water sources. Examples include swimming pools (echovirus 30 aseptic meningitis), campsite swimming pool (echovirus 9 aseptic meningitis), nature-like swimming pond (echoviruses 30 and 13), tap and bottled drinking water (echoviruses 30 and 6, CV-B5) [Reference Faustini106–Reference Amvrosieva109]. Inadequate chlorination might have accounted for some outbreaks; however, the usual levels of chlorination in swimming pools and potable water (2–4 ppm and 1 ppm free chlorine, respectively) do not reliably inactivate picornaviruses. Viable enteroviruses can also be isolated from bottled and tap water (vaccine strain of poliovirus, Coxsackievirus B, echoviruses) [Reference Lee and Kim110–Reference Ehlers, Grabow and Pavlov112]. The waterborne outbreak in Belarus in 2003, mainly caused by Echovirus 30 and to a lesser extent, Echovirus 6, was one of the largest outbreaks, affecting 1222 children; 57·5% of the patients developed meningitis [Reference Amvrosieva109]. Interestingly, during the 1998 Taiwan outbreak, usage of tap water was found to be a risk factor for acquisition of HFMD or herpangina in univariate analysis [Reference Chang56]. However, the study did not specify whether the contact involved drinking unboiled tap water, rinsing of mouth during tooth brushing or face washing. The role of cross-infection caused by backwashing of infected saliva onto drinking fountains used in schools and other public facilities has not been investigated.
In addition to drinking water, sewage contamination of coastal and marine waters can also lead to accumulation of enteroviruses in molluscs. Clams, mussels, oysters, and crabs have been found to have a high prevalence of enteroviruses (ranging from 8% to 40% in some studies), frequently in the presence of a normal coliform count in the samples [Reference Ehlers, Grabow and Pavlov112–Reference Gabrieli116]. Foodborne transmission of HEV-71 in humans has not been demonstrated. However, it would not be surprising if this turns out to be an important route of transmission in settings where raw shellfish is frequently consumed. Apart from hepatitis A virus, another picornavirus, Aichi virus, has been found to be involved in causing outbreaks of gastroenteritis related to consumption of oysters and other seafood in France, and the seroprevalence among adults in the general populations was up to 85% [Reference Le Guyader117–Reference Goyer119].
Although the role of environmental viruses in the transmission and maintenance of HEV-71 infection remains to be defined, their contribution needs to be further studied, especially in developing countries where sewage treatment, quality of potable water supply, and food hygiene could be suboptimal. Nevertheless, these environmental factors will not be the sole determinants of the epidemiology of HEV-71 infections. Some developing countries such as India has not reported major outbreaks, whereas high prevalence of enteroviruses has been found in potable water in developed countries like Korea, and epidemics of HEV-71 HFMD have occurred in developed countries such as Singapore and Taiwan [Reference Saoji120]. The policy of surveillance for HFMD and HEV-71 and class suspension in cases of school outbreaks may have helped to limit the size of outbreaks in several of the endemic Southeast Asian countries [121, 122]. Various control measures at epidemic sites such as school closure or discontinuation of drinking fountains have not been adequately evaluated. Such measures may theoretically reduce interpersonal transmission at these sites and so prevent further community spread of the virus.
The peak season for HFMD and HEV-71 infection is generally the summer months. Epidemics of HEV-71 infection tend to occur once every 2–5 years. The cyclical appearance of epidemics could be due to the accumulation of susceptible individuals in the community. On the other hand, introduction of new genotypes or emergence of new genotypes or strains has led to outbreaks in the Asia Pacific region [Reference Cardosa64]. Studies in Taiwan showed that the level of seroprevalence against HEV-71 in different parts of the island and the age-specific seroprevalence are correlated with the incidence of severe disease and mortality rates [Reference Chang56]. Emergence of a new genotype or subgenotype of HEV-71 could contribute to the occurrence of outbreaks. The two changes in the prevailing genotype of HEV-71 in Taiwan can be seen in Table 3, with the epidemics in 1998 and 2000 being caused by C2 and B4 strains, respectively [Reference Wang123]. Similarly, surveillance studies in Sarawak also demonstrated the emergence of a new subgenotype C1 in the 2003 epidemic [Reference Podin124]. New variants of the virus can also be generated from intra-typic and inter-typic recombinations of the pre-existing viruses (Fig. 2) [Reference Ding22, Reference Huang125, Reference Chan and AbuBakar126]. Nevertheless, epidemic genotypes or subgenotypes of the virus could circulate for a long time before causing outbreaks. Similarly, the introduction of new genotypes into a susceptible population does not always result in large epidemics. The lack of herd immunity towards the prevailing strains of HEV-71 is apparently not sufficient to generate an epidemic. Virulence of the viral strains could also be pivotal. However, this area has not been fully studied. A comparison of the HEV-71 strains from fatal and non-fatal cases in Taiwan showed that they have a very high degree of genomic identity, with only minor differences in the homology of the 3C protease [Reference Shih127]. The 3C protease induces apoptosis in human neural cells in vitro, but its role in determining the virulence of the virus has not been documented [Reference Li128]. Neither has the role of temperature-sensitivity of the virus been shown to be of significance in determining virulence in an animal model [Reference Hagiwara, Yoneyama, Takami and Hashimoto129]. The contribution of micronutrient deficiencies (such as selenium and vitamin E) in determining the outcome of HEV-71 infection is uncertain.
TREATMENT, PREVENTION, AND CONTROL
Most HFMD cases are self-limiting and only required supportive treatment. The case-fatality rate for HFMD of all causes ranges from 0·06% to 0·11% [Reference Chong61]. The rapid progression to neurological and cardiopulmonary complications after the onset of HEV-71-associated disease (usually within 3–5 days) suggests that viral replication and direct cytopathic effects of the virus on the host cells are important in the pathogenesis of severe manifestations [Reference Chan45, Reference Yang67, Reference Pérez-Vélez130]. Thus, early administration of an effective antiviral agent should theoretically be beneficial. There is currently no specific antiviral approved for HEV-71 infections. Of the available antivirals, pleconaril is the best studied agent against picornaviruses. It binds to the hydrophobic pocket in the viral capsid VP1, thereby inhibiting the attachment, entry, and uncoating of the virus [Reference Smith131]. Pleconaril is readily absorbed after oral administration and penetrates well into body fluids including the cerebrospinal fluid. However, the drug is not readily available in most countries and there are limited data showing that it lacks in vitro activity against HEV-71 [Reference Shia132, Reference Nolan133]. Pleconaril has been used in a small number of patients suffering form HEV-71 encephalitis and pulmonary oedema but its efficacy cannot be ascertained to date [Reference Nolan133, Reference Prager134]. In one study, ribavirin showed antiviral activity against HEV-71 in vitro and in animal models but there are no reports of its use for treating human EV-71 infections [Reference Li135]. One animal experiment suggested that type I interferons may have a useful therapeutic role in EV-71 infection [Reference Liu136].
Agammaglobulinaemic individuals are prone to the development of chronic enteroviral meningoencephalitis. Prophylaxis and treatment with intravenous immunoglobulin (IVIG) has been successful in this group of patients and there are anecdotal reports of successful treatment of enteroviral meningoencephalitis in other immuncompromised patients [Reference McKinney, Katz and Wilfert137–Reference Moschovi139]. Since maternal antibody appears to be protective against enteroviruses including HEV-71, transfusion of maternal plasma had been used in a case of neonatal disseminated echovirus infection [Reference Jantausch140]. IVIG has also been used in patients with complicated HEV-71 infections; in addition to the presence of neutralizing antibodies and hence the suppression of viral replication, immunoglobulin may also play a role in limiting organ damage through its anti-inflammatory activities [Reference Nolan133, Reference Wang141–Reference Wang143]. However, its benefits have not yet been demonstrated in randomized controlled trials. Different preparations of IVIG also vary in their titres of antibodies against enteroviruses [Reference Galama144]. Based on the deleterious effects of dexamethasone in animals, control of the inflammatory response using systemic corticosteroids cannot be recommended at present [Reference Liu136]. The use of milrinone – a bipyridine inotropic agent used in the treatment of heart failure – has been recommended based on a historically controlled study in Taiwan which demonstrated clinical and survival benefits in milrinone-treated patients with pulmonary oedema due to severe HEV-71 infection [Reference Wang145, Reference Wang146].
With the observation that neutralizing antibodies offer protection against infection and mortality in humans and animal models, vaccination is an obvious pathway towards prevention of HEV-71 infection and epidemics. Initial work on various models of HEV-71 vaccines (using VP1 subunits, inactivated viruses, DNA vaccines, virus-like particles, or oral vaccination with VP1 protein) in animals appears promising, although still in the early stages of development [Reference Wu147–Reference Chen151]. Animal studies with attenuated EV-71 vaccine and human seroepidemiological studies suggested there may be cross-protecting neutralizing antibodies between different EV-71 genotypes [Reference Arita152, Reference Mizuta153]. However, the relevance of these findings to the development of vaccines (in terms of the choice of and the need periodically to change vaccine strains) is uncertain. Rapid changes in VP1 as a result of recombination necessitate a robust surveillance of circulating viruses. The generally mild disease manifestations, prevalence and lack of monitoring in some developing countries means the incentive for vaccine development could be low. A novel potential means of preventing HEV-71 infection is the use of oral lactoferrin. In vitro, lactoferrin inhibits binding of EV-71 to cells and neonatal mice fed with recombinant porcine lactoferrin were protected against lethal infection by EV-71 [Reference Lin, Chu and Chiu154–Reference Chen156].
In the absence of vaccine, prevention of human EV-71 infections still relies on basic infection control measures, especially in schools and childcare centres. Monitoring for the incidence of HEV-71-associated diseases such as HFMD and herpangina is essential, as well as virological studies to detect emergence of new genotypes of the virus.
Proper environmental disinfection in school and childcare centres and avoidance of possible public sources of viral acquisition may be necessary [Reference Hsu, Chen and Wan104, Reference Faustini106, Reference Hauri108, Reference Keswick, Gerba and Goyal157, Reference Begier158]. Only one study specifically examined the efficacy of a peroxygen disinfectant on HEV-71 [Reference Chan and AbuBakar159]. In general, effective environmental disinfectants against picornaviruses include sodium hypochlorite, glutaraldehyde, chlorine dioxide, and peroxygen compounds [Reference Chan and AbuBakar159–Reference Zoni162]. As an antiseptic, povidone iodine is highly effective against non-enveloped viruses [Reference Kawana163]. Alcohol hand rubs commonly used in healthcare settings contain 60–70% v/v alcohols (such as ethanol, isopropanol, and n-propanol). These preparations are less effective in inactivating non-enveloped viruses than bacteria and enveloped viruses. Higher concentrations of alcohol (such as 85–95% v/v ethanol) are required for reliable virucidal activities against non-enveloped viruses [Reference Kampf and Kramer164]. Alternative hand disinfectants that can be considered include 0·2% peracetic acid with 80% (v/v) ethanol and other newer combination alcohol-based formulations [Reference Wutzler and Sauerbrei165–Reference Macinga, Sattar, Jaykus and Arbogast167]. These products should be used in situations where active outbreaks of enterovirus infection are ongoing.
Countries in the Asia Pacific region in particular should develop national plans for future epidemics of HEV-71 infection. In Hong Kong, for example, the following strategies have been developed in anticipation of possible community outbreaks: surveillance and laboratory support; clinical management and infection control in healthcare settings; emergency preparedness (including stepping up of surveillance and case investigation, mobilization of clinical and intensive-care facilities, enhanced laboratory support, data management, outbreak control measures, and risk communication); health education and capacity building; and applied research [168].
CONCLUSION
In the past decade, HFMD due to HEV-71 has become a major public health concern in the Asia Pacific region, which appeared to be the epicentre for the generation of epidemic genotypes (as with influenza). Control and prevention of the disease is a difficult task because of the stability of the virus in the environment and its high transmissibility, frequency of genetic recombinations, the lack of an effective antiviral and vaccine, and the relatively low priority for vaccine development. Development and studies in the only available anti-picornavirus agent pleconaril have been minimal towards HEV-71, and newer technologies for screening antiviral compounds, for example, using chemical genetics and screening of chemical libraries, may help in the discovery of a novel agent for treatment [Reference Kao169]. The contributions of genetic, environmental, and viral factors in the genesis of epidemics in different regions of the world need to be clarified in order to understand the varying epidemiology of the disease in different countries. Similarly, the role of public health measures to reduce the impact of outbreaks, such as school closure, warrant further studies.
ACKNOWLEDGEMENTS
The authors acknowledge funding from the University Development Fund 2001–2002 (first round) of The University of Hong Kong, Clinical Infectious Diseases Research Endowment Fund from Ms Teresa On-Yik Wong, Providence Foundation Limited in memory of the late Dr Lui Hac Minh, Research Fund for the Control of Infectious Diseases (RFCID) of the Food and Health Bureau of the Hong Kong SAR Government.
DECLARATION OF INTEREST
None.







