Struggles with conflict and hostility in adolescents’ interactions with peers and parents have been linked to numerous mental health difficulties, but might these struggles also have long-term implications for physical health into adulthood? Both life history theory and evidence regarding the plasticity of stress reaction systems in adolescence suggest the likely existence of precisely such linkages (Del Giudice, Ellis, & Shirtcliff, Reference Del Giudice, Ellis and Shirtcliff2011; Romeo, Reference Romeo2010). In adult social relationships, exposure to hostile conflict has long been recognized as a risk factor for cardiovascular disease, with risks likely mediated via health-relevant biological alterations to interpersonal stress (Kiecolt-Glaser et al., Reference Kiecolt-Glaser, Loving, Stowell, Malarkey, Lemeshow, Dickinson and Glaser2005; Smith, Glazer, Ruiz, & Gallo, Reference Smith, Glazer, Ruiz and Gallo2004). Similar findings also appear in early childhood, with exposure to social adversity associated with long-term health difficulties (Fagundes & Way, Reference Fagundes and Way2014; Temcheff et al., Reference Temcheff, Serbin, Martin-Storey, Stack, Ledingham and Schwartzman2011). Within adolescence, close friendship quality and behavior that hews closely to larger social “pack” norms (i.e., behavior that would tend to reduce conflict) have been found to predict self-reported health quality in adulthood (Allen, Uchino, & Hafen, Reference Allen, Uchino and Hafen2015). Conflict and aggression in adolescent social relationships, in contrast, have been linked to an array of internalizing and externalizing behavioral disorders (Collins & Laursen, Reference Collins, Laursen, Lerner and Steinberg2004), but difficulty managing such conflict has not previously been examined as a predictor of future physical health outcomes.
Struggles managing conflict and hostility in adolescent relationships appear most likely to be linked to future physical health outcomes via their effects on the human stress response system. Although human biology has adapted well to accommodate the effects of acute, transient stressors, when stressors become chronic, as in the case of enduring patterns of social conflict, outcomes appear far more problematic. Stress responses that temporarily reallocate resources to meet an immediate physical demand (e.g., suppressed immune functioning and increased inflammation), such as escaping a predator in evolutionary times, become pathogenic when chronically activated (Karatsoreos & McEwen, Reference Karatsoreos and McEwen2011).
The immune system, in particular, appears highly sensitive to the effects of chronic social stress, which can lead to dysregulation of inflammatory pathways in ways that impair long-term health (Hertzman, Reference Hertzman1999; John-Henderson, Stellar, Mendoza-Denton, & Francis, Reference John-Henderson, Stellar, Mendoza-Denton and Francis2015; Miller, Chen, & Parker, Reference Miller, Chen and Parker2011). Extended exposure to stress, and the chronic level of arousal it generates, may reprogram elements of the immune system in the direction of more chronic activation, as reflected in higher levels of proinflammatory cytokines, such as interleukin-6 (IL-6), circulating in the blood stream (Miller et al., Reference Miller, Chen, Fok, Walker, Lim, Nicholls and Kobor2009). Chronic levels of inflammation in turn have been linked to future health difficulties ranging from metabolic syndrome to cardiovascular disease (Dandona, Aljada, Chaudhuri, Mohanty, & Garg, Reference Dandona, Aljada, Chaudhuri, Mohanty and Garg2005; Libby & Theroux, Reference Libby and Theroux2005). More specifically, high IL-6 levels have been associated with lower self-rated health among older adults (Arnberg, Lekander, Morey, & Segerstrom, Reference Arnberg, Lekander, Morey and Segerstrom2016), and with specific disease processes related to premature aging, including tumor formation, arthritis, and osteoporosis (Hunter & Jones, Reference Hunter and Jones2015).
Stress has been directly linked to IL-6 levels at other points in the life span. For example, childhood exposure to early life adversity and parental harshness has been associated with higher future levels of IL-6 (Miller et al., Reference Miller, Chen and Parker2011; Slopen et al., Reference Slopen, Lewis, Gruenewald, Mujahid, Ryff, Albert and Williams2010). In adults, short-term exposure to hostile conflict has also been found to lead to higher levels of IL-6 within 24 hr (Kiecolt-Glaser et al., Reference Kiecolt-Glaser, Loving, Stowell, Malarkey, Lemeshow, Dickinson and Glaser2005). Similarly, adults in chronically stressful situations have been found to experience longer term increases in levels of IL-6 (Lutgendorf et al., Reference Lutgendorf, Garand, Buckwalter, Reimer, Hong and Lubaroff1999). Within adolescence, concurrent stress has been linked to other types of immune-mediated inflammation, such as higher levels of C-reactive protein (Ehrlich, Miller, Rohleder, & Adam, Reference Ehrlich, Miller, Rohleder and Adam2016; Fuligni et al., Reference Fuligni, Telzer, Bower, Cole, Kiang and Irwin2009; Murphy, Slavich, Rohleder, & Miller, Reference Murphy, Slavich, Rohleder and Miller2013). One study has found levels of maternal support had long-range predictions to levels of C-reactive protein (Jones et al., Reference Jones, Ehrlich, Brett, Gross, Mohr, Hopper and Cassidy2016). No research to date, however, has assessed whether social stress in adolescence might have similar long-term implications for immune system functioning. If such long-term links exist, they would suggest a strong need to attend to adolescent social relationship qualities as potential determinants of life course physical health outcomes.
Several lines of reasoning suggest that struggles with conflict and hostility in adolescence will be linked to increased IL-6 levels over time. The combination of hormonal changes, neural development, and social stressors in adolescence makes this one of the most intensely social, yet also intensely lonely and stressful, periods of the life span (Albert, Chein, & Steinberg, Reference Albert, Chein and Steinberg2013; Charles, Reynolds, & Gatz, Reference Charles, Reynolds and Gatz2001; Steinberg & Monahan, Reference Steinberg and Monahan2007). Adolescence also appears as a period in which the stress response system may be particularly susceptible to chronic stress (Romeo, Reference Romeo2010): given growing brain functional connectivity across this period, adolescence may be a “switch point” for the calibration of stress responsivity (Gee et al., Reference Gee, Humphreys, Flannery, Goff, Telzer, Shapiro and Tottenham2013; Goff et al., Reference Goff, Gee, Telzer, Humphreys, Gabard-Durnam, Flannery and Tottenham2013; McEwen, Reference McEwen2007). Chronic stress exposure may even have the potential to alter metabolic systems and anatomic structures related to stress responding in a relatively permanent way (Ben-Shlomo & Kuh, Reference Ben-Shlomo and Kuh2002). A life span approach to understanding physical health suggests a need to attend to exactly these types of potential developmental sensitivities in understanding links between stress and life course health outcomes (Uchino, Reference Uchino2009).
A life span approach also suggests that the timing of effects of exposure to intense stress may be critical to consider (Bremner & Vermetten, Reference Bremner and Vermetten2001). Weathering theory, for example, suggests that chronic stress at a vulnerable stage of development will have long-term health implications, whether it is mediated by later stress or even ultimately subsides (Brody, Miller, Yu, Beach, & Chen, Reference Brody, Miller, Yu, Beach and Chen2016). Alternatively, mediational “chains of risk” theories suggest that early stressors, such as adolescent struggles with hostile conflict, may predict future health outcomes primarily because they forecast the development of hostile conflict in future relationships, which in turn mediates long-term effects (Ben-Shlomo & Kuh, Reference Ben-Shlomo and Kuh2002; Oudekerk, Allen, Hessel, & Molloy, Reference Oudekerk, Allen, Hessel and Molloy2015). This study considered both possibilities.
This 15-year study of a diverse community sample assessed predictions of adult IL-6 levels from independent observations of adolescent exposure to maternal and romantic partner hostility, analogue assessments of adolescent ability to defuse peer aggression, and peer reports of participant skill in avoiding hostile conflict. We also considered whether self-reported personality and behavioral characteristics might serve as alternative explanations, or as potential mediators of effects observed. We examined the following specific hypotheses:
1. Adult IL-6 levels will be predicted by poor early/mid-adolescent conflict resolution ability and skill at defusing peer aggression, and by exposure to hostile maternal conflict behavior.
2. Adult IL-6 levels will be predicted by late adolescent exposure to hostile maternal and romantic partner conflict behavior.
3. Adult IL-6 levels will be predicted by early adult romantic relationship stress and aggressive behavior.
4. Effects of adolescent relationship experiences will potentially be mediated via adult self-reported personality and behavioral attributes.
Method
Participants and statistical power
This report is drawn from a larger longitudinal investigation of adolescent peer influences on adult development. The final sample of 127 participants was a subsample of participants who had levels of IL-6 assessed at age 28 from among 184 participants initially assessed at age 13 (an attrition rate of 2% per year across 15 years). The final sample included 53 males and 74 females and was racially/ethnically and socioeconomically diverse and representative of the community from which it was drawn: 72 adolescents (57%) identified themselves as Caucasian, 38 (30%) as African American, 3 (2%) as Hispanic, 2 (2%) as Asian, 1 (1%) as American Indian, and 11 (9%) as of mixed race/ethnicity. Adolescents’ parents reported a median family income in the $40,000–$59,999 range at the initial assessment. Adolescents were recruited from the seventh and eighth grades of a public middle school drawing from suburban and urban populations in the southeastern United States. Information about the study was provided via an initial mailing to parents with follow-up presentations to students at school lunches. Formal recruitment took place via telephone contact with parents. Students who had already served as close peer informants in the study were not eligible to serve as primary participants. Of students eligible for participation, 63% of adolescents and parents agreed to participation when parents were contacted. Adolescents provided informed assent before each interview session, and parents and adult participants provided informed consent. Interviews took place in private offices within a university academic building.
Assessments in this study were obtained at mean ages 13.3 (SD = 0.64), 16.3 (SD = 0.87), 18.2 (SD = 1.28), 20.9 (SD = 1.07), 23.69 (SD = 0.97), 26.6 (SD = 1.00), 27.6 (SD = 1.00), and 28.5 (SD = 0.96). At the age 16, 27, and 28 assessments, participants also nominated their closest friend to be included in the study (not necessarily the same friend across ages). Close friends participated with informed assent, and parental consent if they were minors. Close friends reported that they had known the adolescents for an average of 5.4 years (SD = 3.4) at the age 16 assessment 13.4 years (SD = 8.00) at the age 27 assessment, and 13.5 years (SD = 7.96) at the age 28 assessment. For age 21 romantic relationship observations, 72 participants had available data; for age 26 reports of romantic relationship stress, 110 participants had available data.
Power for this study (>80% to detect effect sizes as small as d = 0.50, f 2 = 0.078, R 2 = .073) is considered good, both given that a broad range of studies of social dysfunction and physical health find remarkably strong effects (e.g., odds ratios averaging 1.50 (equivalent to effect sizes of d > 0.80) in meta-analyses of mortality risk, using very rudimentary social measures as predictors (Holt-Lunstad, Smith, & Layton, Reference Holt-Lunstad, Smith and Layton2010), and that work within the current sample has also obtained effect sizes above d = 0.50 when predicting adult health outcomes (Allen et al., Reference Allen, Uchino and Hafen2015).
Attrition analyses
Attrition analyses examined missing data for each type of data available at baseline. Females were more likely than males to have continued the study after age 13 and to have provided follow-up IL-6 data (76% continuation rate for females vs. 62% for males, p = .042), and individuals who did not have IL-6 assessments were more likely to have reported greater romantic relationship stress at age 26 than those who did return (p = .009). Adolescents who did not participate in mother–adolescent interaction observations at age 17 had lower baseline family income levels than those who did participate (p < .001).
Other than those few differences, there were no attrition effects on any of the adolescent-era assessments described below for any of our outcome or mediating measures, suggesting that attrition was not likely to have distorted any of the findings reported. Nonetheless, gender and family income were entered as covariates in all analyses and also considered as potential moderators of key findings. Further, to best address any potential biases due to attrition in longitudinal analyses or missing data within waves, full imputation maximum likelihood methods were used with analyses including all variables that were linked to future missing data (i.e., where data were not missing completely at random). Because these procedures have been found to yield the least biased estimates when all available data are used for longitudinal analyses (vs. listwise deletion of missing data; Arbuckle, Reference Arbuckle, Marcoulides and Schumaker1996), the entire original sample was utilized for these analyses. This full sample thus provides the best possible estimates of variances and covariances in measures of interest and was least likely to be biased by missing data.
Procedure
In the initial introduction and throughout all sessions, confidentiality was assured to all study participants, and adolescents/adults were told that no one would be informed of any of the answers they provided. Participants’ data were protected by a Confidentiality Certificate issued by the US Department of Health and Human Services, which protected information from subpoena by federal, state, and local courts. Transportation and childcare were provided if necessary. Adolescent/adult participants and participants’ peer and romantic reporters were all paid for participation.
Measures
Primary measures
IL-6 (age 28)
Approximately 20 ml of blood were collected and treated with EDTA (to prevent clotting) to determine circulating concentrations of IL-6. Plasma was separated via centrifugation, aliquoted, and stored at –80 °C. IL-6 was measured by ELISA (limit of detection = 0.3 pg/ml; R&D Systems, San Diego, CA). Intraassay and interassay coefficients of variation (%CV) are 2.8% and 5.2% for C-reactive protein, and 3.6% and 8.6% for IL-6, respectively. Resulting scores were then log-transformed, as is typical with this measure to address skewness.
Body mass index (BMI)
BMI was assessed at ages 25, 26, and 27 and averaged across these three assessments. Height (in meters) and weight (in kilograms) were assessed with light clothing, and BMI was calculated using the standard formula BMI = weight/height2, which was then log-transformed.
Aggression defusing ability (age 13)
A modified version of the Adolescent Problem Inventory (Freedman, Rosenthal, Donahoe, Schlundt, & McFall, Reference Freedman, Rosenthal, Donahoe, Schlundt and McFall1978) was used at each of ages 13, 14, and 15 to assess adolescents' ability to defuse aggressive peer behavior. Adolescents provided their most likely responses to a series of hypothetical exposures to peer aggressive behavior. Adolescent responses were then rated by coders unfamiliar with other data from the study on a 0–10 scale in terms of competence in resolving/defusing the situation at hand and in making future similar situations of peer aggression less likely. Interrater reliability, calculated using the intraclass correlation coefficient (ICC) was in what has been labeled the “excellent” range for this statistic (ICC = 0.87; Cicchetti & Sparrow, Reference Cicchetti and Sparrow1981).
Conflict resolution with close peer (age 16)
This three-item scale from the Friendship Quality Questionnaire (Parker & Asher, Reference Parker and Asher1993) utilizes a close friend's report about the participant's ability to get over being mad, to resolve arguments quickly, and to make up easily after a fight. Internal consistency was good (Cronbach α = 0.75).
Hostile maternal behavior (ages 13 and 18)
Adolescents and their mothers participated in a revealed differences task in which they discussed an issue in their relationship that they had separately identified as an area of disagreement. The discussion began with the adolescent playing a recording they had made separately with an interviewer describing the problem and their perspective on it. These interactions lasted 8 min and were videotaped, transcribed, and coded with the Autonomy and Relatedness Coding System (Allen et al., Reference Allen, Hauser, Bell, McElhaney, Tate, Insabella and Schlatter2000). Hostile maternal conflict behavior was assessed in terms of mothers’ statements undermining the adolescent's autonomy and sense of relatedness, with a focus on behaviors such as expressing hostility directly toward the adolescent, rudely interrupting or ignoring them, overpersonalizing a disagreement, or pressuring the adolescent to agree. Age 18 interactions were all obtained while adolescents were still living with their family of origin. Reliability for this scale, using the intraclass correlation coefficient, was in the excellent range for this statistic at age 13 (ICC = 0.86) and at the high end of the fair range at age 18 (ICC = 0.56).
Hostile romantic partner behavior (age 21)
Target participants and their romantic partners participated in a revealed differences task at age 21 in which they discussed an issue in their relationship that they had separately identified as an area of disagreement. The discussion began with target participants playing a recording they had made separately with the interviewer describing the problem and their perspective on it. Typical topics of discussion included money, jealousy, moving, friends, and career issues. These interactions lasted 8 min and were videotaped, transcribed, and coded with the Autonomy and Relatedness Coding System for Adolescent–Romantic Partner Interactions (Allen et al., Reference Allen, Porter, McFarland, Hare, Miga and Schad2005). Adolescent exposure to hostile romantic partner conflict behavior was coded using the same constructs as described for hostile maternal behavior above. Interrater reliability was in the good range (ICC = 0.68).
Romantic relationship stress (age 26)
Participants reported their degree of stress, worry, and feeling of a need to make changes in their current romantic life on the three-item Romantic Relationship Stress Scale of the Romantic Life Satisfaction Measure developed for this study with good internal consistency (Cronbach α = 0.83).
Peer-rated participant aggressive behavior (age 27–28)
Participants’ close friends were contacted at both participant age 27 and age 28 and reported on target participant's level of overall aggressive behavior using the 16-item aggressive behavior scale from the Adult Behavior Checklist (Rescorla & Achenbach, Reference Rescorla and Achenbach2004). Scores from this internally consistent scale were aggregated across the 2 years to assess overall aggressive behavior on the part of the participant (Cronbach α = 0.93).
Covariates
Adult anxiety and depressive symptoms were assessed repeatedly at ages 25, 26, and 27, with results averaged across ages to yield an overall measure. The 20-item trait anxiety scale from the State-Trait Anxiety Inventory (Spielberger, Sydeman, Owen, & Marsh, Reference Spielberger, Sydeman, Owen, Marsh and Maruish1999) was used to measure stable individual differences in anxiety proneness. Responses used a 4-point Likert scale to which participants indicated their agreement to statements such as, “I worry too much over something that doesn't really matter.” The overall trait anxiety scale has demonstrated strong psychometric properties and external validity (Spielberger et al., Reference Spielberger, Sydeman, Owen, Marsh and Maruish1999), and internal consistency for the scale in this study was high (Cronbach α = 0.93).
Participants also completed the Beck Depression Inventory, a 21-item measure designed to assess the degree of depressive symptoms in late adolescents and adults (Beck & Steer, Reference Beck and Steer1987). Items were rated on a Likert scale and summed to yield a total depressive symptoms score. Internal consistency for this measure in this study was high (Cronbach α = 0.91). Given that measures of anxious and depressive symptoms were highly correlated (r = .78, p < .001), an overall measure of adult internalizing symptoms was constructed by standardizing and then summing these two measures together.
History of cigarette smoking (ages 16–18)
Cigarette smoking over the past 30 days was assessed on a 4-point scale via self-report annually from ages 16 to 18, with results averaged across years.
Big Five personality traits (ages 24)
At age 24, the Big Five personality traits were assessed with the 50-item International Personality Item Pool (Goldberg et al., Reference Goldberg, Johnson, Eber, Hogan, Ashton, Cloninger and Gough2006), using a 5-point Likert scale, summing across 10 items each assessing constructs of extraversion, agreeableness, conscientiousness, emotional stability, and imagination. This measure has previously demonstrated strong internal consistency, retest reliability, convergence with longer Big Five personality measures, and self-peer agreement (Goldberg et al., Reference Goldberg, Johnson, Eber, Hogan, Ashton, Cloninger and Gough2006). For this sample, internal consistency for the scales ranged from Cronbach α = 0.74 to 0.89.
Trait hostility (age 27)
Trait hostility was assessed via participant reports on the 48-item version of the Buss–Durkee Hostility Inventory (Buss & Durkee, Reference Buss and Durkee1957). The inventory contains six 7-item scales capturing hostile attitudes and verbal aggression, both toward friends and toward strangers, and two 10-item scales capturing physically aggressive behavior toward friends and toward strangers, each with good internal consistency (Cronbach αs = 0.75–0.89).
Results
Preliminary analyses
Means and standard deviations for all substantive variables examined are presented in Table 1. Intercorrelations among the primary variables considered in the study are presented in Table 2. Given initial findings suggesting relations of gender and baseline family income to other variables in the study, gender and baseline family income were included as covariates in all analyses. We also examined possible moderating effects of these factors on each of the relationships described in the primary analyses below. History of cigarette smoking was not related to IL-6 levels and was not considered further.
Table 1. Means and standard deviations of all variables examined

aObserved, other report, or objectively rated.
Table 2. Intercorrelations among primary study variables

*p < .05. **p ≤ .01. ***p < .001.
Moderating effects were assessed by creating interaction terms based on the product of the centered main effect variables. No moderating effects of gender or income were found for any of the analyses reported below.
Primary analyses
Hypothesis 1: Adult IL-6 levels will be predicted by poor early/mid-adolescent conflict resolution ability and skill at defusing peer aggression, and by exposure to hostile maternal conflict behavior.
For all primary analyses, SAS PROC CALIS (version 9.4, SAS Institute, Cary, NC) was employed using full information maximum likelihood handling of missing data for assessment of key relations in hierarchical regression models. Analyses first examined adolescent ability to defuse situations of peer aggression, peer report of target adolescent conflict resolution ability, and exposure to hostile maternal conflict behavior as predictors of future adult IL-6 levels at age 28. Models also accounted for adolescent gender, baseline income in adolescents’ family of origin, and current adult BMI. Results, presented in Table 3, indicate that both poor ability to defuse peer aggression and peer reports of poor adolescent conflict resolution ability contributed to prediction of higher adult IL-6 levels. Hostile maternal conflict behavior was unrelated to future IL-6 levels. Together, the adolescent-era predictors accounted for 8.7% of the observed variation in adult IL-6 levels after accounting for baseline demographic covariates and BMI.
Hypothesis 2: Adult IL-6 levels will be predicted by late adolescent exposure to hostile maternal and romantic partner conflict behavior.
Table 3. Predicting interleukin-6 level from adolescent peer relationship qualities
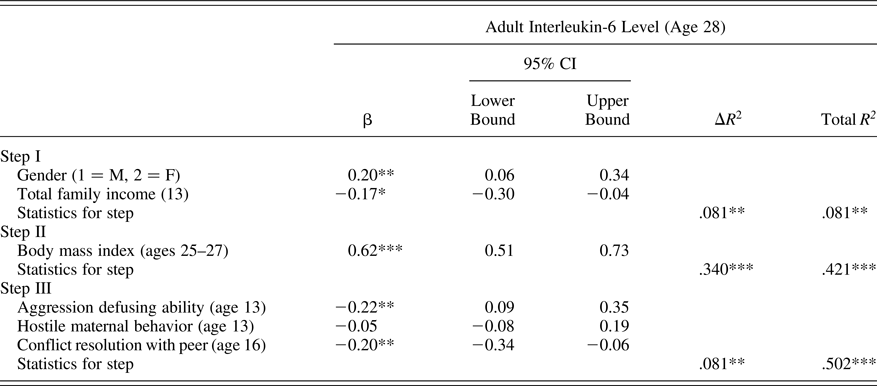
Note: The β values are from the final model.
*p < .05. **p ≤ .01. ***p < .001.
Using the same analytic approach described above, results presented in Table 4 indicate that exposure to hostile maternal and romantic partner conflict behavior in late adolescence each contributed to explaining higher adult IL-6 levels. Together, these two factors accounted for 8.7% of the observed variation in IL-6 levels after accounting for baseline covariates and BMI.
Hypothesis 3: Adult IL-6 levels will be predicted by early adult romantic relationship stress and aggressive behavior.
Table 4. Predicting interleukin-6 level from late-adolescent conflictual relationship patterns
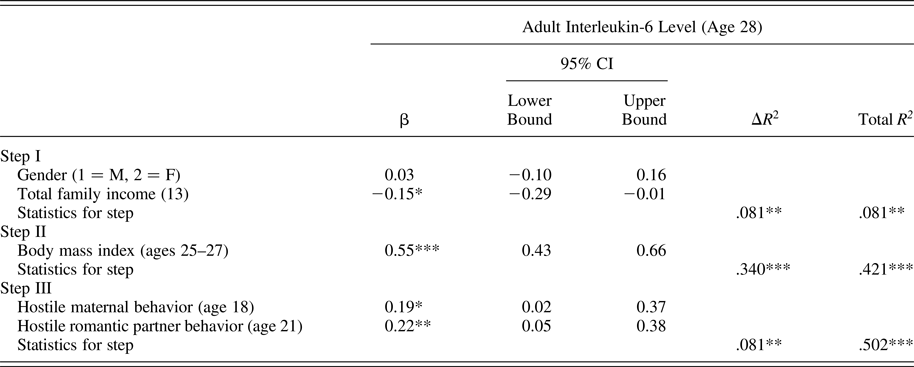
Note: The β values are from the final model.
*p < .05. **p < .01. ***p < .001.
Using the same analytic approach described above, results presented in Table 5 indicate that peer reports of target participant aggressive behavior at ages 27–28 were predictive of higher levels of IL-6 at age 28. Self-reported romantic relationship stress was not a significant predictor of IL-6 levels.
Hypothesis 4: Effects of adolescent relationship experiences will potentially be mediated via adult self-reported personality and behavioral attributes.
Table 5. Predicting interleukin-6 level from early adult conflictual relationship patterns
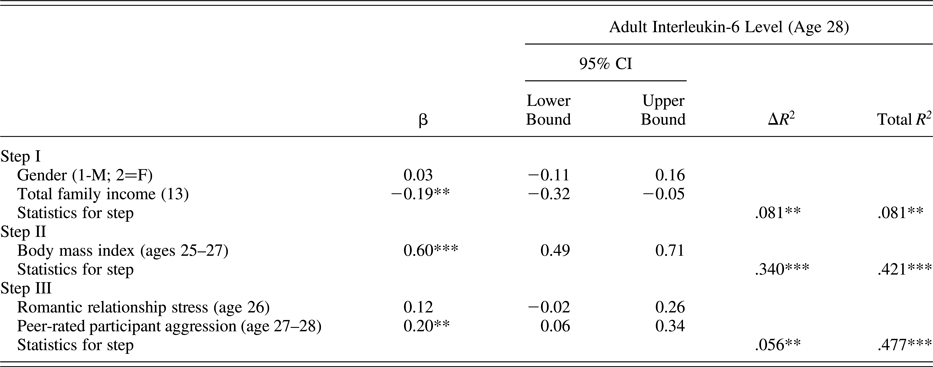
Note: The β values are from the final model.
*p < .05. **p < .01. ***p < .001.
We first examined the Big Five personality measures, self-report markers of adult levels of hostility, concurrent anxiety, and depressive symptoms for their role as potential mediators of relations described above. Initial analyses revealed that neither the Big Five measures (considered either individually or as a block), nor measures of self-reported hostility and aggression, nor concurrent anxiety and depressive symptoms were linked to later IL-6 levels (all ps > .10). Hence, these factors were not considered further.
We next considered the extent to which our indicators of exposure to hostility, conflict, and social stress in adulthood mediated the effects of similar exposure earlier in adolescence. This also allowed us to assess the extent to which predictors from different developmental stages were unique versus redundant in their prediction of future IL-6 levels. Figure 1 presents the significant pathways from a path model that assesses these relations. All temporally sensible paths were considered and the final model fit the data well, goodness of fit index = 0.99, adjusted goodness of fit index = 0.97, root mean square error of approximation = 0.0, χ2 (1) = 0.40, p = .52, and accounted for 58% of the variance in levels of IL-6. In this model, early adolescent ability to defuse situations of peer aggression and conflict resolution ability, and late adolescent exposure to romantic partner hostility each uniquely contributed to the prediction of IL-6 levels at age 28. Although some continuity of early adolescent conflict resolution skill with adult relationship qualities was observed, adult relationship qualities did not appear to mediate effects of adolescent-era qualities, and were not significantly related to adult IL-6 levels when adolescent relationship qualities were included in the model. Taken together, the direct effects from this final model accounted for 57.4% of the variance in adult IL-6 levels, an increment of 15.3% (p < .001) over and above baseline demographics and concurrent BMI.
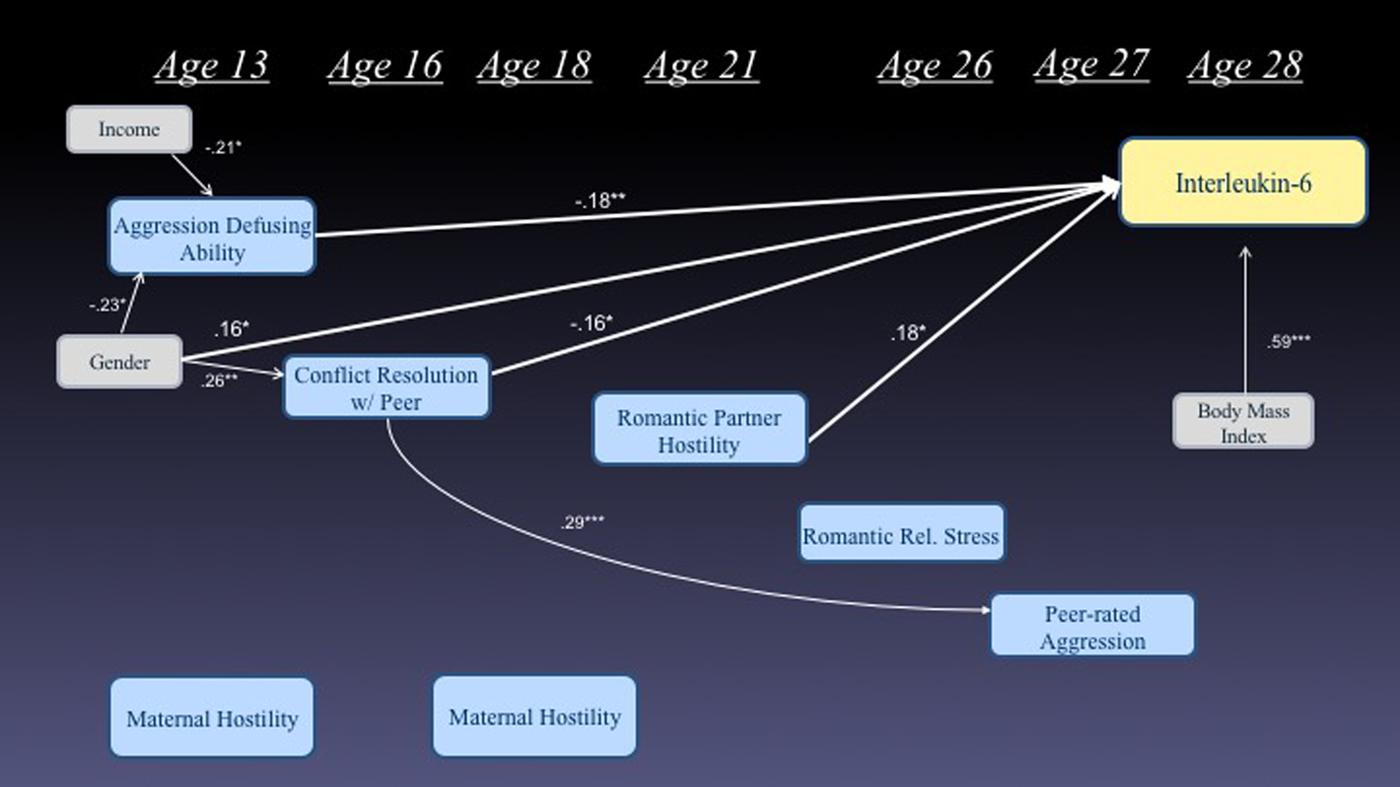
Figure 1. (Color online) Simultaneous model predicting interleukin-6 levels at age 28 from prior markers of struggles with conflict and hostility (only significant paths are depicted).
Discussion
These findings suggest that what happens socially in adolescence, at least in terms of struggles managing conflict and hostility in relationships, may have significant physical health implications extending well into adulthood, across periods spanning as long as 15 years. Within early/mid-adolescence, independent ratings of participants’ inability to defuse instances of aggressive peer behavior and peer ratings of participants’ poor conflict resolution skills each uniquely contributed to predicting higher adult IL-6 levels at age 28. In late adolescence, observations of exposure to maternal and romantic partner hostility also predicted higher adult IL-6 levels. Within adulthood, peer-rated participant aggression predicted subsequent IL-6 levels. When measures at different stages of development were considered simultaneously, two adolescent-era predictors (skill at defusing peer aggression at age 13 and conflict resolution skills at 16) and one late adolescent predictor (exposure to romantic partner hostility at 21) each uniquely contributed to understanding adult IL-6 levels. These findings have significant implications for understanding life course physical health outcomes, as high levels of circulating IL-6 have been linked to a wide array of negative long-term health outcomes up to and including premature aging (Hunter & Jones, Reference Hunter and Jones2015).
Identified predictors of future IL-6 levels all reflected elements of participant struggles with social conflict. These struggles were directly observed in the case of maternal or romantic partner hostility. Earlier in adolescence, predictions were also obtained from analogue assessments and peer-report measures assessing ability to minimize exposure to hostile conflict and peer aggression. Studies of marital discord that find strong concurrent immune reactions to such discord (Kiecolt-Glaser et al., Reference Kiecolt-Glaser, Loving, Stowell, Malarkey, Lemeshow, Dickinson and Glaser2005) suggest that relational conflict may be one of the more potent triggers of stress-related immune system responses. It has even been posited that cytokines such as IL-6 developed in evolutionary times in part because they had the potential to promote withdrawal responses, thus reducing the danger in managing (potentially lethal) conflict situations (Dickerson, Gruenewald, & Kemeny, Reference Dickerson, Gruenewald and Kemeny2004; Simmons & Broderick, Reference Simmons and Broderick2005). The results of this study dramatically expand our understanding of the potential long-term duration of the effects of social conflict by linking IL-6 levels to exposure to conflict at far earlier points in the life span and over a far longer span of time than has been previously examined.
The long duration between observations of exposure to hostile conflict and IL-6 assessments is perhaps the most intriguing and disturbing aspect of the current findings. These long-term linkages were not mediated via more recent conflict exposure nor via adult personality traits, even though both of these potential mediators were assessed more proximately to the IL-6 assessment. These findings are consistent with findings from early childhood research suggesting that immune-related effects of exposure to stress can exist even when such stress does not continue into later life (Pollitt et al., Reference Pollitt, Kaufman, Rose, Diez-Roux, Zeng and Heiss2007). One explanation for these findings is that developmentally, adolescence may be a uniquely sensitive period for exposure to hostile conflict. Adultlike relationships are first being formed and may take on significant prognostic implications for the young adolescent. Emotion regulation and perspective-taking skills are also still developing, leaving adolescents less equipped than adults to manage the dysregulating effects of conflict exposure (Steinberg, Reference Steinberg2005). Exposure to hostile conflict may thus create uniquely intense and enduring stress for adolescents.
In addition, these findings are consistent with both animal and human research suggesting that adolescence may be a period during which stress reaction systems are particularly malleable and thus particularly vulnerable to aversive social experience (Quevedo, Johnson, Loman, Lafavor, & Gunnar, Reference Quevedo, Johnson, Loman, Lafavor and Gunnar2012; Romeo, Reference Romeo2010). Stressors in adolescence have the potential to alter not only metabolic systems and anatomic structures (Ben-Shlomo & Kuh, Reference Ben-Shlomo and Kuh2002) but also more basic patterns of stress responsivity (Goff et al., Reference Goff, Gee, Telzer, Humphreys, Gabard-Durnam, Flannery and Tottenham2013; McEwen, Reference McEwen2007). Whether and how any of these processes are involved in mediating the effects observed is a question future research now needs to address.
It also remains possible, of course, that predictions from adolescent-era conflict struggles may have been stronger for methodological reasons: conflict struggles may be harder to observe in adulthood as adults become more adept at managing external impressions and confining conflicts to venues outside the view of the researcher. In either case, these findings suggest significant, long-term, physical health implications from difficulties managing conflict and hostility in key social relationships in adolescence. At a minimum, these findings suggest a need to deepen our understanding of the role of adolescent stressors in establishing patterns of immune system regulation and dysregulation. Although this study did not have data from preadolescent periods, these findings also raise, but cannot answer, questions as to whether adolescent relational stressors might mediate some of the effects of earlier stressors such as harsh parenting that are known to be linked to later relational difficulties.
Of note, predictions were all obtained from measures that did not depend on participant self-assessment and that did not simply reflect participants’ internal states. In contrast, well-validated self-report, intrapsychic, and personality measures (e.g., hostility, neuroticism, anxiety, and depression) were not predictive of future IL-6 levels. This is consistent with results from the one other long-term study predicting IL-6 from adolescent social relationships, the ADD Health Study, which relied heavily upon self-reports and non-conflict centered measures and did not find significant predictions (Yang et al., Reference Yang, Boen, Gerken, Li, Schorpp and Harris2016). Further, the low correlations among identified predictors makes it more likely that these predictions reflect distinct environmental exposures to conflict struggles, as opposed to manifestations of an internal trait. This raises the possibility that the immune system may be particularly sensitive to overt, experienced hostile conflict, and that individuals may not be skilled at accurately self-reporting their exposure to such conflict (Nisbett & Wilson, Reference Nisbett and Wilson1977; Slavich & Irwin, Reference Slavich and Irwin2014). The importance of moving beyond self-reports is further emphasized by findings that cognitive avoidance in response to a stressor, which would tend to impair self-report accuracy, has been linked to higher IL-6 levels (Lutgendorf et al., Reference Lutgendorf, Garand, Buckwalter, Reimer, Hong and Lubaroff1999).
Several limitations of this study also warrant consideration. Although results are consistent with findings from research in adulthood and extend these to a far earlier stage of the life span, direct causal inferences cannot be supported by these data. Circulating levels of cytokines such as IL-6 have been previously found to influence mood and behavior (Dickerson et al., Reference Dickerson, Gruenewald and Kemeny2004; Simmons & Broderick, Reference Simmons and Broderick2005). The strongest evidence of influence, however, suggests that levels of IL-6 increase the likelihood of social withdrawal and disconnection (Eisenberger, Inagaki, Mashal, & Irwin, Reference Eisenberger, Inagaki, Mashal and Irwin2010). Such social disconnection might be a plausible response to conflictual relationship experiences, though it seems far less likely to be the cause of such experiences. An interpretation of the present findings as reflecting the effects of IL-6 on behavior is also made less likely by the lack of robust relations of IL-6 levels to conflict exposure in more proximal periods of adulthood, and by the fact that several predictions were obtained from adolescents’ partners’ behavior. It is also of course possible that levels of IL-6 were heightened already in adolescence and that what the body is remembering in this study may be linked to prior experiences of stress or other factors leading to long-term IL-6 elevations. Even if this were the case, however, existing research and theory suggests a strong likelihood of a social stress linkage (Miller & Chen, Reference Miller and Chen2010).
An additional limitation is that the observational and other-report assessment procedures used permitted only brief snapshots of struggles with conflict and hostility at various points in adolescence and early adulthood, whereas an enduring pattern of exposure to these struggles is the underlying construct of interest. Similarly, some measures had less than optimal psychometric characteristics (e.g., the measure of maternal hostile conflict had only fair reliability, and the measure of romantic relationship stress was based on only three items). Thus, the present approach would tend to lead to an underestimate of the true effect of such conflict exposure. Relatedly, this study focused primarily on conflict-related stressors, but the theory supporting this work would also suggest value in considering other types of relational stressors such as social isolation or rejection, which were not considered here.
Given these limitations, these data are nonetheless the first to link adolescent struggles with conflict and hostility to physiological markers of immune functioning in adulthood, and they raise new questions regarding the long shadows that problematic adolescent relationship experiences might cast on life span health outcomes. These findings mirror prior research that has established links of adolescent peer relationship qualities to self-reported health in early adulthood (Allen et al., Reference Allen, Uchino and Hafen2015). From a risk and prevention perspective, exposure to hostile conflict in adolescent relationships may now be considered as a potential marker of risk for long-term health difficulties. National pediatric recommendations for the prevention of future health risks in adolescence currently do not address any relational factors (Centers for Disease Control and Prevention, 2017; Institute of Medicine, 2012). Yet, relational factors linked to immune functioning may be at least as modifiable as other identified risks in adolescence (e.g., smoking and obesity; Schreier, Schonert-Reichl, & Chen, Reference Schreier, Schonert-Reichl and Chen2013), and would thus appear to warrant significant attention in efforts to improve life span health outcomes.








