Introduction
Sex differences have been reported across all aspects of the acute ischemic stroke (AIS) care continuum, from initial diagnosis and treatment delivery, to stroke outcomes. Reference Bushnell, Howard and Lisabeth1 While studies examining access to expedient emergency care for AIS have yielded conflicting results regarding sex differences, Reference Park, Shin, Ro, Song and Oh2–Reference Boehme, Carr and Kasner9 the finding that females have worse overall outcomes compared to males has been consistent Reference Kapral, Fang and Hill10 and has been observed regardless of whether they undergo acute treatment or not. Worse outcomes in females following AIS are felt to be at least partially accounted for by differences in their baseline demographic and clinical status at presentation, including the well-established finding that females present at more advanced age and with worse baseline function. Reference Turtzo and McCullough11
To date, most investigation into the impact of sex on treatment-related stroke outcomes has focused on IV thrombolysis, with mixed findings. Reference Gattringer, Ferrari and Knoflach12–Reference Scheitz, Abdul-Rahim and MacIsaac19 More recently, attention has turned to examining whether there are sex-specific differences in stroke outcomes following endovascular treatment (EVT), a highly effective treatment for acute large vessel occlusion (LVO) stroke with a considerably longer treatment window than intravascular thrombolysis. Reference Goyal20–Reference Shekhar22 Despite the emergence of EVT as the standard of care for eligible LVO stroke, there have been few studies to date examining sex differences in the delivery and outcomes of EVT, with conflicting results Reference de Ridder, Fransen and Beumer23–Reference Madsen, DeCroce-Movson and Hemendinger29 with earlier studies examining sex differences in post hoc analyses of large multicentre trial studies designed to investigate the efficacy of EVT as a viable treatment for LVO stroke. Among studies designed specifically to look at the impact of sex on functional outcomes following EVT, there have been contradictory findings, with either no differences observed between sexes, Reference Carvalho, Cunha and Gregório25–Reference Deb-Chatterji, Schlemm and Flottmann27 or with women exhibiting lower likelihood of independence at 90-day post-EVT. Reference Uchida, Yoshimura, Sakai, Yamagami and Morimoto28,Reference Madsen, DeCroce-Movson and Hemendinger29 More recently, a meta-analysis of 33 endovascular therapy studies pooled the data for 7335 patients and when comparing functional outcomes for men and women, it found that women had inferior functional outcomes at 90 days; however, this analysis did not test for interaction between baseline premorbid status and functional outcomes. Reference Dmytriw, Ku and Yang30
While some of the studies designed to examine sex differences in EVT outcomes have accounted for age, premorbid function, and a handful of vascular comorbidities, to our knowledge there have been no studies to date that also account for a comprehensive list of comorbid diseases, including nonvascular conditions such as dementia and malignancies, which are more prevalent in older populations and correlate with baseline function and mortality risk. This relevant gap in the literature served as motivation for this study which aimed to assess for sex differences in functional outcomes following EVT by comparing 90-day modified Rankin Scale (mRS) of males and females while controlling for age, baseline function, and a comprehensive measure of comorbidity burden. We hypothesized that when accounting for these factors, previously observed differences in functional outcome following mechanical thrombectomy would be eliminated.
Methods
Study Design and Participants
We performed a retrospective cohort study reviewing medical records of consecutive patients aged 18 years and older undergoing EVT for LVO stroke at the University Health Network (UHN), a tertiary stroke centre in Toronto, Canada, from October 2014 to July 2019. This included all patients presenting with AIS due to proximal LVO to our centre and excluding inpatient strokes, repeat EVT for strokes within the same admission, and those missing 90-day mRS data. Criteria for EVT included AIS due to LVO in proximal vessels, that is, internal carotid artery, middle cerebral artery (M1 or M2), basilar artery, or vertebral artery as observed on CT angiogram with symptom onset/last seen normal (LSN) time within 24 hours of arrival. The study was reviewed and approved by the UHN research ethics board.
Data Collection and Outcome Measures
Baseline demographic and clinical measures, acute imaging results, and treatment decisions were all retrospectively collected from standardized data entry forms and electronic medical records by two data abstractors, including a stroke specialist and a resident physician proficient, both with proficiency in identifying and classifying key variables and measures collected. A data dictionary with abstraction instructions was utilized to ensure consistency. This data included sex, age, baseline mRS, symptom onset/LSN time, National Institute of Health Stroke Scale (NIHSS), Alberta Stroke Program Early CT score (ASPECTS), vessel occlusion site, intravenous tissue plasminogen activator (tPA) administration data and care delivery data for LSN-to-emergency department (ED) arrival, LSN-to-tPA bolus administration, LSN-to-groin puncture, and arrival-to-groin puncture time delays measured in minutes. Note that the measure of LSN-to-ED arrival was captured for arrival at our tertiary stroke centre and included walk-in patients and patients arriving by EMS from both our local catchment area and patients arriving after bypassing or transferring from peripheral centers.
The primary outcome measure examined in this study, 90-day mRS score, was collected by trained stroke clinic staff at each participant’s in-person 3-month follow-up appointment or, less commonly, over the phone using up-to-date clinical and functional history to guide scoring. A 90-day mRS < 3 was defined as a favorable outcome.
Other demographic and clinical variables, including vessel recanalization following thrombectomy (TICI) score, presumed stroke etiology, discharge mRS, discharge destination (home, rehab, repatriation to home hospital, and long-term care), and Charlson Comorbidity Index (CCI) were collected by trained stroke unit staff or research personal through chart review, including review of scanned paper charts and electronic medical records.
Comorbidity burden was calculated using the CCI, a validated tool for predicting 10-year survival in individuals with multiple comorbidities Reference Charlson, Pompei, Ales and MacKenzie31 which includes a composite score based on age and a comprehensive list of medical comorbidities, including history of myocardial infarction, congestive heart failure, peripheral vascular disease, stroke/transient ischemic attack (TIA), dementia, chronic obstructive pulmonary disease (COPD), connective tissue disease, peptic ulcer disease, liver disease, diabetes, hemiplegia, renal disease (moderate to severe), solid tumor, leukemia, lymphoma, and/or AIDS. High morbidity burden was defined as CCI > 4 which has an estimated 10-year survival of approximately 53% or less.
We explored potential sources of bias in the sample due to exclusion of subjects missing primary outcome measure data by comparing baseline demographic and clinical variables as well as delay to care measures between those with and without 90-day mRS data.
Two of the subjects included in the final study sample had recurrent LVO strokes and underwent EVT at discrete time points during the study period. Given these events were separated in time for each subject and remote from their initial presentations and treatment, they were included as discrete subjects with their history of prior stroke and associated disability accounted for by clinical and demographic measures collected for all subjects.
Statistical Analysis
Data were analyzed using R statistical software v4.0.4. 32 Descriptive statistics were used to summarize baseline demographic and clinical variables between groups. Continuous variables were described using means and standard deviations, and categorical variables were described using counts and percentages. Comparisons of categorical variables across men and women were analyzed using Pearson‘s Chi-square tests and linear model ANOVA’s for continuous variables. P-values < 0.05 were considered statistically significant for all analyses. Exploratory multivariable logistic regression analyses were performed to determine potential risk factors for functional independence at 90 days, defined as an mRS < 3. Associations were presented as odds ratios (OR) and 95% confidence intervals (CIs). The model was selected using Akaike information criterion and covariates included sex (male vs. female), age, baseline mRS > 2, CCI > 4, and NIHSS. Multicollinearity was assessed using variance inflation factor and McFadden’s Pseudo R-squared determined model fit. A univariate regression analysis was also performed to assess whether functional independence at 90 days was significantly different for males and females when the above covariates were not taken into consideration. This was done to compare and contrast results with the multivariable analysis as some of the prior studies that identified sex differences in 90-day mRS performed analyses that excluded the covariates of interest identified in this study.
We explored potential sources of bias in the sample, comparing baseline demographic variables for study participants and those excluded due to missing data for 90-day mRS using Pearson‘s Chi-square tests and linear model ANOVA’s (Supplemental Appendix Table 1). Additionally, sensitivity testing was performed to assess whether the inclusion of data for study participants missing 90-day mRS data would alter results of the multivariate analysis by including these 41 individuals in the model with the following assumptions: 1. All of the 41 individuals had a 90-day mRS < 3, 2. All of the 41 individuals had a 90-day mRS ≥ 3, and 3. An equal proportion of the 41 individuals had a 90-day mRS <3 and ≥ 3 (Supplemental Appendix Tables 2a-c).
Results
Baseline Demographic Differences
A total of 271 patients underwent EVT at our centre during the inclusion period; however, only 230 subjects had primary outcome data (90-day mRS) available. As a result, 41 subjects were excluded from the study following an analysis to assess for systematic differences in demographic data between patients with 90-day mRS data and without. A consort diagram of subjects included in the final analysis can be seen in Figure 1.
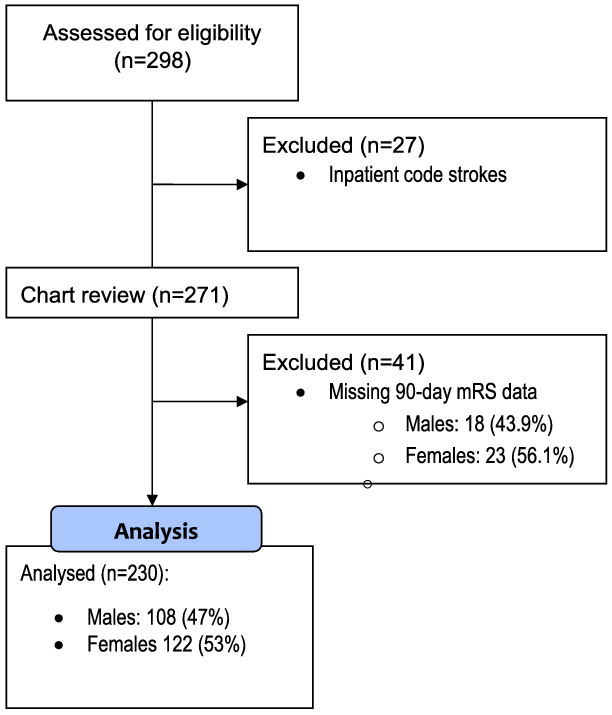
Figure 1: Consort flow diagram for retrospective cohort included in study examining sex differences in 90-day mRS following EVT. Analysis comparing all subjects undergoing chart review by those with and without 90-day mRS data seen in corresponding Supplemental Appendix Table 1. mRS-modified Rankin Scale.
Of the 230 subjects included in this study, 122 (53%) were female and 108 (47%) were male (Table 1). Females were significantly older than males, with a mean difference of 9 years in age (meanfemale = 75 ± 13 vs. meanmale = 66 ± 15). Both functional dependence and comorbidity burden were higher in females than in males prior to presentation with stroke, with 20% of females versus 8% of males presenting with baseline mRS ≥ 2 (p = 0.014) and 43% of females versus 24% males (p = 0.003) presenting with CCI measure >4.
Table 1: Demographic and clinical characteristics of stroke patients undergoing EVT according to sex
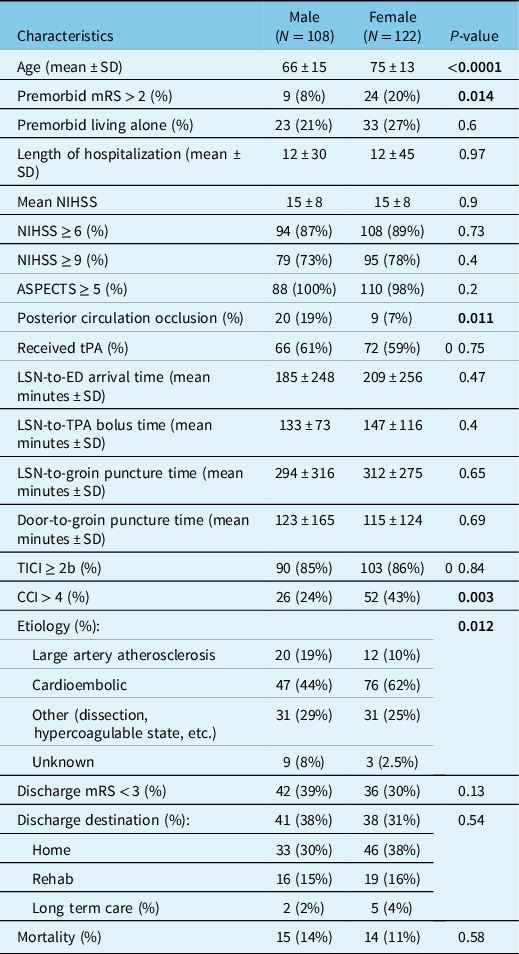
mRS = modified Rankin Scale; NIHSS = NIH Stroke Scale; ASPECTS = Alberta Stroke Program Early CT Score; tPA = tissue plasminogen activator; TICI = thrombolysis in cerebral infarction scale; CCI = modified Charlson Comorbidity Index; LSN = last seen normal; ED = Emergency Department.
Bold indicates that the significant values are <0.05.
Males were more likely than females to present with posterior circulation occlusions (p = 0.011). There was a significant difference in stroke etiology between males and females (p = 0.012), driven by a higher rate of cardioembolic source in females.
There were no significant differences in the remaining demographic and clinical data, including premorbid living arrangement, NIHSS, ASPECTS, tPA status, LSN-to- ED arrival time, LSN-to-tPA bolus time, LSN-to-groin puncture time, door-to-groin puncture time, TICI score, discharge mRS, discharge destination, or mortality.
A number of premorbid health conditions were significantly more common in females than males (Table 2) including hypertension (79% in females vs. 63% in males), atrial fibrillation (45% in females vs. 23% in males), and congestive heart failure (11% in females vs. no cases in males). Conversely, obstructive sleep apnea (11% in males vs. 1% in females), smoking (29% in males vs. 7% in females), and alcohol misuse disorders (8% in males vs. no cases in females) were all more common in males.
Table 2: Premorbid health conditions utilized for Charlson Comorbidity Index score compared by sex
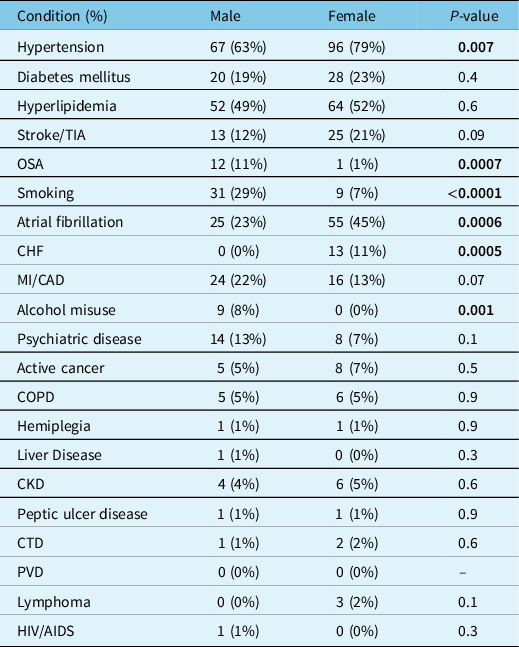
TIA = transient ischemic attack; OSA = obstructive sleep apnea; CHF = congestive heart failure; MI = myocardial infarction; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease (moderate to severe); CTD = connective tissue disease; PVD = peripheral vascular disease; HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome.
Bold indicates that the significant values are <0.05.
We found that subjects missing 90-day mRS data were significantly older (76 ± 13 years vs. 71 ± 15 years; p = 0.027) and had longer delays in LSN-to-ED arrival (350.4 ± 382.7 minutes vs. 197.5 ± 251.8 minutes; p = 0.001), and LSN-to-groin puncture (445.8 ± 456.5 minutes vs. 304.1 ± 294.0 minutes; p = 0.013) times than subjects with primary outcome data. There were no differences in LSN-to-tPA bolus or door-to-groin puncture times between subjects with and without the primary outcome data. Subjects missing 90-day mRS data were also more likely to have been repatriated after undergoing EVT than subjects with 90-day mRS data (78% vs. 15.4%; p < 0.001). There were no other differences between subjects with and without 90-day mRS data (Supplemental Appendix Table 1).
Regression Analysis
In the univariate analysis, 90-day mRS < 3 was more common in males than females [OR = 1.831, 95%CI 1.082–3.098]; however, there was no significant difference in the multivariable model that accounted for age, NIHSS, premorbid mRS, and CCI [OR 1.21, 95%CI 0.61–2.37] (Table 3). The multivariate analysis indicated that all variables other than sex contributed significantly to 90-day mRS outcomes, as older age [OR 0.97, 95%CI 0.94–0.99], NIHSS [OR 0.91, 95%CI 0.87–0.95], premorbid mRS > 2 [OR 0.11, 95%CI 0.02–0.50], and CCI > 4 [ OR 0.40, 95%CI 0.18–0.89] were each independently associated with lower odds of better functional outcome, that is, 90-day mRS < 3.
Table 3: Multivariate logistic regression: odds ratio for 90-day mRS < 3
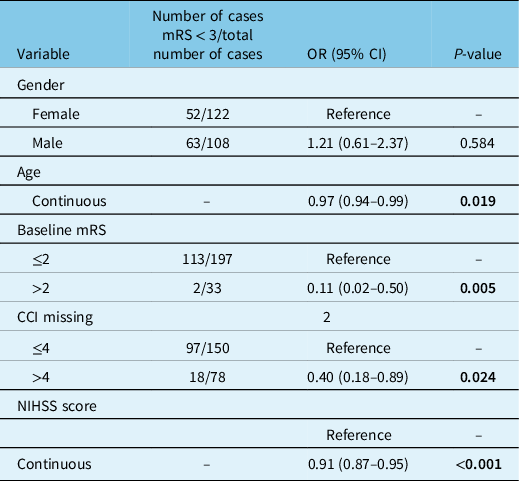
mRS = modified Rankin Scale; NIHSS = NIH Stroke Scale; CCI = modified Charlson Comorbidity Index.
McFadden Pseudo R2 = 0.290359; variance inflation factor: mCCI = 1.298516; premorbid status = 1.026319; NIHSS = 1.021593; sex = 1.113997; age = 1.411629. NIHSS = 1.021597; sex = 1.112242; age = 1.409769.
Bold indicates that the significant values are <0.05.
In the multivariable analysis, age was treated as continuous. Further analyses were performed to investigate for an age-by-sex interaction on the primary outcome both with age dichotomized to <50 or >50 years and with age binning of <50, 50–75, and >75 years old, and in either analysis we did not observe a significant difference in 90-day mRS outcomes between males and females, nor we did identify a pivotal age at which functional outcomes are most impacted.
Sensitivity testing did not show any significant difference in odds of 90-day mRS < 3 related to sex when the multivariate model included subjects excluded from the original analysis due to missing primary outcome data (Supplemental Appendix Table 2a-c). As with the original multivariate analysis, higher NIHSS, premorbid mRS >2, and CCI > 4 were each independently associated with lower odds of better functional outcome, that is, 90-day mRS < 3 (Supplemental Appendix Table 2a-c). In the sensitivity analyses assuming either all or half of the 41 individuals lacking primary outcome data had a favorable functional outcome (90-day mRS < 3), older age was not associated with functional outcome (Supplemental Appendix Table 2a and 2c), whereas older age was predictive of lower odds of 90-day mRS < 3 in both the original analysis excluding participants without primary outcome data (Table 3) and in the sensitivity analysis assuming poor functional outcome (90-day mRS ≥ 3) for all individuals missing 90-day mRS data (Supplemental Appendix Table 2b).
Discussion
Sex differences in AIS have been identified across the continuum of care, from disparities in accurate identification of stroke between males and females to investigations and delivery of acute treatments. Reference Bushnell, Howard and Lisabeth1 Consistent findings that females have overall greater disability and mortality following AIS Reference Reeves, Bushnell and Howard33 have suggested that baseline differences in clinical and functional measures contribute to this disparity in outcomes. These baseline demographic factors include older age and worse functional baseline at stroke presentation. Reference Turtzo and McCullough11 The disparity in AIS outcomes between males and females has raised questions about whether differences in acute stroke treatment efficacy are at play, with most studies focusing on sex-specific differences in functional outcomes following intravenous thrombolysis. Reference Gattringer, Ferrari and Knoflach12–Reference Scheitz, Abdul-Rahim and MacIsaac19 More recently, attention has turned to whether sex differences in AIS care exist for patients treated with EVT. A limited number of studies have examined this question, having produced conflicting results, Reference Carvalho, Cunha and Gregório25–Reference Dmytriw, Ku and Yang30 indicating either no impact of sex on patient outcomes Reference Carvalho, Cunha and Gregório25–Reference Deb-Chatterji, Schlemm and Flottmann27 or worse functional independence in women at 90-day post-EVT Reference Uchida, Yoshimura, Sakai, Yamagami and Morimoto28–Reference Dmytriw, Ku and Yang30 despite controlling for advanced age, functional baseline, and a limited list of vascular risk factors. This study was performed to assist in clarifying these discrepant findings by studying a real-world sample of patients undergoing EVT for AIS. We hypothesized that there would be no difference in 90-day mRS following EVT between males and females when accounting for baseline differences in age, pre-stroke functional independence (baseline mRS) and a comprehensive measure of pre-stroke comorbid disease burden.
As previously demonstrated, we found that females were significantly older than men and had a lower level of functional independence. The proportion of females with a 10-year survival of approximately 53% or less as predicted by a CCI score > 4 was significantly higher than in males. In a univariate analysis comparing 90-day mRS between men and women, we found that women had worse outcomes; however, when accounting for age, baseline mRS, and CCI scores, we found that there was no difference in 90-day mRS between men and women, confirming our initial hypothesis. The use of the CCI as a validated measure of comorbidity burden in our model was, to our knowledge, novel among comparable studies examining sex differences in EVT outcomes. This study also revealed that comorbidity burden, as measured by CCI, was a risk factor for poorer functional outcomes in addition to baseline mRS and age.
There were no differences in measures of delays to care between males and females in the study population, including pre- and post-hospital delays to care, a finding that suggests that despite reports of delayed recognition of stroke symptoms in females compared to males, Reference Bushnell, Howard and Lisabeth1 access to timely management of stroke is no different between sexes undergoing EVT. This finding may be related to the typically more pronounced symptoms of LVO stroke amenable to EVT, which tends to effect a greater vascular distribution as compared to other presentations of AIS such as distal LVO stroke or lacunar stroke.
In keeping with previous studies, we found that females were more likely to have stroke due to cardioembolic etiology. Reference Appelros, Stegmayr and Terént34 This corresponded with our finding of a higher proportion of females presenting with premorbid hypertension and heart failure, both of which are risk factors for atrial fibrillation which was also more frequent in females. Males were more likely to have a history of active smoking, alcohol misuse disorder, and obstructive sleep apnea which has been previously described. Reference Martin-Schild and Samai35 Interestingly, nonvascular risk factors commonly seen with advancing age, such as malignancy and dementia, were similar between groups despite women being significantly older. There was no overall difference in the frequency of any individual nonvascular comorbid disease between sexes; however, the significantly higher proportion of females with a CCI > 4 suggests it is the difference in cumulative disease burden, as opposed to the individual comorbid conditions, that accounts for worse functional outcomes in females.
Prior studies have reported that females present with more severe stroke as indicated by higher NIHSS Reference Bushnell, Howard and Lisabeth1 ; however, mean NIHSS was no different between sexes in our study. This may be the result of selection bias, as only patients deemed eligible for EVT were included in the current study with eligibility for EVT predicated on stroke recency and severity.
A significant limitation of this study was the low sample size and loss of subjects from the original sample due to missing primary outcome data. There were no apparent systematic differences in sex, baseline functional, comorbidity burden, or stroke severity when comparing subjects with and without 90-day mRS data. Furthermore, sensitivity testing revealed that when including subjects missing 90-day mRS data in the multivariate model, results were consistent with the findings of the main analysis, that is, that sex does not significantly impact functional outcomes following EVT when accounting for age, functional baseline, NIHSS, and comorbidity burden. However, it is worth noting that subjects lacking 90-day mRS data tended to be older, had longer pre-hospital delays to care, and had worse function at discharge from our site. While this suggests the possibility of systematic bias in those subjects excluded from this study, we also observed that there was no difference in time elapsed from LSN-to-tPA bolus or time elapsed from arrival at our ED to groin puncture for EVT when comparing subjects with and without 90-day mRS data. Taken together, we suspect that the longer delay from LSN-to- ED arrival at our centre and from LSN-to-groin puncture time in excluded subjects is accounted for by proximity of these individuals to our centre at stroke onset. This is supported by the finding that individuals missing 90-day mRS data were more likely to present from more distant locations where they either bypassed or were transferred from peripheral hospitals. Accordingly, subjects missing 90-day mRS data were more likely to have been repatriated to their local hospital shortly after undergoing EVT as opposed to being discharged to long-term care, rehab, or home following a longer period of recovery at our centre. Given that patients repatriated to their local hospital are not followed up at our centre, there is a lack of 90-day mRS data for this cohort ultimately leading to their exclusion from our primary analysis.
Interestingly, when performing the sensitivity analysis with the assumptions that either all or half of subjects without 90-day mRS data had favorable outcomes, there was no significant relationship between age and odds of 90-day mRS < 3. This was contrary to both the main analysis of 230 study participants and the sensitivity analysis with conditions assuming all of subjects missing 90-day mRS data had poor outcomes, both yielding the result that older age predicted lower odds of a favorable functional outcome following EVT. Any discrepancies identified regarding the relationship between age and post-EVT functional outcomes in the current study should be considered alongside the finding that subjects excluded from the main analysis due to missing outcome data were significantly older and that there is a previously established relationship between older age and worse functional outcomes following EVT. Reference Goyal20 Importantly, despite the finding that advanced age portends worse post-EVT functional outcomes, a persistent benefit of EVT for LVO stroke across all age groups has been demonstrated to persist even when controlling for prognostic factors such as baseline function and NIHSS. Reference Finitsis36
The use of mRS as the sole functional outcome measure is an additional limitation of this study, given that mRS is a crude tool for measuring function, emphasizing mobility and failing to capture non-motor symptoms, such as cognition. Studies of sex differences in cognition at 90-day post-stroke have demonstrated greater cognitive impairment in women, a finding accounted for by age, pre-stroke motor function and cognition, marital status, and level of education. Reference Dong, Briceno, Morgenstern and Lisabeth37 Furthermore, one study has shown that women tend to report worse subjective disability, despite higher functional scores as measured by direct observation of tasks associated with activities of daily living. Greater discrepancy in subjective versus objective function was associated with depressed mood. Reference Dong, Briceno, Morgenstern and Lisabeth37 Taken together, these findings suggest that future studies investigating the impact of sex on post-EVT outcomes should evaluate cognition as well as mood and should include both subjective and objective tools for assessing each.
It is worthwhile noting that patient selection for EVT prior to the publication of landmark EVT trials in 2015 Reference Goyal20,Reference Chong, Lee, Boden-Albala, Paik and Sacco38 was heterogeneous, and that the current study included 6/230 patients who underwent EVT from October 2014 to December 2014, that is, prior to 2015 when EVT became standard of care for eligible LVO strokes. However, no meaningful comparison of patients from before and after 2015 could be made for the current study given the very small sample size. Note is made that during the study period, criteria for performance of mechanical thrombectomy evolved with the publication of landmark EVT trials and operator proficiency, including increased frequency of EVT for LVOs of the posterior circulation and longer treatment windows. Reference Lindsay21,Reference Shekhar22 It is worth noting that subjects with posterior circulation occlusions were excluded from most of the early EVT trials, and that our study found that posterior circulation occlusions were significantly more common in males, a finding that has previously been reported. Reference Hasan39 This poses the question of whether sex differences in post-EVT outcomes should be compared for studies carried out before and after completion of the landmark EVT trials, either in studies using pooled data traversing this period, such as meta-analyses.
Implications for this study include a better appreciation for the ways in which sex differences in premorbid functional clinical factors influence EVT outcomes and how the influence of these factors may have previously been erroneously attributed to sex itself. Prior studies demonstrating worse outcomes in women following EVT have proposed possible biophysiological mechanisms for measured differences in disability following mechanical thrombectomy, such as differences in vascular access or vulnerability to complications of EVT; however, we propose that premorbid differences in age, disability, and health are more relevant in predicting long-term benefits of EVT for AIS. Importantly, these findings further support the notion that sex should not be used as a predictor of the potential benefits of EVT or influence the provision of this critical treatment for eligible individuals.
Beyond the premorbid clinical functional variables explored in the current report, several studies have shown that additional sociodemographic factors intersect with sex to influence stroke risk and outcomes Reference Frid, Drake and Giese40,Reference Reshetnyak, Ntamatungiro and Pinheiro41 including ethnicity/race, gender, socioeconomic status, and primary language spoken. Future efforts to better understand how sex influences stroke outcomes following EVT should take into account the impact of social determinants of health on baseline function and health status and acute stroke care provision.
Conclusion
This retrospective study of consecutive patients undergoing EVT for LVO stroke found that there is no difference in functional independence at 90 days between males and females when accounting for age, baseline functional independence, stroke severity, and comorbidity burden. Females were older with worse baseline function and greater burden of comorbid disease, all factors that were significant predictors of poorer functional outcomes following EVT. Our use of a validated and comprehensive measure of comorbidity burden in the form of theCCI is novel among similar studies that either have not accounted for comorbidity burden or have examined a limited set of vascular risk factors when assessing for sex differences post-EVT. We propose that future studies examining sex differences in EVT outcomes utilize the CCI, or a similarly comprehensive and validated measure of comorbidity burden alongside baseline mRS and age.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2022.22
Acknowledgments
I would like to thank the journal editors for their thoughtful comments and recommendations. I would also like to thank Heather Momen, Ibrahim Momen and Bader Elkhatib for their invaluable support during the writing of this manuscript.
Disclosures
The authors declare that there are no conflicts of interest.
Statement of Authorship
AIM: study conception and design, data retrieval, analysis, and manuscript preparation. TF: study design, data analysis, and manuscript preparation. JDS: study conception and design, data acquisition, and manuscript preparation. VR: study design, data analysis, and manuscript preparation. AB: study conception and design and data acquisition. VMP: study conception and design, data acquisition, and manuscript approval. AP: study conception and design, manuscript preparation, acquisition of data, and final approval of manuscript.






