INTRODUCTION
Cryptosporidiosis is a gastrointestinal illness caused by an intracellular but extracytoplasmic protozoan parasite. The most common clinical presentation consists of watery diarrhoea. Other symptoms include abdominal cramping, low-grade fevers, loss of appetite, dehydration, and severe and long-lasting weight loss [Reference Chappell1–Reference Clark5]. Symptoms are usually self-limiting, lasting for 10–14 days, although symptoms can last longer. In immunocompromised hosts, symptoms can be more severe and can last much longer [Reference Hunter and Nichols6]. Cryptosporidium is an important cause of diarrhoea around the world in both children and adults. Transmission occurs by the oral–fecal route [Reference Chen4, Reference Clark5] via contaminated drinking water or food and also by contact with infected humans and animals. Historically, outbreaks were predominantly linked to drinking water in the USA, but more recently outbreaks in the USA are increasingly being linked to treated recreational water exposures [Reference Yoder and Beach7].
In developed countries, outbreaks associated with swimming pools are common [8–Reference McAnulty, Fleming and Gonzalez16]. Swimming pools efficiently transmit infections because the oocysts excreted by infectious individuals are relatively resistant to chlorine and because ingestion of a relatively small amount of oocysts can lead to infection, with the median infectious dose estimated to range from between 12 and 2066 oocysts [Reference Messner, Chappell and Okhuysen17]. In addition, an infected individual can excrete up to one billion oocysts during an infectious period and a single bowel movement from an infected person can contain 108–109 oocysts, and shedding can continue after the diarrhoea is resolved [Reference Yoder and Beach7, Reference Jokipii and Jokipii18].
In addition to reports of outbreaks associated with swimming pools, population-based surveys have reported risk factors related to endemic cases [Reference Valderrama19, Reference Roy20]. However, few studies have investigated the association of swimming pools with the geographical distribution of Cryptosporidium. All swimming pools contaminated with Cryptosporidium are potential sources of infection; even swimming pools utilizing more advanced purification systems have been associated with the spread of the disease [Reference Boehmer11]. Thus, it is the swimming patterns of where, when, and how often people swim, both infected and susceptible, that helps drive these outbreaks. The purpose of this study was to investigate the county-level association of the distribution of swimming pools with the actual distribution of cases of Cryptosporidium across Iowa during an outbreak.
DATA AND METHODS
We obtained de-identified Cryptosporidium data from the Iowa Department of Public Health and the University Hygienic Laboratory (Iowa's State Public Health Laboratory). The University of Iowa's Institutional Review Board classified this as non-human subjects research. In the summer and autumn of 2007, Iowa experienced a statewide outbreak of cryptosporidiosis impacting 67 of 99 counties. Laboratory-confirmed cases of cryptosporidiosis reported in 2007 represented a 315% increase over the previous 3 years. Cryptosporidiosis is a required reportable condition to the Iowa Department of Public Health (IDPH). Throughout the outbreak, IDPH attempted to assess potentially common sources of infection. Cases increased incrementally, starting in July and peaking in August and September. Numerous sources were considered including drinking water, recreational water, child-care exposure, farm and zoo animal, and person-to-person spread. Of all cases investigated, 61% (257/610) reported recreational water exposure, most commonly swimming pools. Splash parks have been implicated in other outbreaks [8] but this was not identified during the Iowa investigation as a source. The median age of cases was 11 years; however, cases ranged from 0 to 88 years, with 32% aged <5 years and an additional 15% aged between 5 and 9 years. Twenty-six percent of cases were aged between 20 and 39 years. However, most of these cases were females (72%). Note this is in marked contrast to the gender distribution in children, where males and females are evenly represented. The age distribution and the seasonal pattern of this Iowa outbreak are similar to features described in other outbreaks recently reviewed in the literature [Reference Yoder and Beach7].
Diagnosis date and the county of diagnosis for 610 cases of cryptosporidiosis during 2007 were obtained. We restricted analysis to only the 550 cases that were diagnosed between 1 June and 31 October in order to include the cases that could have been infected during the summer swimming season from Memorial Day (28 May) to Labor Day (3 September) with extra time included for incubation. Cases that occurred after or before this period were excluded from our analysis.
A pool registry was provided by the Iowa Department of Public Health that contained owner type, pool category, and location of 2231 pools in Iowa. The county population by 5-year age increments and the county centroids (latitude and longitude) were obtained from the U.S. Census Bureau for all 99 counties in Iowa.
To determine the county-level factors related to the spread of cryptosporidiosis across Iowa, we used generalized linear mixed models (GLMMs) [Reference McCullagh and Nelder21]. Iowa is divided into 99 counties, which are of similar size and shape. The number of Cryptosporidium cases in each county served as the random component (dependent variable), which was assumed to follow the negative binomial distribution. We used the negative binomial distribution rather than the Poisson distribution to allow for overdispersion in the counts of Cryptosporidium cases for each county. A log link was used to relate the mean of the random component to the systematic component, i.e. the linear combination of independent variables. An offset variable, defined as the log of the county population size, was incorporated in the systematic component to adjust for county population differences. (Offset variables are independent variables that have regression coefficients constrained to be 1.) The GLMM allows us to characterize the association between the mean number of Cryptosporidium cases in a county and the county-level independent variables of interest.
Independent variables considered in our investigation included the number of pools in certain pool categories (large, small, spa, wading, waterslide) and pool-owner classes (apartment, camp, country club or health club, hotel, municipal, school, other) as well as the proportion of county residents aged <5 years. (Most cases of Cryptosporidium in the 2007 Iowa outbreak occurred in preschoolers.) Specifically, each model contained a pool category count, a pool-owner type count, or a count for a pool category and pool-owner type combination (e.g. large camp pools). Each model also included the proportion of county residents aged <5 years and the offset variable, which is the log of the county population.
The numbers of cases in nearby counties are presumably correlated, and the strength of this correlation is most likely related to the distance between the two counties. Thus, to account for the correlation between counties, we used a spatial power covariance structure. The Euclidean distance between the centroids of each possible pair of counties was computed. With the imposed covariance structure, the value of the correlation between the number of cases in any two counties decreases as the distance between them increases.
RESULTS
Figure 1 represents the total number of cases in each county and the rate of Cryptosporidium cases for each county per 1000 population, and Figure 2 shows the timeline of cases during the outbreak period. Table 1 provides a summary of pools in the state of Iowa by owner/management type, and for each type, shows the results from the associated GLMM. Table 2 provides the same for pools of different sizes and features. Table 3 provides the same for some of the combined pool attributes described in Tables 1 and 2. GLMM results given in Tables 1–3 are marginal effects: the multiplicative change in the mean number of Cryptosporidium cases corresponding to a 1 unit change in the number of pools of a specific class. We found that in the owner/management pool types examined to predict the number of Cryptosporidium cases, only camp and municipal pools had a marginal effect that differed significantly from 1 with 95% confidence (Table 1). In pool size and features, only wading pools had a marginal effect that differed from 1 with 95% confidence (Table 2). When considering combinations of both owner/management pool type and pool category, big municipal pools, municipal wading pools, and small camp pools all had marginal effects that differed from 1 with 95% confidence (Table 3). Finally, for all pool variables considered, the point estimates for the marginal effects were at least 1. Thus, for any specific pool category or owner type, an increase in the number of pools in a county, adjusted for the proportion of county residents aged <56 years and population size, is generally associated with an increase in the number of cases.
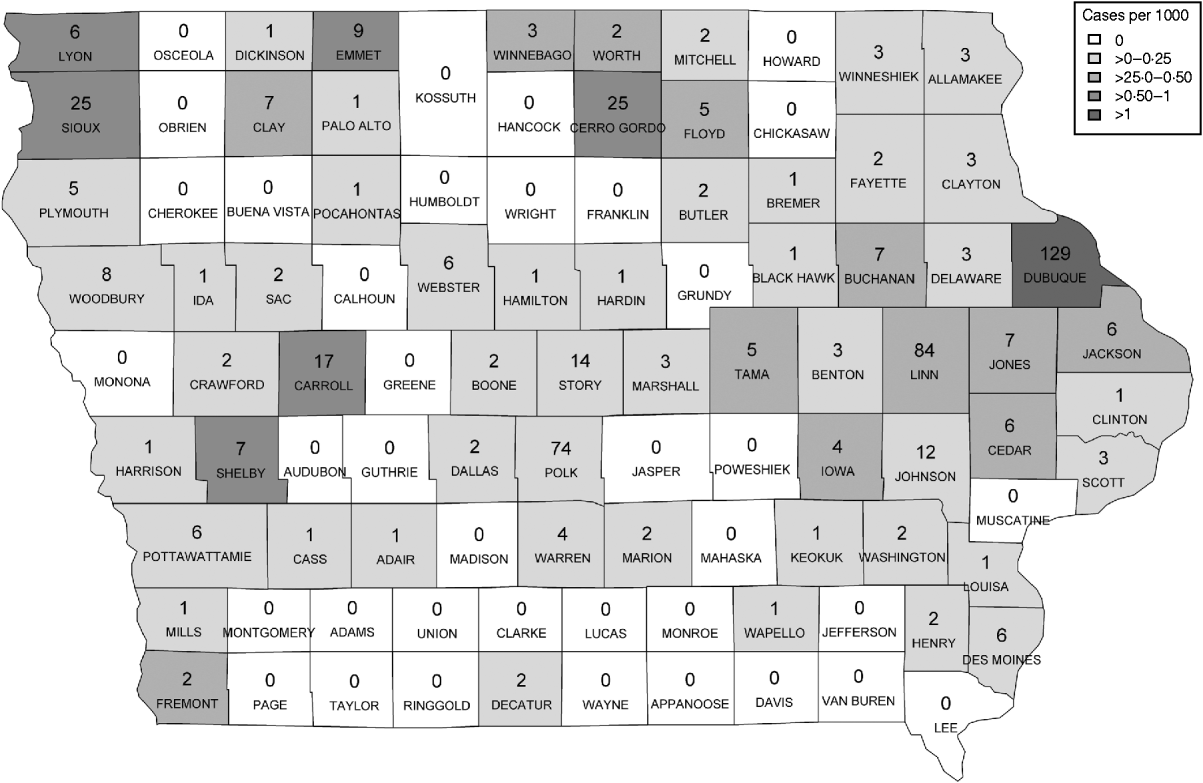
Fig. 1. Cases by county. The number of cases per county appears in each county on the map of Iowa. The rate of cases per 1000 population is demonstrated by the shading of each county according to the legend.
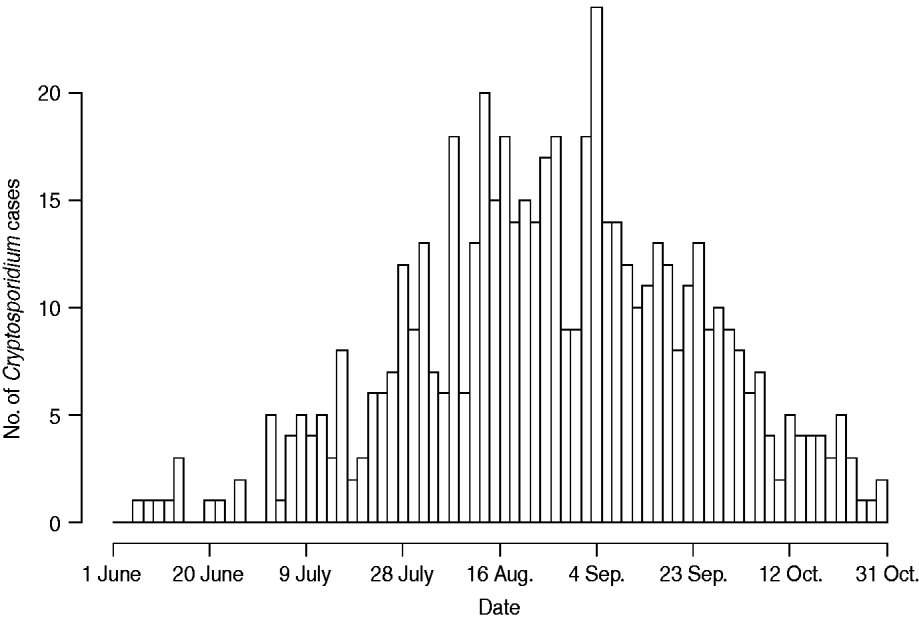
Fig. 2. Cases by time. Number of cases in Iowa from 1 June to 31 October 2007.
Table 1. GLMM results and summary statistics for pools by owner type
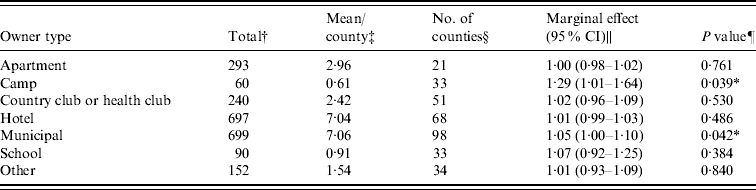
† The total number of each type of pool in Iowa.
‡ The mean number of each type of pool per county.
§ The number of Iowa counties with at least one of the specified pool type.
|| Results from the GLMM: the marginal effect is the multiplicative change in the mean number of Cryptosporidium cases corresponding to a 1 unit change in the number of pools of a specific class. A 95% confidence interval is also included.
¶ The P value associated with the marginal effect.
* Marginal effects that differ from 1 with 95% confidence.
Table 2. GLMM results and summary statistics for pools by categoryFootnote †
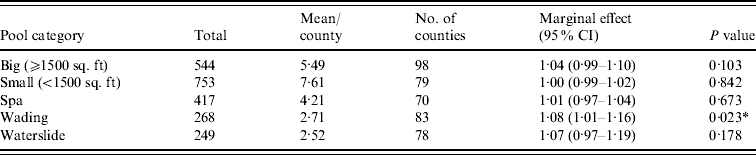
CI, Confidence interval.
† Columns are defined as in Table 1.
* Marginal effects that differ from 1 with 95% confidence.
Table 3. GLMM results and summary statistics for pools by owner and categoryFootnote †
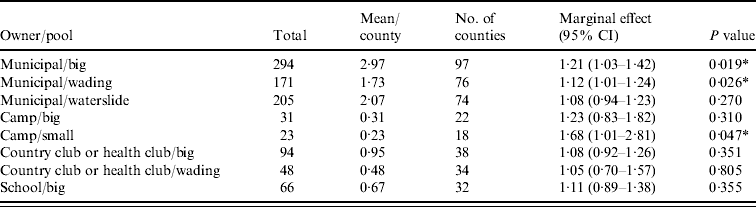
CI, Confidence interval.
† Columns are defined as in Table 1.
* Marginal effects that differ from one with 95% confidence.
DISCUSSION
Our results demonstrate a clear relationship between the geographical distribution of reported Cryptosporidium cases during the Iowa 2007 outbreak and the distribution of swimming pools in Iowa. The association between swimming and the spread of Cryptosporidium has been well established in a number of reports. The contribution of this project is to show that across a large geographical area, the type and nature of pools was associated with and thus may have contributed to the distribution of the outbreak.
We find, at the county level, that in terms of management/owner type, pools that tend to serve more heterogeneous populations, such as municipal pools, are associated with more cases. Conversely, and not surprisingly, pools that serve more homogenous or isolated populations, such as country clubs or apartment buildings, are not. Furthermore, when we consider the combination between pool ownership/management type and the actual category of pools, we generally find that increases in the number of bigger pools, pools with more heterogeneous mixing, and pools catering to young children (wading pools) are associated with more cases at the county level.
Our finding that small camp pools are a county-level risk factor was surprising. We thought that larger camp pools would be more at risk, but perhaps counties with more small camp pools have fewer resources and impose fewer hygienic controls than counties with large camp pools, and maintenance patterns may overwhelm the social network factors (e.g. mixing of populations). In addition, the frequent turnover in summer camp residency may play a role.
There are several limitations to our project. First, we did not use individual-level data, i.e. we did not know the swimming patterns of the citizens of Iowa and people clearly travel to swim. An example in Iowa: two of the larger water parks in the state are in Dubuque County and Blackhawk County, and these counties did indeed have many Cryptosporidium cases. However, because families travel across counties to go to these parks, these parks may have contributed to cases all over Eastern Iowa. Although it would be ideal to have complete swimming histories and exposures for all cases for outbreak investigations, these histories are often incomplete as well as expensive and difficult to collect. There is also a considerable delay in obtaining this information, and it is subject to recall bias. Furthermore, hotel pools were not associated with more Cryptosporidium cases, but the cases originating from these pools may have ended up in distant states. Second, there are limitations to using the county-level data. For example, age is only available for 5-year intervals. Moreover, the statewide pool database does not account for how well pools are maintained throughout the season nor does it include the kind of filtration systems used by the pools and whether individual pools are following specific public health recommendations. Third, we did not have information on the volume of swimmers at different pools. Instead we relied on variables related to the size of the pool and owner type to approximate attendance and mixing patterns. Fourth, swimming pools are certainly not the only way Cryptosporidium is spread during an outbreak. For example, if all swimming stopped, Cryptosporidium could continue to be spread by other venues (i.e. children in daycare). Our analysis does not account for these other opportunities for transmission. However, swimming pool exposures were clearly identified as a risk factor and this probably was then amplified by other exposures (i.e. daycare). In fact, transmission that has started in daycare centres has been amplified across communities and age groups via swimming pools [Reference Turabelidze22]. Although drinking water has been linked to other outbreaks in the USA, especially in more urban areas, we do not believe that drinking water was the major driver for several reasons: the diffuse distribution of the cases, the young ages of the cases, the seasonal pattern, and the diffuse distribution of municipal drinking water in Iowa. In addition, drinking water was not implicated in the public health investigation. Fifth, cases were more common in adult women than adult men. This may have been because the women had more exposure to ill children. However, we would need more detailed individual-level data to confirm this explanation. Finally, our analysis included only reported cases instead of all cases. Thus, the cases we included in our analysis may have just represented the ‘tip of the iceberg’ of the outbreak. Although the data collection was coordinated at the state level, the data collection was actually done at the county level introducing a possible source of bias.
Despite these limitations, our results provide a framework for preventing and controlling the spread of Cryptosporidium via swimming pools. Furthermore, we believe this framework is useful for both outbreaks and for endemic disease. In ideal settings such analysis would be accompanied by detailed individual exposure data; but resources are often limited, and the results of such investigations are not available at the start of outbreaks. During an outbreak there are often data that would be ideal and data that are actually available to public health investigators. Our analysis took advantage of the location of cases recorded during the outbreak but the other data were ‘population based’ and were already readily available. Thus we believe our approach is generalizable to other outbreaks other regions.
There are several measures that public health authorities recommend for controlling pool-related cryptosporidiosis outbreaks [14, 23]. For example, after a case is epidemiologically linked to a swimming facility, the CDC recommends hyperchlorination of the pool water [24]. For prevention, the CDC suggests posters and community outreach/education, stressing regular bathroom breaks for small children, not swimming while suffering from diarrhoea, changing diapers in the changing room rather than the pool area, and proper hand hygiene [Reference Kaye25]. Further, they recommend that pool operators should develop policies to react to pool ‘accidents’ and maintain water quality. Temporarily stopping all swimming or swimming of young children could also be implemented at a state-wide or individual-pool level. We strongly believe that all of the aforementioned control measures are important and should be applied uniformly for all pools open to the public as per public health recommendations. However, public health resources are scarce, particularly during an outbreak, and our results help guide public health officials and practitioners to allocate these resources for control in the most efficient manner, especially in an outbreak setting. Our results also suggest a strategy for targeting surveillance for new cases and also surveillance for adherence to recommendations. During summer outbreaks even if swimming pools are not the start of an outbreak, they have the potential to amplify and or accelerate the spread of the outbreak. Indeed, recreational water exposures are increasingly associated with Cryptosporidium outbreaks [Reference Yoder and Beach7]. Our results indicate that current recommendations for preventing and controlling the spread are especially critical for pools that are most likely to have the highest mixing of swimmers as well as the largest number of small children, e.g. large public pools, especially those with wading pools. Furthermore, closing one pool may unintentionally shift swimmers to other pools, increasing the potential of the outbreak to spread to other populations that previously might not have been exposed. Our results should encourage public health officials and investigators to increase their understanding of when and where people swim, especially younger children.
ACKNOWLEDGEMENTS
Support for this research was provided by a National Institutes of Health Career Investigator Award (Research Grant K01 AI75089: P.M.P.) and a National Institute for General Medical Sciences Training Grant (T32 GM077973).
DECLARATION OF INTEREST
None.







