The vulnerability of patients with long-term mental illness to reduced levels of satisfaction with their living situation, family and social relations, leisure activities, work, finances, safety and health, compared with the general population, has been recognised for many years (Reference Lehman, Ward and LinnLehman et al, 1982). Although there have been advances in treatment following the introduction into clinical practice of the novel antipsychotic drugs, concerns about patients' functioning and well-being are still valid today (Reference Bengtsson-Tops and HanssonBengtsson-Tops & Hansson, 1999; Reference Doyle, Flanagan and BrowneDoyle et al, 1999; Reference Aksaray, Oflu and KaptanogluAksaray et al, 2002), particularly in those with residual symptoms and in those who have not achieved an optimal response to treatment (Reference KaneKane, 1999). Improvement in these outcomes is therefore an important dimension of successful treatment alongside symptom improvement. Our study examined self-rated functioning and well-being among clinically stable patients with schizophrenia during a year-long trial that evaluated the long-term safety and efficacy of long-acting risperidone, and investigated the relationship between subjective functioning, well-being and objective measures of schizophrenia symptoms.
METHOD
An open-label, international multicentre trial was conducted to evaluate the long-term outcome of treatment with long-acting risperidone given as an intramuscular injection every 2 weeks, for 1 year. A more detailed description of the study methodology is provided elsewhere (Reference Fleischhacker, Eerdekens and KarcherFleischhacker et al, 2003).
Participants
Eligible patients were at least 18 years old and had a DSM-IV diagnosis of schizophrenia (American Psychiatric Association, 1994). They were required to be symptomatically stable, as judged by the treating physician, be receiving a stable dose of an antipsychotic drug for at least 4 weeks before the trial and be in good general physical health. The main exclusion criteria were substance dependence, a history of tardive dyskinesia, neuroleptic malignant syndrome or clinically significant physical abnormalities. Patients who had been treated with clozapine within 2 months of entry into the trial or with a conventional depot antipsychotic drug within one treatment cycle were also ineligible.
Study medication
After a 2-week run-in period during which antipsychotic agents other than risperidone were discontinued, patients were started on oral risperidone at a daily dosage of 1-6 mg based on the judgement of the treating physician. The oral dosage was used as a guide to the starting dose of long-acting risperidone of 25 mg, 50 mg or 75 mg. Supplementation with oral risperidone was given for 2-3 weeks following the first injection of long-acting risperidone to provide antipsychotic coverage during the 3-week latency period after the first injection (Eeredekens et al, 2004). Concomitant medications that could be initiated or continued, at the discretion of the investigator, were those to assist sleep, anti-Parkinsonian agents, antidepressants, mood stabilisers, propranolol for akathisia and benzodiazepines for agitation and insomnia.
Measures
Both the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987; Reference Kay and SinghKay & Singh, 1989), which was the primary measure of treatment efficacy, and the Medical Outcomes Study Short Form 36-item questionnaire (SF-36; Reference Ware, Snow and KosinksiWare et al, 1993), which measures patient-rated functioning and well-being, were administered every 3 months. The SF-36 is a multipurpose generic measure of functional health and well-being with psychometrically based physical and mental health summary measures, comprising eight domains which assess the following quality of life parameters:
-
(a) limitations in physical functioning because of health problems (physical functioning);
-
(b) role limitations due to physical health problems (role - physical);
-
(c) bodily pain;
-
(d) general health;
-
(e) energy and fatigue (vitality);
-
(f) limitations in social activities because of physical or emotional problems (social functioning);
-
(g) role limitations because of emotional problems (role - emotional);
-
(h) general mental health (mental health).
Scores range from 0 to 100, with the higher scores indicating a better quality of life. From these eight domains, a physical component summary score (composed of items a-d) and a mental component summary score (composed of items e-h) were calculated and compared with benchmark scores from a general population (Reference WareWare, 2003). Scores above or below 50 represent quality of life above or below the population average, respectively. Patients completed the questionnaire prior to being interviewed by the investigator. A 1-month recall was used for all questions. The SF-36 has been found to be suitable for use in multinational studies (Reference Ware, Kosinski and GandekWare et al, 1998).
The Structured Clinical Interview for PANSS (Reference Kay, Fiszbein and OplerKay et al, 1987) was used to rate schizophrenia symptoms. In addition to the total PANSS score (sum of all 30 items, maximum score 7 per item), five sub-scales were calculated based on the findings of Marder et al (Reference Marder, Davis and Chouinard1997). These sub-scales were:
-
(a) positive symptoms: delusions, hallucinatory behaviour, grandiosity, suspiciousness (items 1, 3, 5 and 6 on the positive sub-scale); stereotyped thinking (item 7 on the negative sub-scale); somatic concern, unusual thought content, lack of judgement and insight (items 1, 9 and 12 on the general psychopathology sub-scale);
-
(b) negative symptoms: blunted affect, emotional withdrawal, poor rapport, passive social withdrawal, lack of spontaneity (items 1, 2, 3, 4 and 6 on the negative sub-scale); motor retardation, active social avoidance (items 7 and 16 on the general psychopathology sub-scale);
-
(c) disorganised thoughts: conceptual disorganisation (item 2 on the positive sub-scale); difficulty in abstract thinking (item 5 on the negative sub-scale); mannerisms and posturing, disorientation, poor attention, disturbance of volition, preoccupation (items 5, 10, 11, 13 and 15 on the general psychopathology sub-scale);
-
(d) uncontrolled hostility/excitement: excitement, hostility (items 4 and 7 of the positive sub-scale); uncooperativeness and poor impulse control (items 8 and 14 on the general psychopathology sub-scale);
-
(e) anxiety/depression: anxiety, guilt feelings, tension, depression (items 2, 3, 4 and 6 on the general psychopathology sub-scale).
Sample
A total of 615 patients with schizophrenia were recruited into this trial (422 men, 193 women). The majority (92%) of them were White, and their mean age was 42 years (s.e.=0.6, range 18-84). The mean PANSS total score at baseline was 67.1 (s.e.=0.8). Eighty-seven per cent of the total cohort completed the first 3 months, and 65% completed the 1-year trial period. Tolerability, safety and efficacy have been reported elsewhere (Reference Fleischhacker, Eerdekens and KarcherFleischhacker et al, 2003), and suggest that in schizophrenia patients with stable symptoms can achieve further improvement in terms of both safety and efficacy by having their medication switched to long-acting risperidone.
Statistical analysis
The primary analysis of the study explored changes in self-rated functioning and well-being from baseline at 12 weeks, 24 weeks, 36 weeks and 50 weeks and at end-point. Changes from baseline in self-rated functioning and well-being, as measured by the SF-36, were analysed for each measurement point and for the last observation for each patient using a paired samples t-test. Differences on the SF-36 between population norms and study participants are expressed as effect size scores using Cohen's formula (Reference CohenCohen, 1976), which consists of subtracting the mean differences of the two groups and dividing it by the pooled standard deviation. Cohen (Reference Cohen1976) has termed effect sizes of 0.20-0.50 as small, 0.50-0.80 as medium and greater than 0.80 as large. For example, an effect size of 0.20 corresponds to the difference between the heights of 15-year-old and 16-year-old girls in the USA, an effect size of 0.50 corresponds to the difference between the heights of 14-year-old and 18-year-old girls and an effect size of 0.80 corresponds to the difference between the heights of 13-year-old and 18-year-old girls (Reference CohenCohen, 1976).
A second planned analysis examined the overlap of self-rated functioning and well-being as measured by the four SF-36 mental health domains and the mental component summary scores, and psychopathology as measured by the five PANSS sub-scale scores. This was done by examining correlations of the measures and then by maximum likelihood factor analysis with promax rotation. The number of factors was determined by examining the scree plots. Factor analysis permitted a test of the extent to which the PANSS sub-scale scores and SF-36 mental health domain scores overlapped and the goodness of fit of the factor model obtained. Root mean square error of approximation (RMSEA) was used to indicate the goodness of fit; values below 0.1 are considered to be a ‘good’ fit and those below 0.05 a ‘very good’ fit (Reference SteigerSteiger, 1989).
RESULTS
Self-rated functioning and well-being at baseline and comparison with norms
Table 1 shows the mean, standard deviation and normalised scores at baseline. The internal consistency of the scales (Cronbach's α=0.73-0.90) suggested that there was good internal consistency of responses. On the mental component summary score participants were substantially below norms by a large effect size of 0.80. On the physical component summary score there was a much smaller difference from norms (effect size 0.22), but scores were still below the norms. Almost half (49%) of the patients had scores above normal on the physical component summary measure and 29% were above normal on the mental component summary measure, with few patients having values considerably higher than normal levels. Except for bodily pain and the physical component summary, scores on all the scales differed in effect size moderately to very strongly from the norms.
Table 1 Quality of life assessed using the SF-36 in the study sample (n=550), compared with normalised scores

| SF-36 domain | Sample score Mean (s.d.) | Normalised score | Effect size (difference from norms) |
|---|---|---|---|
| Physical functioning | 76.5 (24.0) | 46.5 | −0.35 |
| Role-physical | 61.0 (38.3) | 44.0 | −0.60 |
| Bodily pain | 77.9 (25.9) | 51.0 | −0.10 |
| General health | 58.5 (21.3) | 43.2 | −0.68 |
| Vitality | 51.5 (22.4) | 45.4 | −0.46 |
| Social functioning | 66.0 (27.4) | 42.1 | −0.79 |
| Role-emotional | 56.8 (42.0) | 42.6 | −0.74 |
| Mental health | 63.2 (20.3) | 43.6 | −0.64 |
| Mental component summary score | 42.0 (12.2) | 42.0 | −0.80 |
| Physical component summary score | 47.8 (9.2) | 47.8 | −0.22 |
Improvement in self-rated functioning and well-being during antipsychotic treatment
Table 2 presents mean change on the SF-36 domains and summary scores. On the physical health measures there was no significant change to end-point based on last value carried forward when examining all participants. On the mental health measures there were significant improvements at all time points on the mental component summary, the vitality score and, with the exception of week 12 (P=0.08), also on social functioning. On ‘role - emotional’ there were significant improvements at all time points but not at end-point derived by last observation carried forward analysis.
Table 2 Change in SF-36 scores from baseline

| Week 12 (n=482) Mean (s.e.) | Week 24 (n=434) Mean (s.e.) | Week 36 (n=348) Mean (s.e.) | Week 50 (n=312) Mean (s.e.) | End-point1 (n=536) Mean (s.e.) | |
|---|---|---|---|---|---|
| Physical component summary score | 0.2 (0.37) | 0.3 (0.43) | 0.9 (0.45) | 0.9 (0.45)* | -0.0 (0.36) |
| Physical functioning | 0.4 (0.92) | 1.6 (1.02) | 1.3 (1.03) | 2.2 (1.16) | 0.7 (0.90) |
| Role - physical | 3.7 (2.00) | 3.3 (2.26) | 6.7 (2.44)** | 6.3 (2.54)* | 1.7 (1.97) |
| Bodily pain | 0.5 (1.10) | -0.0 (1.21) | 1.6 (1.34) | 1.5 (1.39) | -0.2 (1.08) |
| General health | 0.4 (0.81) | 1.6 (0.95) | 2.4 (1.04) * | 4.0 (1.07)*** | 1.3 (0.89) |
| Mental component summary score | 1.3 (0.49)** | 2.2 (0.56)*** | 2.6 (0.61)*** | 2.6 (0.64)*** | 1.5 (0.51)** |
| Vitality | 2.3 (0.92)* | 4.5 (1.05)** | 5.5 (1.15)*** | 5.7 (1.22)*** | 3.1 (0.98)** |
| Social functioning | 2.1 (1.22) | 4.1 (1.32)** | 5.0 (1.45)*** | 5.9 (1.55)*** | 3.5 (1.25)** |
| Role - emotional | 4.3 (2.03)* | 7.1 (2.29)** | 8.7 (2.41)*** | 8.7 (2.61)*** | 3.2 (2.06) |
| Mental health | 1.3 (0.8) | 1.8 (0.95) | 2.8 (0.97)** | 2.0 (1.08) | 0.8 (0.85) |
Association of self-rated functioning and well-being and PANSS
Table 3 shows the correlations of the mental component summary, and the four SF-36 mental health domain scores it comprises, with the PANSS sub-scale scores at baseline and the correlations among change from baseline to end-point on these measures. At baseline and on change, the highest correlations were found between the PANSS anxiety/depression scale and the SF-36 domain ‘general mental health’; at baseline the correlation coefficient was r= -0.53 and for change it was r= -0.42. Correlations of PANSS anxiety/depression with mental component summary were 0.49 at baseline and 0.36 for the change scores. Despite the fact that both the total PANSS score, as presented elsewhere (Reference Fleischhacker, Eerdekens and KarcherFleischhacker et al, 2003), and the mental component summary score of the SF-36 improved significantly over the course of the trial, changes in the two measures were only moderately correlated (r= -0.4).
Table 3 Correlations of SF-36 mental health items and PANSS items at baseline and change from baseline to end-point (n=563)

| SF-36 items | PANSS items | |||||
|---|---|---|---|---|---|---|
| Total PANSS | Positive symptoms | Negative symptoms | Disorganised thoughts | Hostility/excitement | Anxiety/depression | |
| Baseline | ||||||
| Mental health | −0.31 | −0.20 | −0.23 | −0.13 | −0.14 | −0.53 |
| Vitality | −0.19 | −0.10 | −0.22 | −0.05 | −0.02 | −0.33 |
| Social functioning | −0.35 | −0.26 | −0.27 | −0.20 | −0.22 | −0.37 |
| Role - emotional | −0.22 | −0.12 | −0.18 | −0.10 | −0.15 | −0.34 |
| Mental component summary score | −0.30 | −0.17 | −0.25 | −0.11 | −0.16 | −0.49 |
| Change from baseline | ||||||
| Mental health | −0.38 | −0.24 | −0.27 | −0.23 | −0.15 | −0.42 |
| Vitality | −0.27 | −0.13 | −0.28 | −0.21 | −0.04 | −0.30 |
| Social functioning | −0.28 | −0.26 | −0.21 | −0.11 | −0.13 | −0.29 |
| Role - emotional | −0.27 | −0.19 | −0.26 | −0.19 | −0.13 | −0.19 |
| Mental component summary score | −0.40 | −0.29 | −0.33 | −0.25 | −0.17 | −0.36 |
To examine further the extent to which the domains measured by the two instruments overlapped, maximum likelihood factor analysis was conducted with promax rotation. The factor analysis included the five PANSS sub-scales and the four SF-36 mental health domains. Analyses of the baseline data and change scores both produced two factors, one of which was associated with the five PANSS sub-scales, the other with the four mental health domains of the SF-36. At baseline the factors cumulatively accounted for 47.2% of the variance and, for change to end-point, for 39.5% of the variance. The correlation between the factors at baseline was r= -0.34, suggesting that the SF-36 and PANSS have an overlap of 12% at baseline. For change to end-point the correlation was r= -0.52, suggesting an overlap of 27%. The factor plot presenting the results of the factor analysis for the baseline measures is presented in Fig. 1. The mental health domains of the SF-36 were more tightly clustered than the sub-scales of the PANSS.
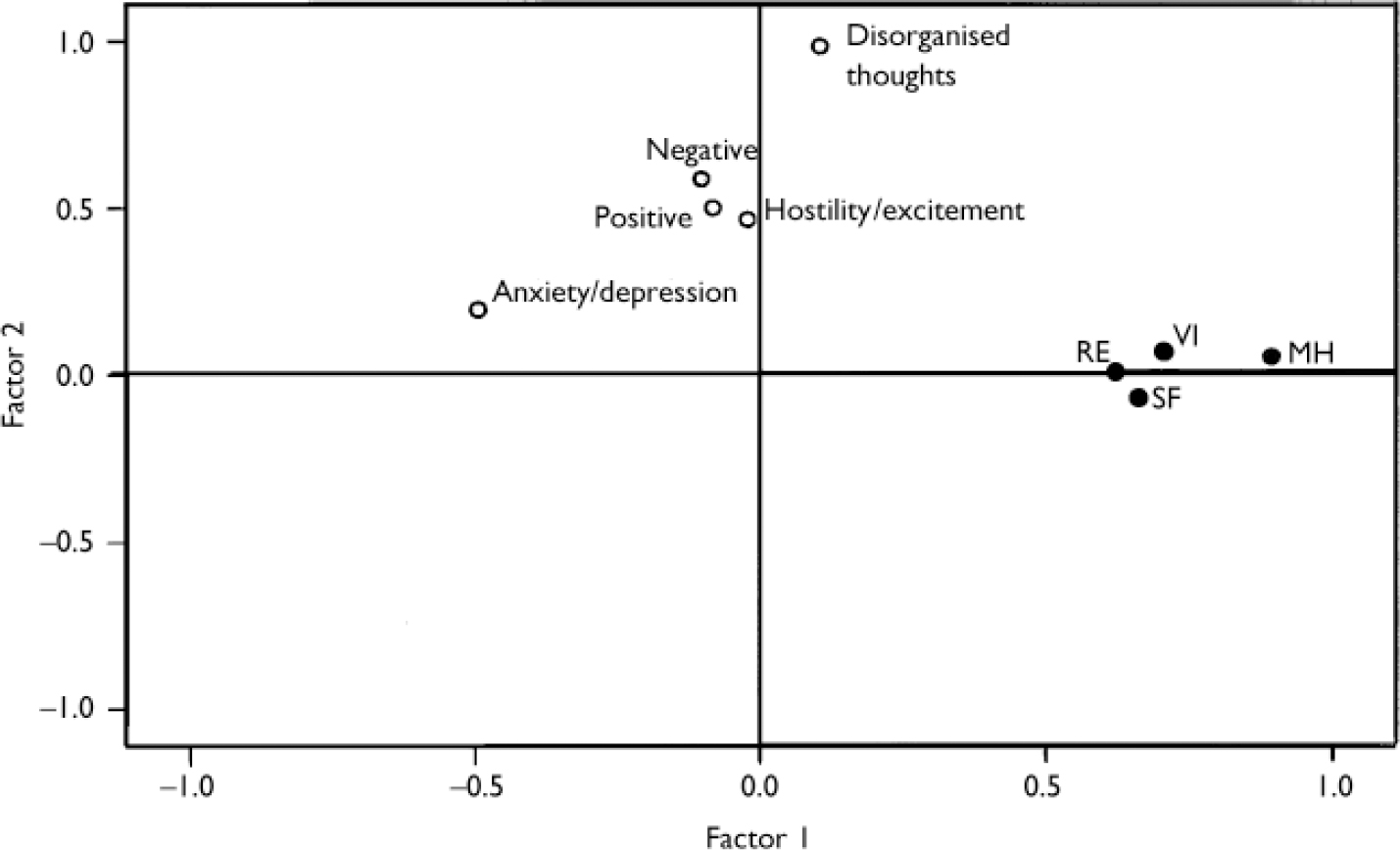
Fig. 1 Factor plots in rotated factor space: baseline scores on five sub-scales of the Positive and Negative Syndrome Scale (open circles) and four domains of the 36-item Short Form questionnaire (black circles: MH, mental health; RE, role - emotional; SF, social functioning; VI, vitality).
The RMSEA values for both baseline and change were 0.09, suggesting an acceptable fit of the factors (Reference SteigerSteiger, 1989). The results thus suggest that although the PANSS (a measure of investigator-rated psychopathology) and the mental health domains of the SF-36 (a patient self-rating measure of functioning and well-being) are related, they do not overlap to a great extent, perhaps due to being different methods of assessment, with different strengths and weaknesses. This demonstrates the need for more than one method for the comprehensive assessment of outcomes.
DISCUSSION
The principal aim of the treatment of schizophrenia is to bring about a rapid reduction of symptoms in the short term and to minimise the risk of relapse and side-effects in the longer term. Traditional assessment instruments, such as PANSS and the Clinical Global Impression (CGI; Reference Guy and RushGuy, 2000), are widely used - notably in clinical trials - to measure and monitor objective changes in symptoms during treatment. These instruments, however, do not provide a wider picture of the subjective impact of treatment on the patient, particularly in terms of the effect on self-rated functioning and well-being. Achievement of the highest possible level of these parameters is an outcome that is of major importance, not only to patients, but also to their family, caregivers and to payers. Despite the recognised importance of patient-reported outcomes, surprisingly little information on such measures has been collected in large samples of people with severe mental illness treated with novel antipsychotic drugs.
Baseline illness severity and outcome
Even though our study recruited symptomatically stable patients, baseline scores (particularly in the mental health domains) were significantly below population norms. Improvements both in psychopathology and in the mental health domains and the mental component summary score during treatment with long-acting risperidone were apparent early in treatment and were maintained throughout the long-term treatment period. Given that the population in this study were required to be physically healthy, the physical component summary score and scores on its individual physical health domains (physical functioning, role - physical, bodily pain and general health) were high at baseline and approached normative scores. Consequently, the effect of treatment on the physical components of the SF-36 was modest over the course of the study, which may reflect the fact that treatment with long-acting risperidone generally induces few side-effects that might interfere with the subjective physical well-being of patients with schizophrenia. These findings are consistent with the observed increments in a smaller double-blind, placebo-controlled study, involving more severely symptomatic patients with schizophrenia treated with long-acting risperidone for a shorter period (Reference Nasrallah, Duchesne and MehnertNasrallah et al, 2004).
Correlation results in the light of previous studies
In this study correlations between objective symptom ratings and subjective ratings of functioning and well-being at baseline were only modest. Also, although patients in this trial improved both in terms of SF-36 mental health domain scores and the PANSS scores (Reference Fleischhacker, Eerdekens and KarcherFleischhacker et al, 2003), there was only a moderate correlation between changes measured on the PANSS and the SF-36.
We are not aware of any other published data on perceived functioning and well-being, as measured by the SF-36, in clinically stable patients with schizophrenia treated with novel antipsychotic drugs. The SF-36 was, however, used in another published large-scale study in this area, conducted by Tunis et al (Reference Tunis, Croghan and Heilman1999) in a large cohort of people with schizophrenia who participated in a clinical trial of olanzapine v. haloperidol. The trial sample consisted of patients who had clinically significant psychosis and who had demonstrated less than an optimal response to their previous medication. The study found that the SF-36 exhibited good reliability and validity among people with schizophrenia. Tunis et al (Reference Tunis, Croghan and Heilman1999) also found that, compared with population norms, the study participants had marked deficits in the SF-36 domains in general health, vitality, general mental health, social functioning and in both physical and emotional role limitation; furthermore, correlations between the Brief Psychiatric Rating Scale total score and the SF-36 domain scores were non-significant for all physical component scores and ranged from -0.20 to -0.31 for the mental health domain scores (vitality, r= -0.21; social functioning, r= -0.29; role - emotional, r= -0.20; general mental health, r= -0.31). Similar to our study, these correlations suggested - also in poorly responding patients - little overlap between the psychopathology measures and the SF-36 mental health domains; however, the association of each component of the SF-36 with specific BPRS item clusters was not examined, thus not allowing more detailed examination of the association of change in symptoms as rated in a clinical interview and changes in patients' perceived mental and physical health.
These results support our finding that patient-reported improvement in functioning and well-being is a different outcome dimension from investigator-rated improvement of psychopathology, which suggests that it is advisable to use more than just one method when assessing outcomes.
Limitations
The major limitation of our study was that it did not have a control group, so that although improvements in well-being were noted it is not possible to know whether these changes would have occurred in the absence of treatment with long-acting risperidone. Still, regardless of any potential drug effects, the finding that symptom improvement, as measured by an observer-based rating scale, has little in common with subjectively experienced functioning and well-being still stands. Another limitation of the study is that the SF-36 is a generic instrument used across all disease states, and its usefulness in schizophrenia is constrained by the fact that it does not adequately cover the social domains, notably social functioning and psychosocial reintegration, that are relevant outcomes in this illness. On the other hand, the SF-36 is a patient-rated instrument that is well validated and sensitive to changes in the level of functioning in schizophrenia (Reference Russo, Trujillo and WingersonRusso et al, 1998; Reference Tunis, Croghan and HeilmanTunis et al, 1999). It is short, simple, practical and widely used, with associated normative data to enable comparisons with the general population.
Implications for further research
There is evolving evidence that in comparison with conventional antipsychotics, novel antipsychotics are more effective in improving health-related quality of life in patients with schizophrenia (Reference Hamilton, Edgell and RevickiHamilton et al, 2000; Reference Cook, Goldberg and Van LieshoutCook et al, 2002; Reference Karow and NaberKarow & Naber, 2002). This may be a reflection of the efficacy, tolerability and relapse prevention benefits of these drugs over conventional drugs (Reference Csernansky, Mahmoud and BrennerCsernansky et al, 2002; Sartorius et al, Reference Sartorius, Fleischhacker and Gjerris2002, Reference Sartorius, Fleischhacker and Gjerris2003; Reference Leucht, Barnes and KisslingLeucht et al, 2003). Whatever the cause of these improvements, this outcome domain is very relevant to people with schizophrenia.
Given the importance of health-related quality of life, particularly among people with schizophrenia, and the apparent lack of overlap between symptom improvement and improvement in this domain, future clinical trials of antipsychotic medications should routinely apply measures such as the SF-36, optimally in conjunction with a disease-specific, patient-rated instrument. Studies are also needed of the extent to which perceived functioning and well-being are associated with longer-term outcomes such as relapse. In addition, more work is needed in identifying which domains and measures of health-related quality of life are most germane to treating schizophrenia.
In summary, maintenance treatment with long-acting risperidone appears to contribute to an improvement of outcomes beyond a reduction of symptoms in patients with schizophrenia. Clinically meaningful improvements were observed in our study participants; these findings for self-rated functioning and well-being are distinct from symptomatic improvement. Therefore, patient functioning and well-being are relevant parameters to assess in addition to symptom improvement.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ In addition to psychiatric symptoms, people with schizophrenia suffer from reduced reduced levels levels of functioning and perceived well-being. perceived well-being.
-
▪ In symptomatically stable patients with schizophrenia treated with long-acting risperidone, statistically significant improvement was noted in the 36-item Short Form (SF-36) mental health domains social functioning and vitality, and in the mental component summary score.
-
▪ Improvements in psychopathology and perceived functioning and well-being during antipsychotic treatment were only moderately associated, therefore both should be assessed when measuring treatment outcomes.
LIMITATIONS
-
▪ Without a control group, it is not possible to know whether changes in self-rated functioning and well-being would have occurred in the absence of treatment with long-acting risperidone.
-
▪ The SF-36 is a generic measure and may not adequately capture all areas of functioning and well-being that are relevant to people with schizophrenia; optimally, a disease-specific instrument should be used alongside it.
-
▪ The correlational design of the analyses leaves the potential for other unmeasured factors to contribute to, or confound, the observed relationships.
Acknowledgements
We thank Anita Hsu and Margaret Rothman (J&J Pharmaceutical Services LLC) for their initial correlation and regression analyses, Stuart Donovan for editorial assistance and Robert A. Lasser, MD, for helpful comments on drafts.







eLetters
No eLetters have been published for this article.