Impact statement
As the world comes together to address plastic waste and pollution, and with the March 2, 2022, United Nations Resolution 5/14 to End Plastic Pollution with an International Legally Binding Instrument by 2024, many strategies are being considered for how to best address this immense problem. We argue that the simplification of the additives and the heterogeneity in the polymer universe is the most promising solution. As plastic formulations have become much more variable, post-consumer plastic becomes increasingly hard to recycle. Simplification of additives and formulations could allow for much more effective recovery methods resulting in a more circular lifecycle for these plastics. There is a significant gap in current policy on addressing these additives and their use in plastics. Based on these foundations, this review identifies possible repetition in the additive class of phenolic antioxidants, addresses possible pathways forward for policymaking and discusses future research that needs to be executed on this topic.
Introduction
History
Since the 1950s, plastics have been mass-produced and sold to consumers around the globe as cheap, easily manufactured alternatives to traditional materials. The inert nature of plastic, which resulted in its use for an increasingly broad range of applications, was not considered hazardous or toxic pursuant to the United States 1960s era environmental laws. In the evolution of the plastic industry, manufacturers sought to enhance specific performance characteristics for different uses. Innovations were made in the form of polymers as well as polymer additives, used in the production of the polymer as well as incorporated in the final product to enhance a broad range of characteristics. Scientists then found that adding chemicals to plastics during the manufacturing process increased their performance to expand their applications. Chemical additives as a revolutionary way to package and produce materials paved the way for widespread use and popularity. Today, chemical additives are fully incorporated into polymer formulations to meet market demand because they can meet product performance specifications and expectations. Chemical additives are now part of nearly all marketable plastics to create durable materials for a low price without consideration of post-use management (Basuhi et al., Reference Basuhi, Moore, Gregory, Kirchain, Gesing and Olivetti2021). The proliferation of chemical additives has made post-consumer plastic increasingly hard to recycle. Simplification of additives and formulations could allow for much more effective recovery methods and a potential reduction in environmental and public health impacts. This review will identify possible repetition in the phenolic antioxidants additives class and address possible pathways for policymaking to simplify usage.
The problems of shedding potential, variability and complexity of plastic products as well as the amount of single-use items interfere with collection and recovery resulting in a planet inundated with plastic pollution. Microplastics containing toxic chemical additives are entering our ecosystems and bodies as they have been found in human blood, lungs and placentas in addition to our waterways, soil, air and rain (Azeem et al., Reference Azeem, Adeel, Ahmad, Shakoor, Jiangcuo, Azeem, Ishfaq, Shakoor, Ayaz, Xu and Rui2021). The severity of the plastic pollution crisis resulted in the March 2, 2022, United Nations Resolution 5/14 to End Plastic Pollution by 2024 (UNEP, 2023b). This provides a step forward for countries and industries to negotiate an end to plastic pollution with an international legally binding agreement to be finalized by 2024, calling for an end to plastic pollution by 2040. Of the primary goals discussed in these negotiations, reducing plastic production through the adoption of use and design standards is a priority to simplify the variety of plastic products for resource recovery.
Recent reports have indicated that over 70,000 different chemical formulations using over 16,000 chemical additives are associated with plastics and plastic production (Wagner et al., Reference Wagner, Monclús, Arp, Groh, Løseth, Muncke, Wang, Wolf and Zimmermann2024; UNEP, 2023c). According to Wagner et al. (Reference Wagner, Monclús, Arp, Groh, Løseth, Muncke, Wang, Wolf and Zimmermann2024), fewer than 6% of additives are subject to regulation, and 4,200 are chemicals of concern since they are persistent, bioaccumulative, mobile or toxic (PBMT). Simplification of the universe of plastic additives is a critical part of addressing this global problem. Recognizing common chemicals, structures and functions to allow identification, narrowing and simplification, free of the obfuscation currently resulting from chemical marketing strategies with duplicative trade names and CAS numbers, can help to achieve this goal. A developing body of data regarding the human health impacts of chemical additives recognizes micro- and nanoplastics as delivery devices for chemical additives when ingested or inhaled or absorbed (Qian et al., Reference Qian, Gao, Lang and Min2024). These additives are then directly delivered into the tissues of the exposed host from the surfaces of the internalized particles - magnifying the toxicity and making chemical additive management, including simplification as well as banning the most hazardous, as key features of effective policy.
1.2 Lack of global regulation
Despite the widespread use of plastics, there continues to be a lack of regulation in plastic production, marketing and waste around the world, including the United States (Nagtzaam and Kourabas, Reference Nagtzaam and Kourabas2023). While monomer and most polymer manufacturing are pervasively regulated in the United States through the environmental authority applicable to chemical manufacturers mitigating pollution from the production process itself, down the value chain, production steps such as molding and extrusion, and final sale of plastic products, are generally not regulated at all. Thus, most of the value chain post initial polymer production remains invisible to regulators, including the incorporation of additives through the value chain (Groh et al., Reference Groh, Backhaus, Carney-Almroth, Geueke, Inostroza, Lennquist, Leslie, Maffini, Slunge, Trasande, Warhurst and Muncke2019). It is inexpensive to produce, loosely regulated and convenient to use. However, plastic production continues to accelerate in the context of inadequate waste management and leakage of plastics and microplastics into the environment through use and waste management failures, has led to global economic, environmental and social consequences, particularly in the developing world.
Chemical additives
Additives generally
In the manufacturing of plastics, chemical additives play a crucial role by enhancing the production process and tailoring the material properties to suit the intended application of the final product. The modification of various physical and chemical properties of plastics aids in the manufacturing processes to ensure the functionality of the final product from both contemporary and historical consumer safety perspectives. More than 16,000 chemicals (Wagner et al., Reference Wagner, Monclús, Arp, Groh, Løseth, Muncke, Wang, Wolf and Zimmermann2024) are associated with plastics and plastic production across a wide range of applications. More than 3,200 monomers, additives, processing aids and non-intentionally added substances are of potential concern due to their hazardous properties including carcinogenicity, mutagenicity, reproductive toxicity, specific target organ toxicity, endocrine disruption, ecotoxicity, bioaccumulation potential, environmental persistence and mobility (Weber et al., Reference Weber, Ashta, Aurisano, Wang, Outters, Miguel, Schlummer, Blepp, Wiesinger, Andrade, Scheringer and Fantke2023). Weber et al.’s (Reference Weber, Ashta, Aurisano, Wang, Outters, Miguel, Schlummer, Blepp, Wiesinger, Andrade, Scheringer and Fantke2023) analysis reveals that out of these 16,000 additives detected in plastics, only about 7,000 have been scrutinized for their hazardous properties. This underscores the diversity and vast number of chemicals used as polymer additives in the plastic industry and raises concerns about the need to understand their hazardous impacts. The absence of stringent governance thus hampers transparency in plastic products, complicating the recovery and recycling processes.
Weber et al. (Reference Weber, Ashta, Aurisano, Wang, Outters, Miguel, Schlummer, Blepp, Wiesinger, Andrade, Scheringer and Fantke2023) also delve into the challenges posed by the patenting and marketing strategies within the industry, where minor variations in additive molecules are patented to create “unique” marketable products. This practice not only stymies the development of safer chemical alternatives but also increases the complexity of the additive landscape, making it difficult to phase out hazardous substances in the absence of stringent regulations. Additionally, this lack of regulatory oversight means consumers have little to no control or even knowledge about the specific additives present in the plastic products they use, contributing to potential health and environmental risks. Addressing these issues requires a multifaceted approach: enhancing regulations on plastic production, improving supply chain transparency and reducing the plethora of chemical additives used. Such measures are essential to diminish the adverse effects of plastic waste and pave the way for a sustainable and healthier environment for future generations.
Shortcomings in additive regulation
The extensive variety in post-use plastics can be traced back to a market-driven approach that encourages the continuous development of new products for patent protection and market differentiation. This has led to a situation where numerous additives, especially those developed for marketing purposes, are now overlapping in function and composition. The current regulatory framework’s inadequacy in addressing the proliferation of polymers and chemical additives contributes to the inefficiency of recycling processes and poses potential toxicity risks to both the environment and human health.
A key example of the current shortcomings related to polymer regulations include FDA regulations in the United States which limit certain additives in plastics intended for food contact, but not to other plastic products. “Globally” specific chemicals, on the other hand, are regulated under various national laws including the US Toxic Substances Control Act (TSCA) and the EU’s Registration, Evaluation, Authorization and Restriction of Chemicals (REACH). However, these regulations often do not extend comprehensively to polymers or their additives, allowing for significant variability in plastic products. In the EU, certain chemicals like styrenated phenols are not classified as persistently bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB), according to the European Chemicals Agency (ECHA, 2023). In contrast, the U.S. TSCA has a “polymer exemption” and focuses on manufacturing processes, excluding high molecular weight polymers and their additives due to their inert nature, from stringent regulatory oversight. In the United States, the use of industrial chemicals is regulated through the Toxic Substances Control Act (TSCA) implemented by the EPA. The rationale is that TSCA includes a “polymer exemption” to extend only to “manufacturing.” As such, polymers with high molecular weight are considered of low concern within TSCA’s scope due to their general inert (nonreactive) nature. Chemical additives used to achieve specific performance characteristics and aid in manufacturing are not considered within the scope of TSCA because when added to the polymer, neither the polymer itself nor the additives are being manufactured. There is no chemical reaction and thus no manufacturing subject to TSCA (Le Roy-Gleizes et al., Reference Le Roy-Gleizes, Messin-Roizard, Ternes, Jaffe, Bergeson, Hester, Porter, Riesel and Main2022).
Overall, the vast array of individually marketed chemicals appears largely redundant and could be substantially consolidated without compromising the functionality or utility of the plastic products, thus promoting a more sustainable and circular approach to plastic use. In this work, we scrutinize the core chemical functions within the category of phenolic antioxidants, identifying the apparent superfluous diversity and redundancies among the additives marketed to the plastic industry. In response, we propose strategies to significantly streamline the assortment of polymers and additives. Such a simplification could facilitate the recycling process and also reduce the leakage of plastics into the environment, thereby curtailing overall plastic pollution. By optimizing and reducing the range of additives, it could be possible to mitigate the environmental impact of plastic waste, reduce the exposure of humans and wildlife to harmful substances and alleviate the economic costs associated with plastic waste-related hazards.
Lowering the apparent unnecessary diversity of plastic additives that complicate resource recovery and result in post-use plastics into a near-infinite mix of waste-like materials will be critical (Landrigan et al., Reference Landrigan, Raps, Cropper, Bald, Brunner, Canonizado, Charles, Chiles, Donohue, Enck, Fenichel, Fleming, Ferrier-Pages, Fordham and Gozt2023). This research in identifying apparent duplication of additives provides important insights for the creation of the UNEA Resolution 5/14 and the forthcoming Plastic Treaty. These global policies seek to introduce new and comprehensive regulations that target the entire lifecycle of polymers and their additives, including post-use recovery. Simplification will significantly impact the heterogeneity of post-use plastics, improving their recyclability and supporting a circular economy. This research will help inform the future regulatory landscape, shaped by these international initiatives and holds the potential to transform the plastic industry by standardizing the use of polymers and additives, thus mitigating the environmental and health impacts of plastic pollution.
Phenolic antioxidant background
Antioxidants are crucial additives in various polymers, serving to prevent oxidative degradation and thus extend the materials’ lifespan, a key factor in promoting circular economies. These additives play a vital role in enhancing the durability and utility of plastic products. However, the extensive array of antioxidants, particularly phenolic antioxidants, raises concerns due to their toxicological effects.
Phenolic antioxidants are part of a broader group of antioxidants that also include phosphites, amines and thioesters (see Figure 1). Despite their known toxicity (Xu et al., Reference Xu, Liu, Hu, Ares, Martínez-Larrañaga, Wang, Martínez, Anadón and Martínez2021; Liu and Mabury, Reference Liu and Mabury2020), phenolic compounds, such as sterically hindered styrenated phenols, are widely used in industry due to their ability to react with free radicals. They function by reacting with free radicals, acting as hydrogen donors, inhibiting enzyme activities through protein interactions and chelating metal ions, thereby thwarting the oxidation process. However, the environmental and health implications of using such toxic substances necessitate a reevaluation of their use in polymer manufacturing.
Figure 1. Antioxidant categories.
Phenolic antioxidants, specifically sterically hindered phenols, are often used with secondary antioxidants to protect plastics in various environmental conditions (Landrigan et al., Reference Landrigan, Raps, Cropper, Bald, Brunner, Canonizado, Charles, Chiles, Donohue, Enck, Fenichel, Fleming, Ferrier-Pages, Fordham and Gozt2023 and Pospisil, Reference Pospisil1998). Phenolic antioxidants used to enhance polymer performance integrate secondary antioxidants like hydrolysis-resistant phosphites or photo-antioxidants (hindered amine stabilizers) and absorbers of ultraviolet light (light stabilizers). These preserve the plastic when exposed to environmental conditions and cause plastic to never fully biodegrade. Manufacturers create these slight molecular variations to circumvent patent laws and regulatory measures that apply to the base chemical molecules, contributing to an unnecessary amount of the types of phenolic antioxidants in the market. This practice complicates intellectual property landscapes and poses challenges for resource recovery from post-use plastics, as the variability in chemical composition complicates the potential for resource recovery from post-use plastics. Unchecked growth and diversification of these additives, particularly phenolic antioxidants, are leading causes of improperly managed plastic waste and a reduction of its ability to be recycled a threat to human and environmental health.
Our research informs strategies for reducing and regulating the chemical substances employed in plastic production. As indicated in Figure 1, commercialized additives are categorized based on their functional roles, providing a structured overview that can assist in guiding future regulatory and reduction efforts in plastic additive use.
Food grade phenolics
For regulatory orientation purposes, there are two types of phenolic antioxidants most often recognized: food grade (preservatives or plastic in contact with food) and durable plastic additives. Food-grade phenolic antioxidants utilized in food for food packaging and pharmaceuticals include butylated hydroxytoluene (BHT) as well as butylated hydroxyanisole (BHA, mixture of isomers). BHT has been found in high concentrations in urine samples in Japan, India and the United States. Its metabolite (BHT-acid) has been detected in 98% of samples in the German Environmental Specimen Bank, with median levels being slightly higher in women than men (Schmidtkunz et al., Reference Schmidtkunz, Kupper, Weber, Leng and Kolossa-Gehriung2020). Due to its potential endocrine-disrupting properties, the European Union strictly regulates BHT in consumer goods that could be incidentally consumed such as mouthwash and toothpaste. Science is ongoing to link BHT exposure to cancer and other health issues (Wang and Kannan, Reference Wang and Kannan2019). Despite this both BHT and BHA are still Generally Recognized as Safe (GRAS) in the United States provided their use complies with FDA regulations. Other food-grade antioxidants include catechin, epicatechin, quercetin and kaempferol (flavonoids), gallic acid, caffeic acid and coumaric acid (phenolic acids) and umbelliferone (coumarine). This illustrates how additives are pervasive and make their way into human bodies.
Industrial grade phenolics
Regarding plastic additive antioxidants, styrenated phenol (Chemical Abstract Service (CAS) number 61788-44-1), referenced under the more general subclass of compounds “sterically hindered phenolic antioxidants,” can be used alone or in combination with other phenolic analogs. Many polymer additive phenolic antioxidants are substituted with carrier or additional functional groups, one example of which is pentaerythritol tetrakis(3,5-di-tert-butyl-4-hydroxyhydrocinnamate), with the synonym pentaerythritol tetrakis (3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate), the structure of which is also shown in Figure 2. Another common industrial antioxidant, used primarily for stabilizing polyamides (nylons), is N,N′-(hexane-1,6-diyl)bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanamide] (trade name Irganox 1,098). In both cases, one can clearly notice the presence of the key phenolic functional groups which are highlighted in blue in Figure 2.
Figure 2. Examples of food grade and phenolic-functionalized industrial antioxidants. The phenolic substructure is highlighted in blue to show the structural similarities between these materials.
Olefin-based antioxidant phenolics are better suited for extreme environments and durability expectations for which plastics are designed and unlike plant-based phenolics, they are less water soluble. These olefin-based molecules deliver one or more phenolic groups when acting as antioxidants, whereby they scavenge free radicals. Free radicals are atoms, molecules or ions with unpaired electrons and are highly reactive. In biological systems, radicals play important roles in functions such as cell signaling and gene expression; however, excessive amounts of radicals can lead to detrimental effects, for example, the degradation of DNA, RNA and other critical biomolecules (Lu et al., Reference Lu, Lin, Yao and Chen2010). The body can combat this through intracellular enzymes, and several natural antioxidants such as Vitamins C and E. It is worth noting that Vitamin E, like several of the food and plastic additive antioxidants mentioned above, contains the key phenolic functional group. In food and plastics, antioxidant additives will react with radical intermediates to prevent degradation. For example, BHT will react with radicals via a hydrogen abstraction process to generate resonance-stabilized phenoxy radicals, effectively reducing the radical’s activity, as shown in Figure 3. The tert-butyl groups provide steric hindrance which further stabilizes the resulting radical. Sterically hindered phenolic antioxidants often take the form of mono-, di- and tri-styrenated phenols.
Figure 3. Reaction of BHT with a radical through a process called hydrogen abstraction, resulting in a resonance-stabilized radical and a stable, neutral molecule.
Tri-styrenated sterically hindered phenolic antioxidants are also problematic, as discussed by Brooke et al. (Reference Brooke, Burns, Cartwright and Pearson2009). Sometimes these styrenated phenols are misleadingly marketed as “pollution free,” given their potential high toxicity to aquatic organisms, as well as bioaccumulation and degradation challenges in some forms, classifying styrenated phenol as very bioaccumulative (vZB), persistent (P) and possibly very persistent (vP), though toxicity criteria are unavailable due to insufficient data and risks to humans have not yet been assessed.
Nonylphenols are used as antioxidants and plasticizers in various resins. Concern about the endocrine-disrupting properties of nonylphenols led to increasing concern for human health, particularly if used in food contact materials. In fact, migration of nonylphenols from bottles into the water they contained was shown for HDPE and PVC bottles and caps (Loyo-Rosales et al., Reference Loyo-Rosales, Rosales-Rivera, Lynch, Rice and Torrents2004).
Methodology
The analysis of additives was methodically structured into three primary phases. Initially, we pinpointed the pertinent class of additives—antioxidants—by selecting those exhibiting a broad variation and employed them across numerous polymer types in significant weight percentages. This initial data was sourced from various industry catalogs and databases. Within the antioxidant category, five subcategories were examined: phenolics, amines, phosphites, thioesters and blends. Phenolics were chosen for in-depth study due to their prevalence, with over 500 phenolic antioxidant products identified in the market.
The next phase involved a detailed examination and categorization of these phenolic antioxidant products based on their chemical structures. By leveraging the International Union of Pure and Applied Chemistry (IUPAC) chemical names, CAS numbers and physical attributes of the products, we analyzed the structural similarities and differences, focusing on the primary chemical mechanisms and functional groups. This analysis revealed that out of the 500+ marketed products, there were only 79 unique and individual CAS numbers. After excluding blends, this number was further narrowed down to 66 unique CAS numbers.
In the final step, we compared these unique phenolic antioxidants, scrutinizing their chemical structures, functional groups and associated hazard levels using Wang and Kannan (Reference Wang and Kannan2019) as a source of data. The comparison aimed to identify structural similarities and assess hazard levels, particularly noting instances where a high-hazard phenolic antioxidant had structural similarities to an unresearched or less-documented phenolic antioxidant. Our final objective was to uncover areas of redundancy within the phenolic antioxidants market, providing a foundation for future research aimed at reducing the number of unnecessary phenolic antioxidants, thereby streamlining the marketplace and enhancing safety and sustainability in polymer production.
Results
A thorough internet search on additive manufacturers globally yielded the total number or global manufacturers and total number of trade names used. These results are tabulated in Table 1, along with the level of concern, which was taken from Weber et al. (Reference Weber, Ashta, Aurisano, Wang, Outters, Miguel, Schlummer, Blepp, Wiesinger, Andrade, Scheringer and Fantke2023). In Table 1 notes significantly the vast number of global trade names for many of the same individual phenolic additives. This marketing tactic creates unnecessary confusion and complexity in attempts to regulate these materials. A structural overview of selected phenolic antioxidants is provided in Figure 4. Note that the phenolic substructure can be found in each example.
Table 1. Duplication of CAS No. for phenolic antioxidant additives
Figure 4. Illustrations of similarity among phenolic antioxidants.
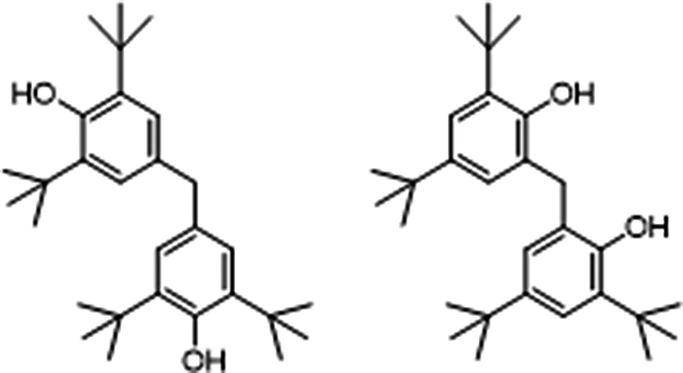
Figure 4 Illustrates some of the similarities noted between molecules with differing CAS numbers. The purpose of these figures is to illustrate the proliferation of structurally similar molecules with only minor differences to the non-phenolic parts of the structure. These illustrations demonstrate the problem with regulating chemical additives by CAS number or trade name, rather than functionality.
In Figure 4(a) Two chemicals, 4,4′-methylenebis(2,6-di-tert-butylphenol) and 2,2′-methylenebis(4,6-di-tert-butylphenol), are displayed. The difference in these chemicals is the location of the tert-butyl and the methylene groups with respect to the alcohol group. In 4,4′-methylenebis(2,6-di-tert-butylphenol) this results in the alcohol being on opposite sides of the structure whereas the 2,2′-methylenebis(4,6-di-tert-butylphenol) have them much closer. In Figure 4(b), the difference between these two chemical species is the length of their carbon chains. For CAS No. 110553-27-0 there is an octyl group off of each sulfur and in CAS No. 110675-26-8 there is a dodecyl group. As shown in Figure 4(c), the difference between the chemicals comes from the propyl linker between the amide nitrogens in CAS #69851-61-2. In CAS #32687-78-8, the nitrogens are bonded directly to each other. As shown in Figure 4(d), the difference in the chemicals here is the nature of the alkyl chain off the hydrocinnamate. In CAS #125643-61-0, it is a straight-chain octyl group whereas in CAS #144429-84-5 it is a branched 2-ethylhexyl group.
In Figure 4(e), the difference in the structures is the substitution of the sulfur that connects the phenols as well as the methyl group substituent. In CAS 90-66-4 the sulfur is at the 2-position with respect to the hydroxyl group and the methyl group is at the para-position, but in CAS 96-69-5 the sulfur is at the 4-position and the methyl group is located at the meta-position. In Figure 4(f), the main difference between the structures lies in the type of alkyl substituents located on the phenolic rings. In CAS 27676-62-6 each phenolic ring has two tert-butyl groups attached at positions 3 and 5 whereas in CAS 40601-76-1 there is only one tert-butyl group at the 4-position and two methyl groups attached at positions 2 and 6. In Figure 4(f), the difference between the two chemicals is once again the nature of the alkyl substituents on the phenolic rings. CAS #88-58-4 has two tert-butyl groups while CAS #79-74-3 has two tert-pentyl groups. Finally, in Figure 4(g), five different substituted phenols are shown. These chemicals are all slightly different from one another insofar as the nature and bonding pattern of the alkyl substituents. CAS #2409-55-4 bears a methyl group and a tert-butyl group. CAS #1879-09-0 has an additional methyl group while CAS #128–37-0 swaps the methyl group in the 6-position for another tert-butyl group. Then in CAS #4130-42-1, the last methyl group (the 4-position) is swapped for an ethyl group. Lastly in CAS #732–26-3, all three groups are tert-butyl.
In summary, as one reviews the different structures presented in Figure 4 there is an obvious, overarching structural theme, that of the phenol group. While some of these phenolic structures bear more elaborate side chains (Figure 4(b)) or are themselves substituents of a larger molecule with a central core (Figure 3(c) and (f)), the majority of these additives are simply phenols with slight variabilities in the nature of the alkyl substituents and/or substitution pattern. As a result of this analysis, each of the examples in Figure 4 warrant further analysis to determine whether these slight changes result in significant functional differences or are simply the result of attempts by manufacturers to repatent a currently marketed additive. The lack of data on the functional differences among the different phenolic antioxidant additives on the market illustrates a critical research need.
Discussion
Potential mitigating strategies
Reducing or eliminating chemical additives in plastics is essential for public health and environmental sustainability. This necessitates concerted efforts from both manufacturers and policymakers. While the Fair Packaging and Labeling Act mandates the disclosure of ingredients in consumer goods, it seldom encompasses chemical additives in plastics, leaving consumers largely uninformed about potential health risks (Fair Packaging and Labeling Act, 1966).
Implementing universal policies that mandate comprehensive labeling of products with detailed information on chemical additives and associated health risks can enhance consumer awareness and reduce exposure to harmful substances. Grouping similar chemical additives like biocides, flame retardants, plasticizers, antioxidants and colorants for regulatory purposes can streamline regulation, improve recyclability and foster transparency. Banning the most harmful and hazardous additives, such as endocrine-disrupting phthalates found in high concentrations in certain plastics like polyvinyl chloride is the best way to significantly mitigate health risks (Hlisníková et al., Reference Hlisníková, Petrovičová, Kolena, Šidlovská and Sirotkin2020). Phasing out harmful chemicals and reevaluating the necessity and design of plastic products are critical steps toward safeguarding public health and the environment.
The United Nations Environment Programme’s “Zero Draft” for the International Legally Binding Agreement to End Plastic Pollution by 2040 (UNEP, 2023a) outlines comprehensive strategies to manage plastic production and the use of chemical additives. It proposes identifying and eliminating hazardous chemicals and polymers, reducing reliance on short-lived and single-use plastics, promoting safer product designs and encouraging the use of alternative materials. Furthermore, the draft addresses the entire lifecycle of plastics, from production to waste management, and emphasizes transparency, just transition, public education, stakeholder engagement and international cooperation to combat plastic pollution effectively. We also emphasize the need to provide sufficient infrastructure and resources for developing countries to manage their plastic waste. Such comprehensive approaches underscore the importance of global collaboration, informed policymaking and adopting sustainable practices to address the challenges posed by plastic additives and pollution, paving the way for a healthier planet and future generations.
Alternative strategies
The complexity of the chemicals used in plastics poses significant challenges, making it crucial to implement strategies to reduce their use and exposure. We argue that one key strategy is simplification to enhance recyclability. A key example to substantiate this is our finding that the numerous chemicals categorized as phenolic antioxidants that perform similar functions can be simplified and reduced. By reducing the variety of these additives, we can streamline recycling processes and mitigate negative human health and environmental impacts. These insights provide valuable information for the current momentum toward effective regulation of plastic additives including global efforts to forge a legally binding agreement to curb plastic pollution by 2040 and extensive resources dedicated by the United Nations (BRS, 2023; UNEP, 2023a, 2023b). In addition, the UNEP Zero Draft (UNEP, 2023a) and subsequent compilations (UNEP, 2024) highlight the high priority on limiting chemical additive categories in ongoing international negotiations.
Another important component of the ongoing negotiations to solve the plastic waste crisis is to decrease the production and use of unnecessary plastics, like single-use items, to combat the growing issue of plastic waste and its effects on human and ecosystem health. We support exploring alternative materials such as paper, glass, cotton and seaweed could pave the way for reusable or more sustainable single-use products. Embracing sustainable innovations and integrating them into daily practices can significantly reduce the detrimental effects of plastics.
Education also plays a vital role in effecting change. The public health impacts of microplastic exposure are still being defined, exposure can include ingestion and its potential health risks, including impacts on future generations (de Witde and Bigaud, Reference Wit and Bigaud2019). Raising awareness about the risks associated with plastics and the global plastic waste crisis is essential for fostering informed consumer choices and advocating for policy reforms. Additionally, the environmental injustices of massive plastic pollution in developing countries where people, most of whom are the poorest of the poor, are living next to mountains of unmanaged plastic waste. As an example, in 2017, the Koshe Landfill located in the capital city of Addis Ababa, Ethiopia, collapsed and killed more than 100 people who lived by the landfill and represented the most impoverished demographic in the city (Ahmed and Fortin, Reference Ahmed and Fortin2017). By enhancing public understanding of the human health and ecosystem threats of plastics and additives, corporations and governments will be held more accountable and potentially forced to mitigate these impacts.
Robust policy interventions will yield the most significant impact. Various global initiatives that ban, restrict or enhance transparency regarding plastic additives are crucial for safeguarding public health and preserving ecosystems. Collaborative efforts between scientists and policymakers, like the European Chemicals Agency’s initiative on plastic additives, are instrumental in developing comprehensive strategies to address the adverse effects of plastic additives, underscoring the importance of informed and decisive action in the fight against plastic pollution.
Conclusions and recommendations
By delving deeper into the various categories of chemical additives, our work aligns with the objectives of the United Nations’ efforts to forge a robust International Agreement to End Plastic Pollution. This endeavor is not only crucial for enhancing the understanding of additive contributions to plastic pollution but also instrumental in shaping policies that aim to eliminate the use of unnecessary and harmful chemicals in plastic production.
We underscore the urgent need to address the redundancy in chemical additives used in plastics, aligning with the UN Sustainable Development Goals, particularly Target 12.4. Our research reveals that 500 products correspond to only 66 unique CAS numbers, falling into 8 main functional groups. This finding suggests a significant potential for simplification, which could greatly benefit environmental and human health. We advocate for stronger regulatory actions to manage chemical additives in plastics, drawing on properly targeted consumer protection requirements as well as parallels with successful environmental policies like the U.S. Clean Air Act and Clean Water Act, and the multilateral environmental agreements like the Montreal Protocol’s ban on substances that deplete the ozone layer by the cooperation from 197 countries, emphasizes the necessity of collaborative efforts to ensure a sustainable future.
Given the grave implications, enhancing legislative efforts to regulate plastic additives is crucial. Our research emphasizes the importance of continuing to investigate various additive categories to remove harmful chemicals from plastic production to protect environmental and human health. In addition to addressing the full lifecycle of plastics, promoting safer product designs, improving waste management practices and enhancing transparency and stakeholder engagement, we argue the reduction or elimination of chemical additives in plastics must be part of the UNEP’s International Legally Binding Agreement to End Plastic Pollution (UNEP 2023a). Our research team plans to continue to review the remaining categories of plastic additives to address the threats posed by chemical exposure in addition to pollution and environmental injustice to safeguard current and future generations to ensure a just transition toward a more sustainable and less polluting plastic industry.








Impact statement
As the world comes together to address plastic waste and pollution, and with the March 2, 2022, United Nations Resolution 5/14 to End Plastic Pollution with an International Legally Binding Instrument by 2024, many strategies are being considered for how to best address this immense problem. We argue that the simplification of the additives and the heterogeneity in the polymer universe is the most promising solution. As plastic formulations have become much more variable, post-consumer plastic becomes increasingly hard to recycle. Simplification of additives and formulations could allow for much more effective recovery methods resulting in a more circular lifecycle for these plastics. There is a significant gap in current policy on addressing these additives and their use in plastics. Based on these foundations, this review identifies possible repetition in the additive class of phenolic antioxidants, addresses possible pathways forward for policymaking and discusses future research that needs to be executed on this topic.
Introduction
History
Since the 1950s, plastics have been mass-produced and sold to consumers around the globe as cheap, easily manufactured alternatives to traditional materials. The inert nature of plastic, which resulted in its use for an increasingly broad range of applications, was not considered hazardous or toxic pursuant to the United States 1960s era environmental laws. In the evolution of the plastic industry, manufacturers sought to enhance specific performance characteristics for different uses. Innovations were made in the form of polymers as well as polymer additives, used in the production of the polymer as well as incorporated in the final product to enhance a broad range of characteristics. Scientists then found that adding chemicals to plastics during the manufacturing process increased their performance to expand their applications. Chemical additives as a revolutionary way to package and produce materials paved the way for widespread use and popularity. Today, chemical additives are fully incorporated into polymer formulations to meet market demand because they can meet product performance specifications and expectations. Chemical additives are now part of nearly all marketable plastics to create durable materials for a low price without consideration of post-use management (Basuhi et al., Reference Basuhi, Moore, Gregory, Kirchain, Gesing and Olivetti2021). The proliferation of chemical additives has made post-consumer plastic increasingly hard to recycle. Simplification of additives and formulations could allow for much more effective recovery methods and a potential reduction in environmental and public health impacts. This review will identify possible repetition in the phenolic antioxidants additives class and address possible pathways for policymaking to simplify usage.
The problems of shedding potential, variability and complexity of plastic products as well as the amount of single-use items interfere with collection and recovery resulting in a planet inundated with plastic pollution. Microplastics containing toxic chemical additives are entering our ecosystems and bodies as they have been found in human blood, lungs and placentas in addition to our waterways, soil, air and rain (Azeem et al., Reference Azeem, Adeel, Ahmad, Shakoor, Jiangcuo, Azeem, Ishfaq, Shakoor, Ayaz, Xu and Rui2021). The severity of the plastic pollution crisis resulted in the March 2, 2022, United Nations Resolution 5/14 to End Plastic Pollution by 2024 (UNEP, 2023b). This provides a step forward for countries and industries to negotiate an end to plastic pollution with an international legally binding agreement to be finalized by 2024, calling for an end to plastic pollution by 2040. Of the primary goals discussed in these negotiations, reducing plastic production through the adoption of use and design standards is a priority to simplify the variety of plastic products for resource recovery.
Recent reports have indicated that over 70,000 different chemical formulations using over 16,000 chemical additives are associated with plastics and plastic production (Wagner et al., Reference Wagner, Monclús, Arp, Groh, Løseth, Muncke, Wang, Wolf and Zimmermann2024; UNEP, 2023c). According to Wagner et al. (Reference Wagner, Monclús, Arp, Groh, Løseth, Muncke, Wang, Wolf and Zimmermann2024), fewer than 6% of additives are subject to regulation, and 4,200 are chemicals of concern since they are persistent, bioaccumulative, mobile or toxic (PBMT). Simplification of the universe of plastic additives is a critical part of addressing this global problem. Recognizing common chemicals, structures and functions to allow identification, narrowing and simplification, free of the obfuscation currently resulting from chemical marketing strategies with duplicative trade names and CAS numbers, can help to achieve this goal. A developing body of data regarding the human health impacts of chemical additives recognizes micro- and nanoplastics as delivery devices for chemical additives when ingested or inhaled or absorbed (Qian et al., Reference Qian, Gao, Lang and Min2024). These additives are then directly delivered into the tissues of the exposed host from the surfaces of the internalized particles - magnifying the toxicity and making chemical additive management, including simplification as well as banning the most hazardous, as key features of effective policy.
1.2 Lack of global regulation
Despite the widespread use of plastics, there continues to be a lack of regulation in plastic production, marketing and waste around the world, including the United States (Nagtzaam and Kourabas, Reference Nagtzaam and Kourabas2023). While monomer and most polymer manufacturing are pervasively regulated in the United States through the environmental authority applicable to chemical manufacturers mitigating pollution from the production process itself, down the value chain, production steps such as molding and extrusion, and final sale of plastic products, are generally not regulated at all. Thus, most of the value chain post initial polymer production remains invisible to regulators, including the incorporation of additives through the value chain (Groh et al., Reference Groh, Backhaus, Carney-Almroth, Geueke, Inostroza, Lennquist, Leslie, Maffini, Slunge, Trasande, Warhurst and Muncke2019). It is inexpensive to produce, loosely regulated and convenient to use. However, plastic production continues to accelerate in the context of inadequate waste management and leakage of plastics and microplastics into the environment through use and waste management failures, has led to global economic, environmental and social consequences, particularly in the developing world.
Chemical additives
Additives generally
In the manufacturing of plastics, chemical additives play a crucial role by enhancing the production process and tailoring the material properties to suit the intended application of the final product. The modification of various physical and chemical properties of plastics aids in the manufacturing processes to ensure the functionality of the final product from both contemporary and historical consumer safety perspectives. More than 16,000 chemicals (Wagner et al., Reference Wagner, Monclús, Arp, Groh, Løseth, Muncke, Wang, Wolf and Zimmermann2024) are associated with plastics and plastic production across a wide range of applications. More than 3,200 monomers, additives, processing aids and non-intentionally added substances are of potential concern due to their hazardous properties including carcinogenicity, mutagenicity, reproductive toxicity, specific target organ toxicity, endocrine disruption, ecotoxicity, bioaccumulation potential, environmental persistence and mobility (Weber et al., Reference Weber, Ashta, Aurisano, Wang, Outters, Miguel, Schlummer, Blepp, Wiesinger, Andrade, Scheringer and Fantke2023). Weber et al.’s (Reference Weber, Ashta, Aurisano, Wang, Outters, Miguel, Schlummer, Blepp, Wiesinger, Andrade, Scheringer and Fantke2023) analysis reveals that out of these 16,000 additives detected in plastics, only about 7,000 have been scrutinized for their hazardous properties. This underscores the diversity and vast number of chemicals used as polymer additives in the plastic industry and raises concerns about the need to understand their hazardous impacts. The absence of stringent governance thus hampers transparency in plastic products, complicating the recovery and recycling processes.
Weber et al. (Reference Weber, Ashta, Aurisano, Wang, Outters, Miguel, Schlummer, Blepp, Wiesinger, Andrade, Scheringer and Fantke2023) also delve into the challenges posed by the patenting and marketing strategies within the industry, where minor variations in additive molecules are patented to create “unique” marketable products. This practice not only stymies the development of safer chemical alternatives but also increases the complexity of the additive landscape, making it difficult to phase out hazardous substances in the absence of stringent regulations. Additionally, this lack of regulatory oversight means consumers have little to no control or even knowledge about the specific additives present in the plastic products they use, contributing to potential health and environmental risks. Addressing these issues requires a multifaceted approach: enhancing regulations on plastic production, improving supply chain transparency and reducing the plethora of chemical additives used. Such measures are essential to diminish the adverse effects of plastic waste and pave the way for a sustainable and healthier environment for future generations.
Shortcomings in additive regulation
The extensive variety in post-use plastics can be traced back to a market-driven approach that encourages the continuous development of new products for patent protection and market differentiation. This has led to a situation where numerous additives, especially those developed for marketing purposes, are now overlapping in function and composition. The current regulatory framework’s inadequacy in addressing the proliferation of polymers and chemical additives contributes to the inefficiency of recycling processes and poses potential toxicity risks to both the environment and human health.
A key example of the current shortcomings related to polymer regulations include FDA regulations in the United States which limit certain additives in plastics intended for food contact, but not to other plastic products. “Globally” specific chemicals, on the other hand, are regulated under various national laws including the US Toxic Substances Control Act (TSCA) and the EU’s Registration, Evaluation, Authorization and Restriction of Chemicals (REACH). However, these regulations often do not extend comprehensively to polymers or their additives, allowing for significant variability in plastic products. In the EU, certain chemicals like styrenated phenols are not classified as persistently bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB), according to the European Chemicals Agency (ECHA, 2023). In contrast, the U.S. TSCA has a “polymer exemption” and focuses on manufacturing processes, excluding high molecular weight polymers and their additives due to their inert nature, from stringent regulatory oversight. In the United States, the use of industrial chemicals is regulated through the Toxic Substances Control Act (TSCA) implemented by the EPA. The rationale is that TSCA includes a “polymer exemption” to extend only to “manufacturing.” As such, polymers with high molecular weight are considered of low concern within TSCA’s scope due to their general inert (nonreactive) nature. Chemical additives used to achieve specific performance characteristics and aid in manufacturing are not considered within the scope of TSCA because when added to the polymer, neither the polymer itself nor the additives are being manufactured. There is no chemical reaction and thus no manufacturing subject to TSCA (Le Roy-Gleizes et al., Reference Le Roy-Gleizes, Messin-Roizard, Ternes, Jaffe, Bergeson, Hester, Porter, Riesel and Main2022).
Overall, the vast array of individually marketed chemicals appears largely redundant and could be substantially consolidated without compromising the functionality or utility of the plastic products, thus promoting a more sustainable and circular approach to plastic use. In this work, we scrutinize the core chemical functions within the category of phenolic antioxidants, identifying the apparent superfluous diversity and redundancies among the additives marketed to the plastic industry. In response, we propose strategies to significantly streamline the assortment of polymers and additives. Such a simplification could facilitate the recycling process and also reduce the leakage of plastics into the environment, thereby curtailing overall plastic pollution. By optimizing and reducing the range of additives, it could be possible to mitigate the environmental impact of plastic waste, reduce the exposure of humans and wildlife to harmful substances and alleviate the economic costs associated with plastic waste-related hazards.
Lowering the apparent unnecessary diversity of plastic additives that complicate resource recovery and result in post-use plastics into a near-infinite mix of waste-like materials will be critical (Landrigan et al., Reference Landrigan, Raps, Cropper, Bald, Brunner, Canonizado, Charles, Chiles, Donohue, Enck, Fenichel, Fleming, Ferrier-Pages, Fordham and Gozt2023). This research in identifying apparent duplication of additives provides important insights for the creation of the UNEA Resolution 5/14 and the forthcoming Plastic Treaty. These global policies seek to introduce new and comprehensive regulations that target the entire lifecycle of polymers and their additives, including post-use recovery. Simplification will significantly impact the heterogeneity of post-use plastics, improving their recyclability and supporting a circular economy. This research will help inform the future regulatory landscape, shaped by these international initiatives and holds the potential to transform the plastic industry by standardizing the use of polymers and additives, thus mitigating the environmental and health impacts of plastic pollution.
Phenolic antioxidant background
Antioxidants are crucial additives in various polymers, serving to prevent oxidative degradation and thus extend the materials’ lifespan, a key factor in promoting circular economies. These additives play a vital role in enhancing the durability and utility of plastic products. However, the extensive array of antioxidants, particularly phenolic antioxidants, raises concerns due to their toxicological effects.
Phenolic antioxidants are part of a broader group of antioxidants that also include phosphites, amines and thioesters (see Figure 1). Despite their known toxicity (Xu et al., Reference Xu, Liu, Hu, Ares, Martínez-Larrañaga, Wang, Martínez, Anadón and Martínez2021; Liu and Mabury, Reference Liu and Mabury2020), phenolic compounds, such as sterically hindered styrenated phenols, are widely used in industry due to their ability to react with free radicals. They function by reacting with free radicals, acting as hydrogen donors, inhibiting enzyme activities through protein interactions and chelating metal ions, thereby thwarting the oxidation process. However, the environmental and health implications of using such toxic substances necessitate a reevaluation of their use in polymer manufacturing.
Figure 1. Antioxidant categories.
Phenolic antioxidants, specifically sterically hindered phenols, are often used with secondary antioxidants to protect plastics in various environmental conditions (Landrigan et al., Reference Landrigan, Raps, Cropper, Bald, Brunner, Canonizado, Charles, Chiles, Donohue, Enck, Fenichel, Fleming, Ferrier-Pages, Fordham and Gozt2023 and Pospisil, Reference Pospisil1998). Phenolic antioxidants used to enhance polymer performance integrate secondary antioxidants like hydrolysis-resistant phosphites or photo-antioxidants (hindered amine stabilizers) and absorbers of ultraviolet light (light stabilizers). These preserve the plastic when exposed to environmental conditions and cause plastic to never fully biodegrade. Manufacturers create these slight molecular variations to circumvent patent laws and regulatory measures that apply to the base chemical molecules, contributing to an unnecessary amount of the types of phenolic antioxidants in the market. This practice complicates intellectual property landscapes and poses challenges for resource recovery from post-use plastics, as the variability in chemical composition complicates the potential for resource recovery from post-use plastics. Unchecked growth and diversification of these additives, particularly phenolic antioxidants, are leading causes of improperly managed plastic waste and a reduction of its ability to be recycled a threat to human and environmental health.
Our research informs strategies for reducing and regulating the chemical substances employed in plastic production. As indicated in Figure 1, commercialized additives are categorized based on their functional roles, providing a structured overview that can assist in guiding future regulatory and reduction efforts in plastic additive use.
Food grade phenolics
For regulatory orientation purposes, there are two types of phenolic antioxidants most often recognized: food grade (preservatives or plastic in contact with food) and durable plastic additives. Food-grade phenolic antioxidants utilized in food for food packaging and pharmaceuticals include butylated hydroxytoluene (BHT) as well as butylated hydroxyanisole (BHA, mixture of isomers). BHT has been found in high concentrations in urine samples in Japan, India and the United States. Its metabolite (BHT-acid) has been detected in 98% of samples in the German Environmental Specimen Bank, with median levels being slightly higher in women than men (Schmidtkunz et al., Reference Schmidtkunz, Kupper, Weber, Leng and Kolossa-Gehriung2020). Due to its potential endocrine-disrupting properties, the European Union strictly regulates BHT in consumer goods that could be incidentally consumed such as mouthwash and toothpaste. Science is ongoing to link BHT exposure to cancer and other health issues (Wang and Kannan, Reference Wang and Kannan2019). Despite this both BHT and BHA are still Generally Recognized as Safe (GRAS) in the United States provided their use complies with FDA regulations. Other food-grade antioxidants include catechin, epicatechin, quercetin and kaempferol (flavonoids), gallic acid, caffeic acid and coumaric acid (phenolic acids) and umbelliferone (coumarine). This illustrates how additives are pervasive and make their way into human bodies.
Industrial grade phenolics
Regarding plastic additive antioxidants, styrenated phenol (Chemical Abstract Service (CAS) number 61788-44-1), referenced under the more general subclass of compounds “sterically hindered phenolic antioxidants,” can be used alone or in combination with other phenolic analogs. Many polymer additive phenolic antioxidants are substituted with carrier or additional functional groups, one example of which is pentaerythritol tetrakis(3,5-di-tert-butyl-4-hydroxyhydrocinnamate), with the synonym pentaerythritol tetrakis (3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate), the structure of which is also shown in Figure 2. Another common industrial antioxidant, used primarily for stabilizing polyamides (nylons), is N,N′-(hexane-1,6-diyl)bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanamide] (trade name Irganox 1,098). In both cases, one can clearly notice the presence of the key phenolic functional groups which are highlighted in blue in Figure 2.
Figure 2. Examples of food grade and phenolic-functionalized industrial antioxidants. The phenolic substructure is highlighted in blue to show the structural similarities between these materials.
Olefin-based antioxidant phenolics are better suited for extreme environments and durability expectations for which plastics are designed and unlike plant-based phenolics, they are less water soluble. These olefin-based molecules deliver one or more phenolic groups when acting as antioxidants, whereby they scavenge free radicals. Free radicals are atoms, molecules or ions with unpaired electrons and are highly reactive. In biological systems, radicals play important roles in functions such as cell signaling and gene expression; however, excessive amounts of radicals can lead to detrimental effects, for example, the degradation of DNA, RNA and other critical biomolecules (Lu et al., Reference Lu, Lin, Yao and Chen2010). The body can combat this through intracellular enzymes, and several natural antioxidants such as Vitamins C and E. It is worth noting that Vitamin E, like several of the food and plastic additive antioxidants mentioned above, contains the key phenolic functional group. In food and plastics, antioxidant additives will react with radical intermediates to prevent degradation. For example, BHT will react with radicals via a hydrogen abstraction process to generate resonance-stabilized phenoxy radicals, effectively reducing the radical’s activity, as shown in Figure 3. The tert-butyl groups provide steric hindrance which further stabilizes the resulting radical. Sterically hindered phenolic antioxidants often take the form of mono-, di- and tri-styrenated phenols.
Figure 3. Reaction of BHT with a radical through a process called hydrogen abstraction, resulting in a resonance-stabilized radical and a stable, neutral molecule.
Tri-styrenated sterically hindered phenolic antioxidants are also problematic, as discussed by Brooke et al. (Reference Brooke, Burns, Cartwright and Pearson2009). Sometimes these styrenated phenols are misleadingly marketed as “pollution free,” given their potential high toxicity to aquatic organisms, as well as bioaccumulation and degradation challenges in some forms, classifying styrenated phenol as very bioaccumulative (vZB), persistent (P) and possibly very persistent (vP), though toxicity criteria are unavailable due to insufficient data and risks to humans have not yet been assessed.
Nonylphenols are used as antioxidants and plasticizers in various resins. Concern about the endocrine-disrupting properties of nonylphenols led to increasing concern for human health, particularly if used in food contact materials. In fact, migration of nonylphenols from bottles into the water they contained was shown for HDPE and PVC bottles and caps (Loyo-Rosales et al., Reference Loyo-Rosales, Rosales-Rivera, Lynch, Rice and Torrents2004).
Methodology
The analysis of additives was methodically structured into three primary phases. Initially, we pinpointed the pertinent class of additives—antioxidants—by selecting those exhibiting a broad variation and employed them across numerous polymer types in significant weight percentages. This initial data was sourced from various industry catalogs and databases. Within the antioxidant category, five subcategories were examined: phenolics, amines, phosphites, thioesters and blends. Phenolics were chosen for in-depth study due to their prevalence, with over 500 phenolic antioxidant products identified in the market.
The next phase involved a detailed examination and categorization of these phenolic antioxidant products based on their chemical structures. By leveraging the International Union of Pure and Applied Chemistry (IUPAC) chemical names, CAS numbers and physical attributes of the products, we analyzed the structural similarities and differences, focusing on the primary chemical mechanisms and functional groups. This analysis revealed that out of the 500+ marketed products, there were only 79 unique and individual CAS numbers. After excluding blends, this number was further narrowed down to 66 unique CAS numbers.
In the final step, we compared these unique phenolic antioxidants, scrutinizing their chemical structures, functional groups and associated hazard levels using Wang and Kannan (Reference Wang and Kannan2019) as a source of data. The comparison aimed to identify structural similarities and assess hazard levels, particularly noting instances where a high-hazard phenolic antioxidant had structural similarities to an unresearched or less-documented phenolic antioxidant. Our final objective was to uncover areas of redundancy within the phenolic antioxidants market, providing a foundation for future research aimed at reducing the number of unnecessary phenolic antioxidants, thereby streamlining the marketplace and enhancing safety and sustainability in polymer production.
Results
A thorough internet search on additive manufacturers globally yielded the total number or global manufacturers and total number of trade names used. These results are tabulated in Table 1, along with the level of concern, which was taken from Weber et al. (Reference Weber, Ashta, Aurisano, Wang, Outters, Miguel, Schlummer, Blepp, Wiesinger, Andrade, Scheringer and Fantke2023). In Table 1 notes significantly the vast number of global trade names for many of the same individual phenolic additives. This marketing tactic creates unnecessary confusion and complexity in attempts to regulate these materials. A structural overview of selected phenolic antioxidants is provided in Figure 4. Note that the phenolic substructure can be found in each example.
Table 1. Duplication of CAS No. for phenolic antioxidant additives
* 91 trade names had no reported CAS Number.
Figure 4. Illustrations of similarity among phenolic antioxidants.
Figure 4 Illustrates some of the similarities noted between molecules with differing CAS numbers. The purpose of these figures is to illustrate the proliferation of structurally similar molecules with only minor differences to the non-phenolic parts of the structure. These illustrations demonstrate the problem with regulating chemical additives by CAS number or trade name, rather than functionality.
In Figure 4(a) Two chemicals, 4,4′-methylenebis(2,6-di-tert-butylphenol) and 2,2′-methylenebis(4,6-di-tert-butylphenol), are displayed. The difference in these chemicals is the location of the tert-butyl and the methylene groups with respect to the alcohol group. In 4,4′-methylenebis(2,6-di-tert-butylphenol) this results in the alcohol being on opposite sides of the structure whereas the 2,2′-methylenebis(4,6-di-tert-butylphenol) have them much closer. In Figure 4(b), the difference between these two chemical species is the length of their carbon chains. For CAS No. 110553-27-0 there is an octyl group off of each sulfur and in CAS No. 110675-26-8 there is a dodecyl group. As shown in Figure 4(c), the difference between the chemicals comes from the propyl linker between the amide nitrogens in CAS #69851-61-2. In CAS #32687-78-8, the nitrogens are bonded directly to each other. As shown in Figure 4(d), the difference in the chemicals here is the nature of the alkyl chain off the hydrocinnamate. In CAS #125643-61-0, it is a straight-chain octyl group whereas in CAS #144429-84-5 it is a branched 2-ethylhexyl group.
In Figure 4(e), the difference in the structures is the substitution of the sulfur that connects the phenols as well as the methyl group substituent. In CAS 90-66-4 the sulfur is at the 2-position with respect to the hydroxyl group and the methyl group is at the para-position, but in CAS 96-69-5 the sulfur is at the 4-position and the methyl group is located at the meta-position. In Figure 4(f), the main difference between the structures lies in the type of alkyl substituents located on the phenolic rings. In CAS 27676-62-6 each phenolic ring has two tert-butyl groups attached at positions 3 and 5 whereas in CAS 40601-76-1 there is only one tert-butyl group at the 4-position and two methyl groups attached at positions 2 and 6. In Figure 4(f), the difference between the two chemicals is once again the nature of the alkyl substituents on the phenolic rings. CAS #88-58-4 has two tert-butyl groups while CAS #79-74-3 has two tert-pentyl groups. Finally, in Figure 4(g), five different substituted phenols are shown. These chemicals are all slightly different from one another insofar as the nature and bonding pattern of the alkyl substituents. CAS #2409-55-4 bears a methyl group and a tert-butyl group. CAS #1879-09-0 has an additional methyl group while CAS #128–37-0 swaps the methyl group in the 6-position for another tert-butyl group. Then in CAS #4130-42-1, the last methyl group (the 4-position) is swapped for an ethyl group. Lastly in CAS #732–26-3, all three groups are tert-butyl.
In summary, as one reviews the different structures presented in Figure 4 there is an obvious, overarching structural theme, that of the phenol group. While some of these phenolic structures bear more elaborate side chains (Figure 4(b)) or are themselves substituents of a larger molecule with a central core (Figure 3(c) and (f)), the majority of these additives are simply phenols with slight variabilities in the nature of the alkyl substituents and/or substitution pattern. As a result of this analysis, each of the examples in Figure 4 warrant further analysis to determine whether these slight changes result in significant functional differences or are simply the result of attempts by manufacturers to repatent a currently marketed additive. The lack of data on the functional differences among the different phenolic antioxidant additives on the market illustrates a critical research need.
Discussion
Potential mitigating strategies
Reducing or eliminating chemical additives in plastics is essential for public health and environmental sustainability. This necessitates concerted efforts from both manufacturers and policymakers. While the Fair Packaging and Labeling Act mandates the disclosure of ingredients in consumer goods, it seldom encompasses chemical additives in plastics, leaving consumers largely uninformed about potential health risks (Fair Packaging and Labeling Act, 1966).
Implementing universal policies that mandate comprehensive labeling of products with detailed information on chemical additives and associated health risks can enhance consumer awareness and reduce exposure to harmful substances. Grouping similar chemical additives like biocides, flame retardants, plasticizers, antioxidants and colorants for regulatory purposes can streamline regulation, improve recyclability and foster transparency. Banning the most harmful and hazardous additives, such as endocrine-disrupting phthalates found in high concentrations in certain plastics like polyvinyl chloride is the best way to significantly mitigate health risks (Hlisníková et al., Reference Hlisníková, Petrovičová, Kolena, Šidlovská and Sirotkin2020). Phasing out harmful chemicals and reevaluating the necessity and design of plastic products are critical steps toward safeguarding public health and the environment.
The United Nations Environment Programme’s “Zero Draft” for the International Legally Binding Agreement to End Plastic Pollution by 2040 (UNEP, 2023a) outlines comprehensive strategies to manage plastic production and the use of chemical additives. It proposes identifying and eliminating hazardous chemicals and polymers, reducing reliance on short-lived and single-use plastics, promoting safer product designs and encouraging the use of alternative materials. Furthermore, the draft addresses the entire lifecycle of plastics, from production to waste management, and emphasizes transparency, just transition, public education, stakeholder engagement and international cooperation to combat plastic pollution effectively. We also emphasize the need to provide sufficient infrastructure and resources for developing countries to manage their plastic waste. Such comprehensive approaches underscore the importance of global collaboration, informed policymaking and adopting sustainable practices to address the challenges posed by plastic additives and pollution, paving the way for a healthier planet and future generations.
Alternative strategies
The complexity of the chemicals used in plastics poses significant challenges, making it crucial to implement strategies to reduce their use and exposure. We argue that one key strategy is simplification to enhance recyclability. A key example to substantiate this is our finding that the numerous chemicals categorized as phenolic antioxidants that perform similar functions can be simplified and reduced. By reducing the variety of these additives, we can streamline recycling processes and mitigate negative human health and environmental impacts. These insights provide valuable information for the current momentum toward effective regulation of plastic additives including global efforts to forge a legally binding agreement to curb plastic pollution by 2040 and extensive resources dedicated by the United Nations (BRS, 2023; UNEP, 2023a, 2023b). In addition, the UNEP Zero Draft (UNEP, 2023a) and subsequent compilations (UNEP, 2024) highlight the high priority on limiting chemical additive categories in ongoing international negotiations.
Another important component of the ongoing negotiations to solve the plastic waste crisis is to decrease the production and use of unnecessary plastics, like single-use items, to combat the growing issue of plastic waste and its effects on human and ecosystem health. We support exploring alternative materials such as paper, glass, cotton and seaweed could pave the way for reusable or more sustainable single-use products. Embracing sustainable innovations and integrating them into daily practices can significantly reduce the detrimental effects of plastics.
Education also plays a vital role in effecting change. The public health impacts of microplastic exposure are still being defined, exposure can include ingestion and its potential health risks, including impacts on future generations (de Witde and Bigaud, Reference Wit and Bigaud2019). Raising awareness about the risks associated with plastics and the global plastic waste crisis is essential for fostering informed consumer choices and advocating for policy reforms. Additionally, the environmental injustices of massive plastic pollution in developing countries where people, most of whom are the poorest of the poor, are living next to mountains of unmanaged plastic waste. As an example, in 2017, the Koshe Landfill located in the capital city of Addis Ababa, Ethiopia, collapsed and killed more than 100 people who lived by the landfill and represented the most impoverished demographic in the city (Ahmed and Fortin, Reference Ahmed and Fortin2017). By enhancing public understanding of the human health and ecosystem threats of plastics and additives, corporations and governments will be held more accountable and potentially forced to mitigate these impacts.
Robust policy interventions will yield the most significant impact. Various global initiatives that ban, restrict or enhance transparency regarding plastic additives are crucial for safeguarding public health and preserving ecosystems. Collaborative efforts between scientists and policymakers, like the European Chemicals Agency’s initiative on plastic additives, are instrumental in developing comprehensive strategies to address the adverse effects of plastic additives, underscoring the importance of informed and decisive action in the fight against plastic pollution.
Conclusions and recommendations
By delving deeper into the various categories of chemical additives, our work aligns with the objectives of the United Nations’ efforts to forge a robust International Agreement to End Plastic Pollution. This endeavor is not only crucial for enhancing the understanding of additive contributions to plastic pollution but also instrumental in shaping policies that aim to eliminate the use of unnecessary and harmful chemicals in plastic production.
We underscore the urgent need to address the redundancy in chemical additives used in plastics, aligning with the UN Sustainable Development Goals, particularly Target 12.4. Our research reveals that 500 products correspond to only 66 unique CAS numbers, falling into 8 main functional groups. This finding suggests a significant potential for simplification, which could greatly benefit environmental and human health. We advocate for stronger regulatory actions to manage chemical additives in plastics, drawing on properly targeted consumer protection requirements as well as parallels with successful environmental policies like the U.S. Clean Air Act and Clean Water Act, and the multilateral environmental agreements like the Montreal Protocol’s ban on substances that deplete the ozone layer by the cooperation from 197 countries, emphasizes the necessity of collaborative efforts to ensure a sustainable future.
Given the grave implications, enhancing legislative efforts to regulate plastic additives is crucial. Our research emphasizes the importance of continuing to investigate various additive categories to remove harmful chemicals from plastic production to protect environmental and human health. In addition to addressing the full lifecycle of plastics, promoting safer product designs, improving waste management practices and enhancing transparency and stakeholder engagement, we argue the reduction or elimination of chemical additives in plastics must be part of the UNEP’s International Legally Binding Agreement to End Plastic Pollution (UNEP 2023a). Our research team plans to continue to review the remaining categories of plastic additives to address the threats posed by chemical exposure in addition to pollution and environmental injustice to safeguard current and future generations to ensure a just transition toward a more sustainable and less polluting plastic industry.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/plc.2024.36.
Author contribution
Derek Orndoff – Responsible for primary research, writing and editing of the manuscript. Sohail Lone – Responsible for primary research and manuscript editing. Betsy Beymer-Farris – Responsible for writing, review and editing the manuscript, especially sections covering social aspects. Morgan Wood – Responsible for writing, review and editing the manuscript, especially sections covering social aspects. Jennifer Sadler – Responsible for writing, review and editing the manuscript, especially sections covering social aspects. Mary Ellen Ternes – Responsible for writing and editing the manuscript, especially sections covering policy. Tracy Hester – Responsible for writing and editing the manuscript, especially sections covering policy. Kevin M. Miller – Responsible for writing, review and editing the manuscript, especially sections covering organic chemistry. Jeffrey Seay – Corresponding author. Responsible for research supervision, primary writing, review, and editing of the manuscript.
Financial support
No financial support was provided for the preparation of this manuscript.
Competing interest
The authors have no conflicts of interest to report.