Berry seeds, a by-product of juice fabrication with promising health benefits, are currently an unutilised nutritional resource. The seeds are rich in PUFA, tocopherols, fibre and contain polyphenols(Reference Helbig, Böhm and Wagner1, Reference Kapasakalidis, Rastall and Gordon2). Most of these ingredients are known to be associated with oxidation processes.
Oxidation of cell compounds such as DNA, lipids or proteins is described as a promotion of atherogenesis and carcinogenesis(Reference Nakabeppu, Sakumi and Sakamoto3, Reference Willcox, Curb and Rodriguez4). Evidence for the correlation between oxidative DNA damage and human degenerative diseases such as CHD has previously been provided(Reference Kasai and Kawai5). It is presumed that the prevalence of CHD and also of carcinogenesis is associated with tocopherol in serum or with tocopherol intake, although results are ambivalent. However, it has been shown that CHD patients are characterised by a high serum α-:γ-tocopherol ratio(Reference Öhrvall, Sundlöf and Vessby6) and decreased γ-tocopherol serum concentration compared with controls(Reference Kontush, Spranger and Reich7). In addition, the deficiency of α-tocopherol in lipoproteins was not associated with atherosclerosis(Reference Niu, Zammit and Upston8). The results of the few studies dealing with fruit or berry consumption show inconclusive evidence of the effects on DNA damage in leucocytes(Reference Freese9). The findings of the Nurses' Health Study deny an association of vitamin E supplementation and the risk of colon cancer(Reference Wu, Willett and Chan10).
Studies concerning berry consumption basically focused on whole berries or berry juices and extracts, rather than on the seeds and their potential(Reference Kahle, Kraus and Scheppach11–Reference Kay and Holub13). Thus, the present human intervention study was conducted in order to correct omission. Different markers were used to evaluate the physiological and health-beneficial impact of blackcurrant press residue (PR). The most likely effects are probably due to the antioxidant potential of substances in PR. The matrices stool, urine and serum were included. Stool is a very complex and individual matrix, with various substance groups that may affect the antioxidant capacity. Examining one single substance is not always consequential, since synergies and correlations between different substances are very likely. The influence of PR on antioxidant capacity of stool can be evaluated by means of the aromatic hydroxylation of salicylic acid(Reference Owen, Spiegelhalder and Bartsch14). Characterising the DNA-damaging potential in stool can be performed using microgel electrophoresis with faecal water-incubated cells(Reference Collins15, Reference Glei, Habermann and Osswald16). Genotoxicity of faecal water indicates the exposition of colon cells towards genotoxic compounds that lead to increased DNA damage and a risk of colon cancer.
Further, in the present study the influence of PR on oxidative DNA damage of the whole body was measured using the biomarker 8-oxo-2′-deoxyguanosine (8-oxodG) in urine. 8-OxodG, a product of DNA repair excreted in urine, is generated by oxidative stress causing the transformation of the nucleoside guanosine(Reference Cooke, Rozalski and Dove17). The excreted amount of 8-oxodG correlates with the extent of oxidative stress in an individual. Oxidative stress occurs as a result of different factors such as the presence of disease(Reference Cooke, Evans and Dizdaroglu18), smoking(Reference Priemé, Loft and Klarlund19), physical exercise in untrained subjects(Reference Asami, Hirano and Yamaguchi20) and enzymic activities such as that of glutathione-S-transferase(Reference Poulsen, Loft and Prieme21). On the other hand, there are factors that can contribute to decrease the oxidative stress. These factors include a high consumption of fruits and vegetables containing significant concentrations of polyphenols and other substances(Reference Ottaviani, Carrasquedo and Keen22), moderate physical activity(Reference Huang, Appel and Croft23) or the use of hormonal contraceptives(Reference Viña, Sastre and Pallardó24).
The aim of the study was to test whether serum total tocopherol concentrations and parameters linked to oxidative stress are influenced by the intake of blackcurrant PR in human subjects. Secondarily, effects of lifestyle parameters that are associated with oxidative stress such as smoking or the use of hormonal contraceptives were also considered.
Experimental methods
Test substance, diets and experimental design
Berry seed PR contains significant amounts of tocopherols (α, 6·56 μmol/100 g; β, 0·32 μmol/100 g; γ, 14·3 μmol/100 g; δ, 0·44 μmol/100 g) as well as γ-tocotrienol (0·29 μmol/100 g) according to Helbig et al. (Reference Helbig, Böhm and Wagner1). The PR has a gallic acid equivalent of 0·17 g/100 g, and a trolox equivalent of 2·82 mmol/100 g in a hydrophilic- and 67·2 μmol/100 g in a lipophilic solvent. As described previously, the PR contains 25·7 g fat, 48·2 g fibre, 22·5 g crude protein and 19 mg Fe/100 g(Reference Helbig, Böhm and Wagner1). Inositol hexaphosphate (phytic acid) concentration of the applied PR was 0·63 mmol/100 g and no intermediate inositol phosphates were quantified. β-Carotene and plant sterols were not present in significant amounts(Reference Helbig, Böhm and Wagner1). The concentrations of yeasts, mould and bacteria measured (D Helbig, unpublished results) were all below the upper levels considered safe for ground grain products with reference to the values released by the German Society of Hygiene and Microbiology (Regulation (EU) 2073/2005).
Blackcurrant seeds were pressed, ground and sieved to obtain an acceptable mouth feeling. The PR was baked into bread at a maximum dose of 8 % that still allowed the correct preparation of the dough. No significant losses of tocopherol were verified by the baking process. The daily amount of 250 g test bread that had to be consumed by the participants contained 20 g PR. The control bread contained no PR, but was otherwise identical to the test bread. Here too, an amount of 250 g/d had to be ingested. The bread was made using wheat flour, rye flour, crushed rye grain, oat and flax seeds. The total tocopherol concentrations in the control and test bread were 3·86 and 6·32 μmol/100 g, respectively. The sum of inositol hexa-, penta-, tetra- and triphosphate concentration was 0·50 mmol/100 g for control bread and 0·89 mmol/100 g for test bread.
The intervention study comprised three periods: a 5 d baseline period consisting of a normal diet without intervention (period 1; PI) for obtaining baseline data. It was followed by a 4-week period with an intake of control bread (period 2; PII) which was then substituted by test bread in the next 4-week intervention period (period 3; PIII). During baseline and the last 5 d of PII and PIII a standardised diet was administered. At the same time, complete stool and 24 h urine were collected for 3 d as well as one blood sample that was taken by authorised nurses in the morning after overnight fasting. During the standardised diets the consumption of the respective bread was continued. Participants were instructed to eat normally, not to go on a diet and to try to include the bread in their eating habits without increasing their daily energy intake during the study. Except for the control and test bread in the respective periods, the 5 d standardised diet packages were identical between the three study periods and daily included one warm meal, fruits, vegetables, dairy products, bread-spreads and drinks. Participants were free to consume everything provided in the food package, though they were advised to resemble their food intake at each standardised diet period. The uneaten daily portions had to be returned for quantification of the actual food consumption.
If not described differently, nutrient intake was calculated using PRODI® 5.4 software (Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, Germany). At the start and the end of the study, a bioelectrical impedance analysis (BIA) was conducted for purposes of monitoring the physical constitution of the participants (BIA 2000-C; Data Input, Darmstadt, Germany).
Subjects
Initially, we recruited twenty voluntary female smokers and twenty female non-smokers via placards and newspaper advertisements. Subsequently, four participants from the group of smokers dropped out of the study for individual reasons (for example, illness, lack of time). Thus, results presented are based on a total of thirty-six participants; twenty non-smokers and sixteen smokers. Smokers were defined by a consumption of at least five cigarettes/d. The mean cigarette consumption was 9 (sd 5; range 3–20) cigarettes/d. Participants were allowed to smoke before blood withdrawals. Moreover, twenty-two participants (ten smokers and twelve non-smokers) used a hormonal contraceptive, whereas fourteen participants (six smokers, eight non-smokers) did not. Criteria for including subjects into the study were not doing serious sports, no diagnosed diseases, and no use of nutritional supplements 2 weeks before and during the study. Participants were aged 24 (sd 3; range18–33) years. The mean BMI constituted 23·3 (sd 3·7; range 17·9–32·8) kg/m2 with a body size of 170 (sd 7; range 157–186) cm and a body weight of 67 (sd 11; range 53–97) kg. All test individuals were informed as regards the purpose, the course and their responsibilities during the study before they gave their informed written consent. The study was approved by the Ethics Committee of the Medical Faculty of the Friedrich Schiller University of Jena, Germany (approval no. 1485-01/05).
Preparation of human samples
The complete, fresh stool was collected in plastic bags, transported to the laboratory and immediately stored at − 20°C. After receiving all stool samples, stool was defrosted, homogenised and subsequently lyophilised, separately for each participant and period. For gaining faecal water, samples of the homogenised, defrosted stool were weighed into polyethylene tubes (Beckman Coulter, Munich, Germany), and centrifuged for 4 h at 21000 rpm and 4°C (Beckman J2-21). The supernatant fraction representing the faecal water was stored in cryogenic tubes (Roth, Karlsruhe, Germany) at − 20°C. Samples of urine taken from the daily samples were stored at − 20°C. The defrosted samples were mixed according to the proportion of the daily excreted urine volume, separately for each participant and period. Blood was collected into serum tubes (BD Vacutainer Systems, Heidelberg, Germany) and centrifuged for 20 min at 4000 rpm. Serum obtained was frozen at − 20°C until analysis.
Tocopherols
According to Kuhnt et al. lyophilised food and stool were added with ascorbic acid (Fluka, Buchs, Switzerland) and saponified(Reference Kuhnt, Wagner and Kraft25). Extraction was carried out using n-hexane containing 2,6-di-tert-butyl-p-kresol (BHT) (VWR, Leuven, Belgium and Fluka, Buchs, Switzerland, respectively). With each batch executed, a reference milk powder of defined tocopherol concentration was analysed in parallel (BCR-421, Report EUR 18320 EN; Promochem, Wesel, Germany). Serum samples were prepared with ethanol–BHT solution (ethanol: Roth, Karlsruhe, Germany), shaken and extracted using n-hexane containing BHT. Extracts were measured by means of HPLC–fluorescence (Shimadzu, Tokyo, Japan; Nucleosil 100 NH2 column, 250 × 4 mm; Macherey & Nagel, Dueren, Germany). Analysed α-, β-, γ- and δ-tocopherol concentrations were summed up to total tocopherol (tocopherol standards; Calbiochem, Darmstadt, Germany).
Hydroxylation products of salicylic acid in stool (antioxidant capacity of stool)
According to the method of Owen et al. phosphate buffer (100 mm, KH2PO4 and K2HPO4; Merck, Darmstadt, Germany) was prepared with EDTA (500 μm; Roth, Karlsruhe, Germany), FeCl3 (iron 50 μm; Merck, Darmstadt, Germany) and salicylic acid (2 mm; Merck, Darmstadt, Germany) in HPLC-grade water (pH 6·5)(Reference Owen, Spiegelhalder and Bartsch14). Fe3+ and EDTA are added to the test system to support the generation of hydroxyl radicals. These radicals oxidise the salicylic acid to 2,3- and 2,5-dihydroxy benzoic acid (DHBA) and catechol which can all be quantified. Lyophilised stool (0·1 g) was mixed with 10 ml phosphate buffer and incubated in an orbital shaker at 200 rpm for 18 h at 37°C. After centrifugation (4000 rpm, 40 min, Rotina 46; Hettich Zentrifugen, Tuttlingen, Germany), the supernatant fraction was filtered using a sterile, pyrogen-free filter (0·2 μm, Chromafil; Macherey & Nagel, Dueren, Germany) and measured by means of HPLC-UV (diphenols 325 nm, catechol 278 nm; column: Hypersil C18 ODS II, 250 × 4 mm; Agilent, Waldbronn, Germany). Unlike Owen et al. for gradient elution the mobile phase consisted of methanol (VWR, Leuven, Belgium) and ammonium acetate buffer (Merck, Darmstadt, Germany; pH 3·6 with acetic acid, Roth, Karlsruhe, Germany)(Reference Owen, Spiegelhalder and Bartsch14). Standard curves obtained from catechol, 2,5-DHBA and 2,3-DHBA (Sigma-Aldrich, Steinheim, Germany) were utilised for calculating the results.
Iron parameters
For the determination of serum Fe parameters, the Abbott Architect c8000 analyser and the corresponding test kits were used according to the manufacturer's instructions (Abbott, Wiesbaden, Germany and Abbott Laboratories, Baar, Switzerland). The ferritin assay was performed with the chemiluminescent microparticle immunoassay. Serum Fe was analysed colorimetrically by means of the Fe assay; the transferrin was analysed using an immunoturbidimetric assay.
Fe concentrations in test and control bread as well as in the stool were analysed via ICP-OES (Spectroflame, Spectro, Kleve, Germany) according to DIN 38406 (E22). The stool sample was reduced to ash and mixed with water and HCl (Roth, Karlsruhe, Germany), then heated and filtrated. Before measuring, the sample was acidified with ultrapure HNO3 (Roth, Karlsruhe, Germany) to a final concentration of 2 % of the sample and diluted at a ratio of 1:2 with ultrapure water. Unlike described in the used DIN method, the calibration range was adjusted according to the expected concentrations (0·005–5·0 mg/l, multi-element standard Merck IV; Merck, Darmstadt, Germany).
The Fe intake from the foods during the standardised diet that were consumed besides the breads was calculated using the PRODI® 5.4 software (Wissenschaftliche Verlagsgesellschaft mbH).
Cyto- and genotoxicity of faecal water
Cyto- and genotoxicity of faecal water were tested using HT29 clone cells treated with 10 % faecal water for 30 min at 37°C. The determination of cell viability before and after incubation with faecal water was accomplished via the Trypan Blue exclusion(Reference Sandström26). Analyses on genotoxicity of the faecal water were performed using single-cell micro-gel-electrophoresis (comet assay) measuring tail intensity according to Oberreuther-Moschner et al. (Reference Oberreuther-Moschner, Jahreis and Rechkemmer27).
Urinary 8-oxo-2′-deoxyguanosine
The urine samples were purified on a C18 EC column (Macherey & Nagel, Dueren, Germany). 8-OxodG was eluted with methanol (VWR, Leuven, Belgium), concentrated under a stream of N2, resolved in HPLC-grade water and measured by means of HPLC–electrochemical detection (Shimadzu, Tokyo, Japan; column: Hypersil C18 ODS II, 250 × 4 mm; Agilent, Waldbronn, Germany) according to Kuhnt et al. (Reference Kuhnt, Wagner and Kraft25). For calibration purposes, 8-oxodG was purchased from Sigma-Aldrich (Munich, Germany).
F2-isoprostanes and prostaglandin F2α metabolite
8-Iso-PGF2α, a standard marker of oxidative stress, and 15-keto-dihydro-PGF2α, a reliable marker of inflammatory response formed through the cyclo-oxygenase pathway, were analysed in urine samples using two separate RIA, as described previously(Reference Basu28, Reference Basu29).
Statistical analysis
Statistical analysis was carried out using the SPSS 14.0 software package (SPSS, Inc., Chicago, IL, USA). Results were tested for normal distribution by means of the Kolmogorov–Smirnov test. If not described differently, significance was checked using repeated-measures ANOVA. The one-sided Mann–Whitney U test was used to compare subgroups. For correlation analysis, the Pearson's correlation coefficient was determined. Values were referred to as significant at P < 0·05.
Results
The physical constitution of subjects measured assessed via bioelectrical impedance analysis remained unchanged during the study. The mean energy intake with the standardised diets was 7350 (sd 811) kJ/d (baseline). This value significantly increased during control (8000 (sd 1063) kJ/d) and test bread consumption (7771 (sd 1185) kJ/d; P < 0·05). The results were only split into subgroups of smokers, non-smokers as well as users and non-users of hormonal contraceptives when regarded as necessary, i.e. if there were any significant changes apparent between the periods.
Tocopherols
The total tocopherol intake at baseline, control and intervention was 42·0 (sd 5·9), 43·3 (sd 6·7) and 51·4 (sd 8·2) μmol/d, respectively (P < 0·001). The total tocopherol intake was the same for smokers and non-smokers.
Faecal total tocopherol excretion was significantly increased from control to intervention (P = 0·046) (Table 1). With respect to the subgroups, the total tocopherol excretion in smokers significantly increased from baseline and control to intervention (P < 0·05). At intervention, excretion significantly differed between smokers and non-smokers (P = 0·008).
Table 1 Changes in serum and stool total tocopherol concentrations after control bread consumption and intervention with blackcurrant seed press residue-enriched test bread considering smoking habits
(Mean values and standard deviations)
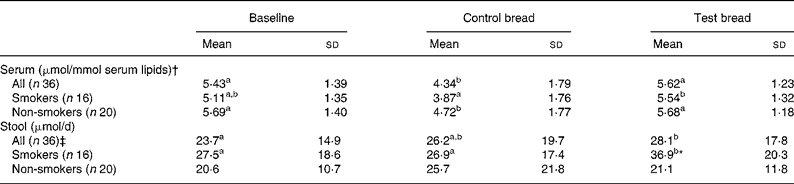
a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* Mean value was significantly different from that of the non-smokers (P ≤ 0·05).
† Tocopherols were adjusted to serum lipids (TAG + cholesterol).
‡ Values were not distributed normally; significance was calculated by means of the Wilcoxon test.
For the whole study group, lipid-adjusted total tocopherol concentration in serum was significantly decreased at control compared with baseline and intervention period (P < 0·01). Similar relationships between the periods were present in non-smokers (P < 0·05) and in smokers, though in smokers the decrease from baseline to control was a tendency only (P = 0·057).
Diphenols in stool
Although the analyses were carried out on lyophilised stool samples, the results were extrapolated to the daily excreted amount of fresh matter, because of the increased daily stool mass and DM (D Helbig, unpublished results) at intervention (Table 2). The generation of the diphenols 2,3- and 2,5-DHBA in faeces was significantly increased from baseline to intervention (P < 0·01). The subgroup of non-smokers showed no changes regarding the diphenols, whereas in smokers concentrations during intervention increased compared with baseline and control (P < 0·05).
Table 2 Concentration (mmol/d fresh matter) of 2,3- and 2,5-dihydroxy benzoic acid (DHBA) in stool, generated from salicylic acid in vitro after control bread consumption and intervention with blackcurrant seed press residue-enriched test bread considering smoking habits
(Mean values and standard deviations)
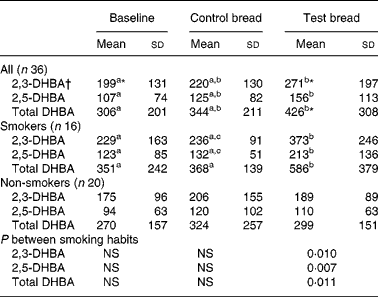
a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* Mean value was marginally significantly different from that for the control bread (P ≤ 0·1).
† Values were not distributed normally; significance was calculated by means of the Wilcoxon test.
Blind tests (without faecal samples), tests with additional antioxidants (gallic acid, trolox; 0·04 m being the tenfold of the expected molarity of each diphenol) and tests without Fe and EDTA were also conducted. Diphenols were not detected in any of the incubation batches. The salicylic acid peak in the faecal chromatogram confirmed that salicylic acid was present in abundance. In contrast, catechol could not be detected. The correlation determined between total DHBA (based on 100 mg fresh matter) and 8-oxodG was r 0·328 during PI (P = 0·051), r 0·354 during PII (P = 0·034) and r 0·324 during PIII (P = 0·054). Further, total DHBA (extrapolated to daily excreted fresh matter) and faecal Fe excretion were highly correlated (r 0·707 for PI (P < 0·001), r 0·568 for PII (P < 0·001) and r 0·744 for PIII (P < 0·001)). Total DHBA (extrapolated to daily excreted fresh matter) and faecal water genotoxicity were negatively associated (r − 0·273 for PI (P = 0·107), r − 0·313 for PII (P = 0·063) and r − 0·134 for PIII (P = 0·434)).
Iron parameters
Fe intake increased steadily from baseline to control and again to intervention (P < 0·001) (Table 3). Similarly, Fe excretion increased with control and test bread v. baseline (P < 0·01). However, serum ferritin concentration was significantly lower at intervention than at baseline (P < 0·05). Excluding anaemic participants, ferritin concentrations were 27·0 (sd 17·2) μg/l (PI, n 31), 27·8 (sd 16·1) μg/l (PII, n 26) and 22·2 (sd 11·6) μg/l (PIII, n 30) with P < 0·05 for both, baseline and control compared with intervention (Wilcoxon test).
Table 3 Iron intake, iron excretion, and iron serum parameters after control bread consumption and intervention with blackcurrant seed press residue-enriched test bread
(Mean values and standard deviations for thirty-six subjects)
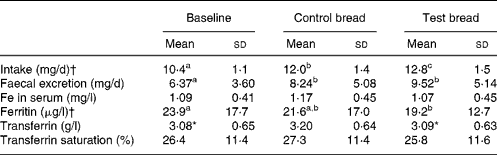
a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05). Results without superscripts in a row had no significant differences.
* Mean value was marginally significantly different from that for the control bread (P ≤ 0·1).
† Intake achieved using PRODI® 5.4 software (Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, Germany).
Cyto- and genotoxicity of faecal water
To evaluate genotoxicity, HT29 clone cell viability was assessed before and after incubation with faecal water. Viability at intervention was significantly reduced compared with baseline and control (P < 0·05) (Table 4). In non-smokers, cell viability was significantly decreased from baseline to intervention (P < 0·001). There were no changes for smokers and non-smokers between the control and intervention periods. After cell incubation with faecal water, tail intensity significantly increased during intervention compared with both baseline and control (P < 0·05). In smokers, no changes occurred throughout the study periods. In non-smokers, the tail intensity increased with intervention v. baseline period (P < 0·05). Cell viability and genotoxicity of faecal water correlated negatively at the control and intervention periods (PI: r − 0·208 (P = 0·228); PII: r − 0·764 (P < 0·001); PIII: r − 0·301 (P = 0·075)).
Table 4 Cyto- and genotoxicity of faecal water (comet assay, given in fluorescence tail intensity) and 8-oxo-2′-deoxyguanosine (8-oxodG) in urine after control bread consumption and intervention with blackcurrant seed press residue-enriched test bread considering smoking habits
(Mean values and standard deviations)
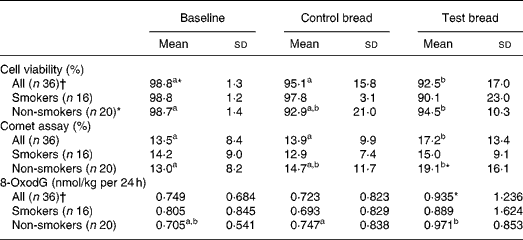
a,b Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05).
* Mean value was marginally significantly different from that for the control bread (P ≤ 0·1).
† Values were not distributed normally; significance was calculated by means of the Wilcoxon test.
Urinary 8-oxo-2′-deoxyguanosine
The daily urinary 8-oxodG excretion was not affected by the control bread compared with baseline, both in the whole study population and in the subgroups of smokers and non-smokers (Table 4). There was a tendency in the daily excretion towards an increase for the whole study population after test bread compared with control (P < 0·1) and it was significantly increased in non-smokers (P < 0·05). There were no significant changes between the periods within the subgroups of users and non-users of hormonal contraceptives, although the 8-oxodG excretion at intervention was significantly higher in the non-users (1·21 nmol/kg per 24 h) compared with the users (0·76 nmol/kg per 24 h) (P = 0·004).
F2-isoprostanes and prostaglandin F2α metabolite
Urinary 8-iso-PGF2α and 15-keto-dihydro-PGF2α were only analysed in samples collected from the control and intervention periods to follow both oxidative stress and the inflammatory response (Table 5). No significant changes were found for the whole study population, and for the smokers and non-smokers separated. Interestingly, the excretion of urinary 8-iso-PGF2α in volunteers using no hormonal contraceptive was significantly increased by the intervention.
Table 5 8-Iso-PGF2α and 15-keto-dihydro-PGF2α excretion (nmol/24 h) in urine after control bread consumption and intervention with blackcurrant seed press residue-enriched test bread considering smoking habits and the use of hormonal contraceptives
(Mean values and standard deviations)
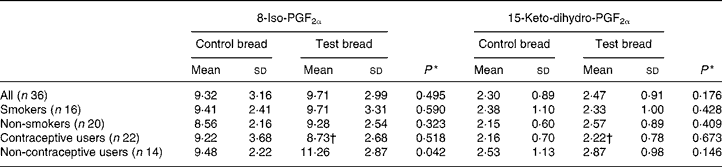
* Between control and test bread for each prostane.
† Mean value was significantly different from that for the non-users of hormonal contraceptives (P ≤ 0·05).
Discussion
The consumption of bread enriched with blackcurrant PR produced effects on biomarkers related to oxidative stress via different PR components, for example, phytic acid, PUFA or Fe. The decreased serum total tocopherol achieved with control bread compared with baseline was possibly a result of replacing the normally consumed foods in favour of the control bread. Though tocopherol intake was increased with the control bread, suggesting an increase of serum tocopherol, single isomers may have been less absorbable, resulting in a decrease. This effect was reduced by the PR intake at intervention and serum total tocopherol concentration increased compared with the control bread. Serum total tocopherol concentrations showed no differences between smokers and non-smokers at any period, confirming the findings of Kontush et al. (Reference Kontush, Spranger and Reich7).
Blackcurrant berries and seeds contain significant quantities of proanthocyanidins(Reference Wu, Gu and Prior30, Reference Lu and Foo31). Because dietary polyphenols are virtually all degraded to monomers by normal gut bacteria, the phenol concentration in faecal water is high enough to bring about physiological changes in the stool(Reference Jenner, Rafter and Halliwell32). Due to low absorption rates of polyphenols, effects in the gastrointestinal tract might be even higher than in the body itself. Cyanidin-3-glycoside, a chief component of blackcurrant anthocyanins, was protective towards H2O2-induced DNA strand breaks in colonocytes in vitro, but failed at physiological dose rates in rats in vivo (Reference Lu and Foo31, Reference Duthie, Gardner and Morrice33). Further, phytic acid from bread and also from PR might improve the antioxidant properties of both the breads.
Contrary to results achieved from studies on polyphenols and phytic acid, the combination of bread and PR increased the formation of diphenols compared with control bread, implying a decreased capacity in the scavenging of hydroxyl radicals with the consumption of the PR bread. Generation of the diphenols 2,3- and 2,5-DHBA remained unaffected by the bread itself. Smoking increased the generation of both diphenols at intervention compared with control and baseline. Thus, the faeces from smokers showed a reduced antioxidant capacity when PR bread was consumed. In addition to the polyphenols, the consumption of tocopherols should also point to an increase in the faecal antioxidant capacity rather than a decrease. However, it has been reported that tocopherols do not effectively scavenge the ubiquitously occurring hydroxyl radicals(Reference Wang and Jiao34).
An analysis of the components of blackcurrant PR exhibited the presence of significant amounts of Fe in the PR(Reference Helbig, Böhm and Wagner1). Therefore, the Fe parameters were also taken into consideration in this human intervention study. Most of the PR Fe probably came from the mill during crushing. Steel dust is a catalyst for Fenton-like oxidations(Reference Lee, Kim and Chang35). The increased Fe intake was not reflected in an improved Fe status of the participants. In fact, most of the Fe remained in the intestinal lumen, able to act as a damaging factor(Reference Knöbel, Weise and Glei36). The concentration of the Fe-storage protein ferritin decreased with consumption of both the control and test bread, indicating an Fe deficiency in the body. An impairment of Fe status has also been described in women consuming wheat bread(Reference Bach Kristensen, Tetens and Alstrup Jørgensen37). The phytic acid in the grain potentially binds faecal Fe and decreases absorption. The ferritin decrease became even more pronounced with the test bread when excluding the anaemic participants, possibly because PR additionally contained phytic acid. In a prior study with a similar study design, 10 or 20 g PR were eaten daily in yoghurt for 4 consecutive weeks (D Helbig, unpublished results). Here, ferritin concentrations also decreased significantly from baseline (pure yoghurt) to both intervention periods, suggesting that ferritin decrease is most probably due to the PR.
Phytic acid prevents the generation of hydroxyl radical by chelating Fe(Reference Graf, Mahoney and Bryant38). Nevertheless, it appears questionable whether phytic acid has an impact on antioxidant capacity in the in vitro test system. First, phytic acid reduces the hydroxyl radical formation by trapping the Fe, but does not scavenge the hydroxyl radicals produced in the test system. However, phytic acid can have an impact on antioxidant conditions in vivo due to the absence of EDTA(Reference Owen, Wimonwatwatee and Spiegelhalder39). Second, when EDTA, which is a stronger Fe scavenger than phytic acid, is added to the test system for in vitro aromatic hydroxylation, the Fe gets detracted from the phytic acid(Reference Owen, Spiegelhalder and Bartsch14). Contrary to phytic acid, EDTA is a promoter of hydroxyl radical generation because of its ability to form a free Fe coordination site, thus contributing to the oxidation processes(Reference Graf, Mahoney and Bryant38, Reference McCord and Day40). It is generally assumed that radicals in stool and oxidation of membrane lipids and its chain-reaction products lead to in vivo DNA damage. The correlations determined between DHBA and 8-oxodG and faecal Fe excretion confirm this assumption. The high Fe content of the digest possibly increased the formation of hydroxyl radicals in vivo, exhausting faecal antioxidants, leading to a reduced antioxidant capacity of the stool. A study using dimethylsulfoxide to scavenge faecal hydroxyl radicals reported that a diet rich in fat and meat and low in fibre showed a thirteen times increased hydroxyl radical production than with a low-fat, vegetarian high-fibre diet(Reference Erhardt, Lim and Bode41). In fact, there resulted a 42 % increased faecal Fe concentration in the high-meat diet. The concentration of faecal Fe is of no consequence in the test system utilising aromatic hydroxylation, since Fe is added in abundance to provoke the radical generation. However, faecal Fe might have exhausted faecal antioxidants in the body already.
It was shown that Fe2+/3+ also accounts for DNA damage(Reference Rieger, Parlesak and Pool-Zobel42). In the present study, faecal Fe concentrations remained unchanged between the control and test breads, but faecal water genotoxicity increased not with the control bread, but only with the test bread. Further, faecal water genotoxicity and faecal Fe concentration did not correlate at test bread consumption, suggesting that increased genotoxicity at intervention was caused by something other than the Fe.
In the present study, faecal water genotoxicity and generated DHBA were negatively associated. Consequently, if both DHBA and faecal water genotoxicity were increased by the intervention, the causes might be attributed to different factors in each case. Further, it has been under discussion that the intake of PUFA promotes DNA damage in the body(Reference Jenkinson, Collins and Duthie43). This is a relevant factor since PR contains high amounts of these fatty acids (79 % PUFA of total fatty acid methyl esters(Reference Helbig, Böhm and Wagner1)) and 4 g PUFA were ingested daily with the test bread. Heat applied during seed processing and bread baking may have induced lipid peroxidation leading to the increased oxidative DNA damage by the faecal water at PR consumption. No correlations could be found between the genotoxicity of faecal water and the total tocopherol concentration of faeces. Testing on the cell viability supports the results of the comet assay. Cell viability decreased after incubating cells with faecal water from test bread consumption and both parameters correlated negatively at the control and intervention periods. Bile acids, not analysed here, are other possible contributors to elevated reactive oxygen species generation and DNA hydroxylation(Reference Allgayer, Kolb and Stuber44). In addition, fibre has a bile acid-binding capacity(Reference Buhman, Furumoto and Donkin45). Consequently, a change in bile acid concentration and pattern at control and intervention is probably due to increased daily fibre intakes compared with baseline (31 % bread, 54 % bread+PR).
Correlation values of serum total tocopherol or total tocopherol intake in the present study confirm that dietary consumption of antioxidants, particularly of vitamin E, is associated with little or no effects on the urine 8-oxodG concentration(Reference Poulsen, Loft and Prieme21, Reference Duthie, Gardner and Morrice33, Reference Loft, Vistisen and Ewertz46). Gackowski et al. described hydroxyl radicals as being the major source of 8-oxodG formation(Reference Gackowski, Ciecierski and Jawieñ47). To attack DNA, these radicals need to be in its immediate vicinity. However, lipophilic vitamins are not usually located near DNA molecules. Non-smokers excreted significantly more 8-oxodG during PR consumption than during intake of control bread. Noticeably, smokers had a two-fold higher standard deviation than non-smokers. Curiously, smokers showed particularly individual reactions to the substances in PR. Generally, 8-oxodG excretion increased with PR bread, although the extent of the increase was influenced by different factors such as smoking habits, the use of hormonal contraceptives, and other, unidentified parameters. There was no correlation between 8-oxodG excretion and faecal water genotoxicity. These results support another study that showed no relationship between faecal water genotoxicity and lymphocyte DNA damage using the comet assay(Reference Glei, Habermann and Osswald16). An Fe-dependent increase of 8-oxodG in lymphocytes was found with acute Fe load(Reference Lucesoli, Caligiuri and Roberti48). In the present study, none of the measured serum or faecal Fe parameters was clearly associated with the urinary 8-oxodG excretion. Thus, the PR component responsible for the increase of urinary 8-oxodG concentration could not be identified.
No differences were shown for prostane excretion in the total study population. However, different outcomes were apparent when looking at the individual subgroups. While hormonal contraceptives obviously protected a PR-induced non-enzymic lipid peroxidation, smoking, contrary to several reports, caused no significant impact(Reference Yin, Gao, Tai and Murphey49). There was no association found between prostane excretions and other tested parameters. England et al. found an association between plasma isoprostane 8-epi-PGF2α and lymphocyte 8-oxodG(Reference England, Beatty and Rehman50). In the present study, only when urinary 8-oxodG and prostanes were analysed in the defined subgroups, strong correlations appeared with PR consumption between 8-oxodG and the oxidative stress marker 8-iso-PGF2α excretion for non-smokers and non-users of hormonal contraceptives. Noticeably, these are the subgroups not influenced by special oxidants or antioxidants, in contrast to smokers (increased reactive oxygen species) or users of hormonal contraceptives (antioxidant protection). Further, it was shown that a PUFA-rich diet may increase plasma PGF2α concentration(Reference Weinberg, VanderWerken and Anderson51). Thus, the 8-iso-PGF2α increase might be attributed to the increased PUFA intake with the PR bread.
Conclusion
Testing complex food matrices makes it difficult to arrive at clear and distinct outcomes compared with single intervention substances. Consequently, effects due to an intervention with berry PR cannot be accredited to one single substance. Nevertheless, the following statements can be presumed: PR consumption resulted in increased faecal Fe concentrations, decreased faecal antioxidant capacity and increased urine 8-oxodG excretion. The increased genotoxicity of faecal water and partially increased prostane excretion could be due to the higher PUFA intake. PR phytic acid is assumed to account for the decrease in serum ferritin. Furthermore, factors associated with lifestyle, such as smoking habits or the use of hormonal contraceptives, have an impact on the correlations described above. In summary, consuming blackcurrant PR-enriched bread for 4 weeks has adverse effects on the antioxidant status in the body, as serum and stool total tocopherol concentrations were increased. The antioxidant properties of tocopherols had no effect on the measured biomarkers associated with oxidative stress.
Acknowledgements
We would like to thank the BMBF (Federal Ministry of Education and Research) which provided financial support for this project in the Rephyna® group. We would also like to thank the IGV GmbH Bergholz-Rehbrücke for preparing the test materials, the Fraunhofer IVV Freising for carrying out the microbiological testing, Claudia Nitsch for performing the tocopherol and 8-oxodG analyses and finally the laboratories of Ralf Greiner (Max Rubner-Institut, Federal Research Institute of Nutrition and Food, Karlsruhe, Germany) for operating the inositol phosphate analyses.
D. H. was responsible for the supervision of the human study, sample handling and analyses conducted; A. W. supported tocopherol, 8-oxodG and DHBA analyses; M. G. supported the analyses of cell viability and the performance of the comet assay; S. B. was responsible for the prostane analyses; R. S. was responsible for obtaining funding and supported the statistical evaluation; D. H. and G. J. were responsible for data interpretation; all authors were responsible for critical revision of the manuscript.
None of the authors had any personal or financial conflict of interest.







