INTRODUCTION
Streptococcus pneumoniae causes serious disease world-wide, especially in infants, young children and the elderly. Pneumococcal conjugate vaccines have become available in recent years and several countries have included these in their routine immunization schedule [Reference Whitney1–Reference Lopalco5]. Data on the incidence of invasive pneumococcal disease (IPD) and of pneumococcal serotype coverage by pneumococcal vaccines is crucial for this decision. Some countries have implemented surveillance of IPD prior to the introduction of routine conjugate pneumococcal vaccination. These countries will be able to assess the impact and costs vs. benefits of pneumococcal vaccination.
In the Czech Republic pneumococcal 23-valent polysaccharide vaccine is recommended for patients with underlying diseases and the elderly population. In 2001 a 7-valent pneumococcal conjugate vaccine (Prevenar) was registered and is recommended for children aged ⩽5 years with an increased risk of pneumococcal infection due to underlying diseases. However, mass vaccination has not yet been introduced.
Within the Czech Republic's list of notifiable diseases, the only IPD syndrome that is included is pneumococcal meningitis. Pneumococcal infection without bacteraemia, constituting a major disease burden both in children and the elderly [Reference Grijalva6], is not notifiable. To better estimate the serotype-specific incidence of IPD, laboratory surveillance of IPD was established in 2000.
We present results of routine notification of pneumococcal meningitis and of laboratory surveillance. Incidence, mortality and serotype-specific vaccine coverage afforded by current and forthcoming pneumococcal vaccines in the Czech Republic are presented for infants, children, adults and the elderly to inform decision making regarding vaccination.
METHODS
Cases of IPD in the Czech Republic were identified using two data sources: mandatory notifications and a dedicated laboratory surveillance system. Pneumococcal meningitis, including deaths, has been notifiable in the Czech Republic since 1994. Electronic data entry transfer from district to national level and data analysis are carried out using the Epidat program. This is part of the National Health Information System and is governed by legislation on the protection of personal data in health information systems. Epidat was created using programs of the Epi-Info system [Reference Dean7], version 3.3.2. Data are entered continuously and are exported to the National Reference Centre for Analysis of Epidemiological Data once a week. The total and age group-specific annual incidence of pneumococcal meningitis, the mortality due to pneumococcal meningitis and case-fatality ratio were calculated. The annual census population data were used as denominator data. Results for the period 1997–2006 are presented.
The National Reference Laboratory (NRL) for Streptococci and Enterococci, which is also a WHO Collaborating Centre for Reference and Research on Streptococci receives pneumococcal isolates from the entire country for confirmation and serotyping. From 2000 onwards, the NRL requested local laboratories to send all pneumococcal isolates from blood and normally sterile sites and to provide information about the size of their catchment population. The total and age-specific incidence of IPD was calculated. Results for the period 2000–2006 are presented.
Case definitions
IPD is defined by the isolation of S. pneumoniae from blood, cerebrospinal fluid, or other normally sterile sites. Only one isolate per disease episode has been included in the analysis. Pneumococcal meningitis cases, being a part of total IPD, are defined by the isolation of S. pneumoniae from cerebrospinal fluid.
Strain identification and serotyping
Each isolate was identified by standard methods and confirmed as S. pneumoniae by optochin sensitivity and bile solubility. S. pneumoniae isolates were serotyped at the NRL by the Quellung reaction using antisera (Statens Serum Institut, Copenhagen, Denmark).
Vaccine coverage
Total and age group-specific coverage afforded by different vaccines was calculated as the percentage of serotypes included in current and forthcoming pneumococcal vaccines in isolates obtained from IPD cases in the Czech Republic during the study period (2000–2006). The vaccine serotypes are presented in Table 1.
Table 1. Serotypes included in current and forthcoming pneumococcal vaccines
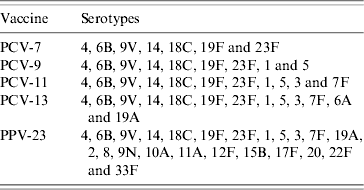
PCV-7, 7-valent pneumococcal conjugate vaccine; PCV-9, 9-valent pneumococcal conjugate vaccine; PCV-11, 11-valent pneumococcal conjugate vaccine; PCV-13, 13-valent pneumococcal conjugate vaccine; PPV-23, 23-valent pneumococcal polysaccharide vaccine.
Statistical analysis
The χ2 test was used to compare the coverage by pneumococcal vaccines in the age groups <5 years and ⩾40 years. Yates' correction was used for χ2 and P values [Reference Dean7]. The trend of incidence and of case fatality was assessed by quadratic regression analysis.
RESULTS
Routine notification of pneumococcal meningitis, 1997–2006
The annual incidence of notified pneumococcal meningitis varied between 0·4 and 0·6/100 000 in the period 1997–2006 (Table 2). The annual incidence of pneumococcal meningitis remained stable during the study period (P=0·317). The age group-specific incidence was highest in the <1-year-olds (4·3/100 000), followed by age groups 1–4 years, 40–64 years and ⩾65 years (Fig. 1). The case-fatality ratio of pneumococcal meningitis in this period was 13·7%. The age-specific case-fatality ratio was highest in the ⩾65 years age group (24%), and lowest in the 5–14 years group (0%). Among children aged <5 years, the case-fatality ratio was <5% [<1 year (2·3%), 1–4 years (4·6%)]. In age groups aged >14 years, case fatality increased with age. This increase was statistically significant (P<0·041).
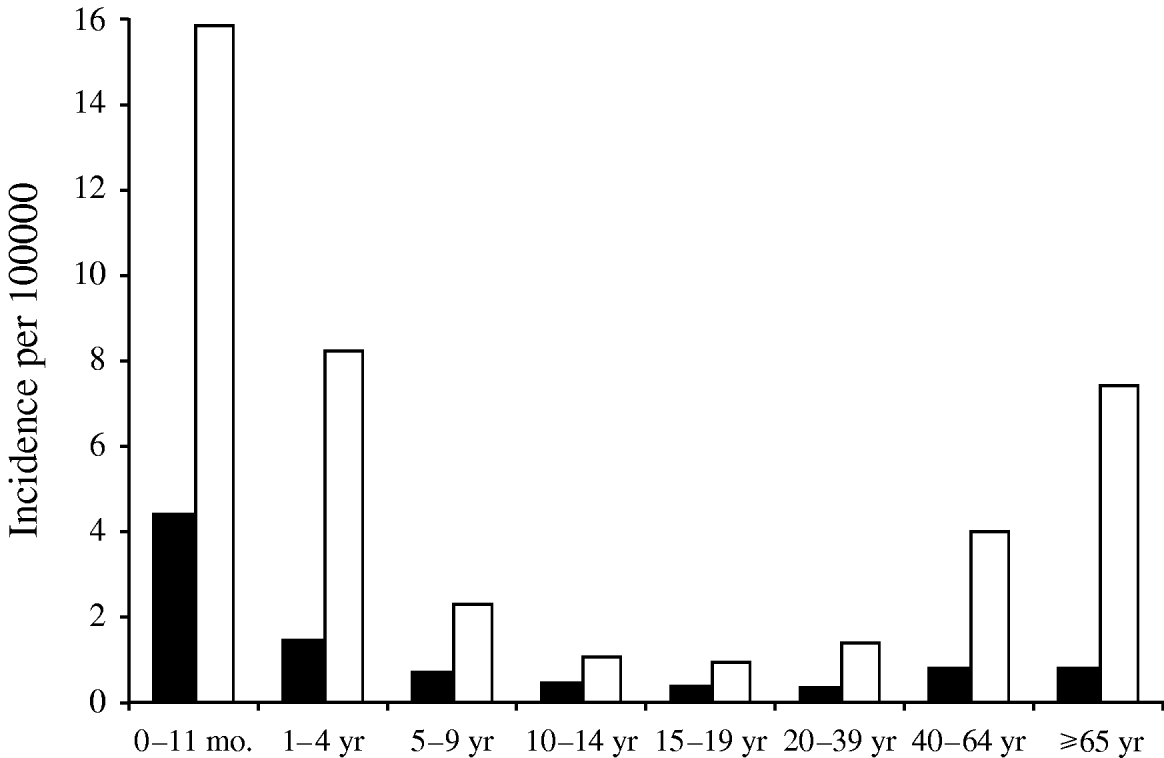
Fig. 1. Pneumococcal meningitis (■) and invasive pneumococcal disease (□), Czech Republic, 2000–2006, age-specific incidence. Pneumococcal meningitis=routine notification data (n=411); invasive pneumococcal meningitis=laboratory-based data (n=1236).
Table 2. Number of cases and incidence per 100 000 population of pneumococcal meningitis and invasive pneumococcal disease by year and age group, the Czech Republic, 1997–2006 (routine notification, laboratory-based data)

RN, Routine notification; LB, laboratory based; PM, pneumococcal meningitis; IPD, invasive pneumococcal disease; n.a., data not available.
Laboratory surveillance of IPD, 2000–2006
Fifty-one local laboratories participated in this prospective study. The population of their catchment area was 5 694 000, representing 55·6% of the Czech Republic's population. In total 1236 S. pneumoniae isolates from IPD cases were received during the study period. Of these, 375 were isolated from cerebrospinal fluid, 829 from blood and 32 from other normally sterile body sites. The annual incidence of IPD ranged from 2·3 to 4·3/100 000. There was no trend in IPD incidence over time during the study period (Table 2). Estimates of the number of IPD cases for the entire country varied from 235 to 437 per year. The age-specific incidence of IPD was highest among children aged <1 year (15·7/100 000), followed by age groups 1–4 years (8·2), 40–64 years (4·0) and those aged ⩾65 years (7·3). The incidence of IPD in children aged <5 years was 9·7/100 000. The annual laboratory-based incidence of pneumococcal meningitis varied between 0·6 and 1·6/100 000 population during the study period. The age group-specific incidence was highest in the <1-year-olds (ranging from 2·1 to 18·4/100 000 population).
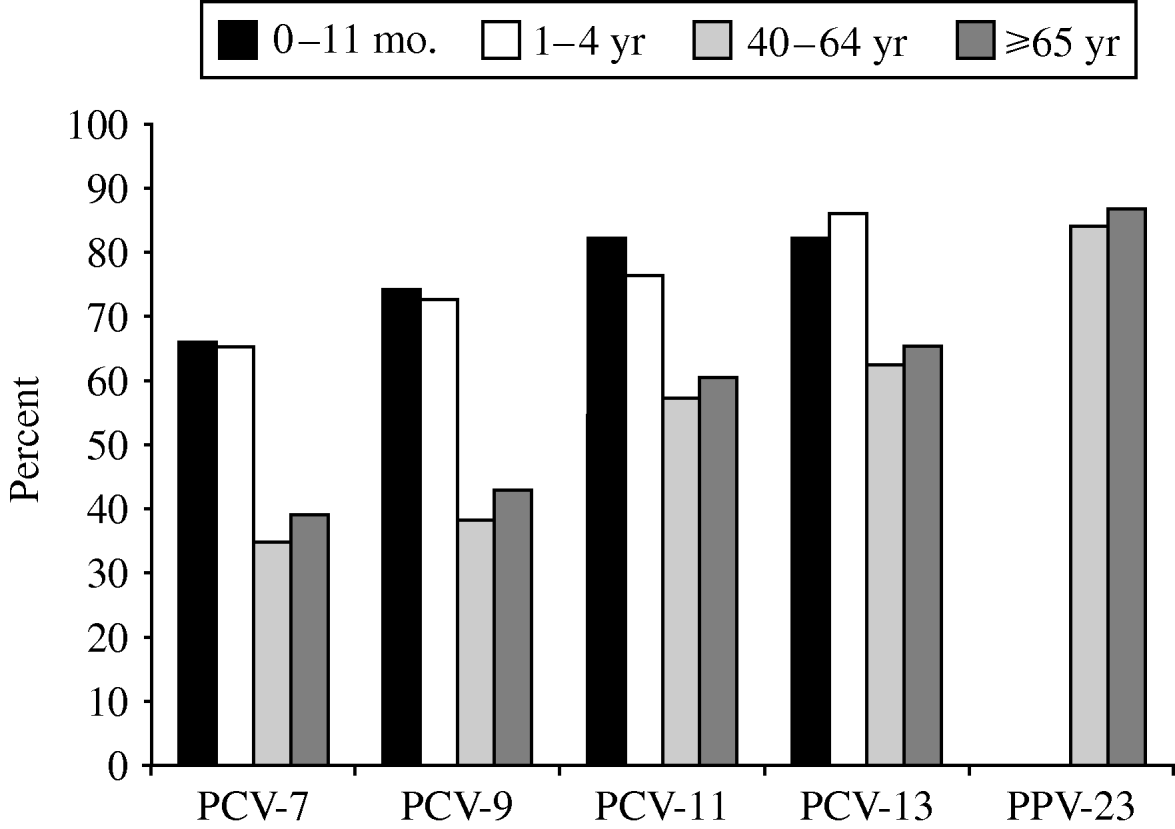
Fig. 2. Age-specific vaccine coverage of serotypes causing invasive pneumococcal disease, Czech Republic, 2000–2006 (laboratory-based data) (n=1000). PCV-7, heptavalent pneumococcal conjugate vaccine; PCV-9, 9-valent pneumococcal conjugate vaccine; PCV-11, 11-valent pneumococcal conjugate vaccine; PCV-13, 13-valent pneumococcal conjugate vaccine; PPV-23, 23-valent pneumococcal polysaccharide vaccine.
Among the 1236 isolates, 52 serotypes were identified (Table 3). Eight isolates could not be typed (two were non-typable and six did not grow). The most frequent serotypes were 3, 4, 14, 8 and 19F. There were differences in the serotype distribution between age groups. The most frequent serotypes by age group were: <1-year-old (6B and 19F), 1–4 years (14, 6B and 23F), 40–64 years (3, 8 and 4), and ⩾65 years (3, 4, 9N and 14). The distribution of serotypes in the total population and individual age groups was stable during the study period.
Table 3. Serotypes of S. pneumoniae causing IPD in the Czech Republic, 2000–2006 (laboratory-based data) and coverage by pneumococcal vaccines
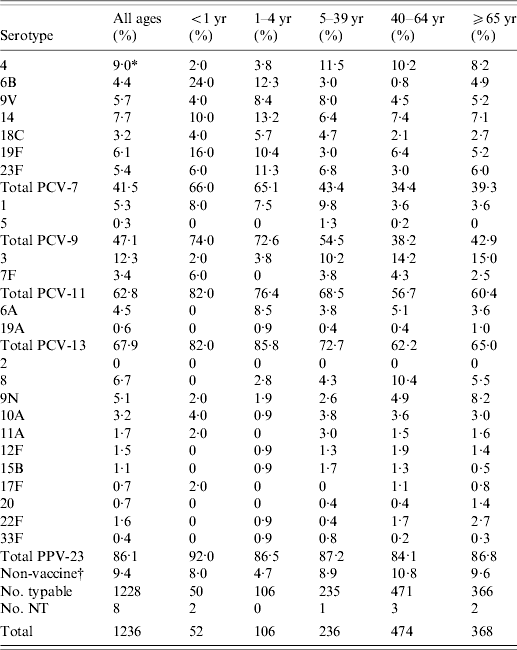
* The percentage of serotype in individual age category was calculated using number of typeable isolates as the denominator; NT, non-typable and/or not typed; PCV-7, 7-valent pneumococcal conjugate vaccine; PCV-9, 9-valent pneumococcal conjugate vaccine; PCV-11, 11-valent pneumococcal conjugate vaccine; PCV-13, 13-valent pneumococcal conjugate vaccine; PPV-23, 23-valent pneumococcal polysaccharide vaccine.
† Non-vaccine serotypes: 7A, 7C, 10F, 13, 15A, 15C, 15F, 16F, 17A, 18A, 18B, 18F, 21, 23A, 24F, 25F, 28A, 28F, 29, 31, 34, 35A, 35B, 35F, 36, 37, 38, 39, 42.
The coverage of serotypes in all age groups was 41·5% for PCV-7, 47·1% for PCV-9, 62·8% for PCV-11, 67·9% for PCV-13 and 86·1% for PPV-23. The coverage of serotypes causing IPD was different between infants/children and adults/elderly (Fig. 2). Coverage by PCV-7 was 34·4% in the 40–64 years age group and 39·3% in the ⩾65 years group, while it was 66·0% in <1-year-olds and 65·1% in the 1–4 years group. Larger differences were found in coverage by PCV-9, PCV-11 and PCV-13 (Table 3). The differences disappeared with PPV-23 for which the coverage was very similar in all age groups. There was no trend during the study period.
DISCUSSION
We present the first report of IPD incidence in the Czech Republic based on two data sources. As routine notification data cover pneumococcal meningitis only, we also used laboratory-based surveillance data. The differences we found in incidence of pneumococcal meningitis between results from laboratory-based surveillance vs. routine notification could be due to underreporting in the latter. As zero reporting is not required in routine notification, it is unclear whether the 50% of non-reporting localities had no cases or simply did not report any cases. For laboratory-based surveillance the denominator was only the population of the catchment area of laboratories actively participating in the study. However, participating laboratories were spread over the entire country and generated data expected to be representative of the Czech Republic. Implementation of active surveillance of IPD covering the whole country is essential in order to assess the epidemiological situation and recommend an appropriate vaccination strategy [Reference Pebody4–Reference Grijalva6, Reference Vergison8].
The incidence of IPD in children aged <5 years in Europe before the introduction of vaccination varied widely from 2·8 to 42/100 000 [Reference Dagan9–Reference Hausdorff19]. The annual incidence of IPD found by laboratory-based surveillance in the Czech Republic in the period 2000–2006 ranged from 2·3 to 4·3/100 000. Age-specific incidence of IPD was highest in children aged <1 year, reaching 15·7, followed by the 1–4 years group (8·2). IPD incidence in children aged <5 years was 9·7, which is consistent with the pre-vaccination incidence in Switzerland (7·6) [Reference Venetz, Schopfer and Muhlemann16] and Germany (8·9) [Reference Von Kries11]. In some countries the pre-vaccination incidence was higher: 14·5 in England and Wales [Reference Miller12], 24·2 in Finland [Reference Escola15], 42 in Israel [Reference Dagan9], while in others it was lower: 2·8–6·3 in Italy [Reference D'Ancona20] and 5·8 in The Netherlands [Reference Spanjaard10]. Our results might be biased by the following: (1) in the laboratory-based surveillance presented in this paper only 55·6% of the total population of the Czech Republic was covered by the participating laboratories; (2) blood-culturing rate is low in the Czech Republic. The geographical and temporal variations of incidence could be explained by real differences in the epidemiological situation in individual countries, however, under-reporting and variability in blood-culturing rates play a significant role [Reference Hausdorff19, Reference D'Ancona20].
In our study, similar age distribution of pneumococcal meningitis was obtained from both data sources, with the highest incidence observed in the lowest age group of <1 year. However, higher numbers of cases resulted from laboratory-based data compared to routine notification.
Differences in serotype distribution in individual age groups were found, with the differences between infants/children vs. adults/elderly especially evident. This finding is consistent with the situation described in other European countries and is a basis for the assessment of coverage by various pneumococcal vaccines and a prediction of their benefit in individual age groups, and consequently for recommendation of vaccination strategy [Reference Ispahani17, Reference Kaltoft, Zeuthen and Konradsen18, Reference Hausdorff21–Reference Von Kries26]. IPD incidence found in age groups 5–9, 10–14, 15–19 and 20–39 years was low and the total number of isolates was only 236. For this reason, the percentage of serotypes for ages 5–39 years was calculated. It was assessed as remaining at a medium level, compared to infants/children vs. adults/elderly.
The coverage of serotypes causing IPD in children aged <5 years in the Czech Republic in 2000–2006 during the study period which was pre-vaccination, was 64·5% for PCV-7 and increased with increasing valency of pneumococcal vaccines, to 83·5% for PCV-13. The coverage by higher-valent pneumococcal vaccines increases less in infants/children than in adults/elderly and it will be necessary to calculate the cost-benefit of higher-valent pneumococcal vaccines carefully, when they are available for age groups showing the highest incidence, i.e. for infants/children. It is desirable to include pneumococcal conjugate vaccines in the routine vaccination scheme of infants in the Czech Republic [27].
An indirect (herd) effect of pneumococcal conjugate vaccine on adults/elderly IPD incidence occurred after its introduction into the routine vaccination scheme of infants [Reference Hammitt28, Reference Isaacman29]. This herd effect is explained by a reduction in the circulation of the pneumococci among vaccinated infants/children and the consequent decrease of IPD incidence in non-vaccinated adults/elderly. It could be expected that this herd effect of pneumococcal conjugate vaccine will also occur in the Czech Republic. Continuing surveillance of IPD will be necessary for the assessment of the effectiveness of PCV-7 and the evaluation of eventual serotype replacement, i.e. emergence of non-vaccine serotypes after introduction of routine PCV vaccination, as was reported in the United States [Reference Byington30, Reference Gonzalez31].
ACKNOWLEDGEMENTS
Part of this work was supported by research grant NR/8770-3 of the Internal Grant Agency of the Ministry of Health of the Czech Republic. We thank all the microbiological laboratories for sending the isolates to the National Reference Laboratory for typing. We thank Dr K. Jolley (University of Oxford, UK) for editing the text.
DECLARATION OF INTEREST
None.







