Alterations in the maternal metabolic milieu during pregnancy influence the development and functional maturation of β-cells (Metzger, Reference Metzger1991; Reusens & Remacle, Reference Reusens and Remacle2006). Protein restriction during pregnancy and/or lactation in rats produces a variety of structural changes in the pancreas of offspring, including a reduction in islet size (Arantes et al. Reference Arantes, Teixeira, Reis, Latorraca, Leite, Carneiro, Yamada and Boschero2002) and in the vascular bed (Snoeck al. Reference Snoeck, Remacle, Reusens and Hoet1990). In addition, there is a persistent alteration in β-cell mass (Swenne et al. Reference Swenne, Crace and Milner1987; Snoeck et al. Reference Snoeck, Remacle, Reusens and Hoet1990; Metzger, Reference Metzger1991; Garofano et al. Reference Garofano, Czernichow and Breant2000) and insulin secretion (Latorraca et al. Reference Latorraca, Carneiro, Mello and Boschero1999). Impaired insulin secretion in protein-restricted rats has also been related to alterations in different stages of the secretory mechanism itself (Rasschaert et al. Reference Rasschaert, Reusens, Dahri, Sener, Remacle, Hoet and Malaisse1995; Latorraca et al. Reference Latorraca, Reis, Carneiro, Mello, Velloso, Saad and Boschero1998; Barbosa et al. Reference Barbosa, Capito, Kogod and Thams2002).
Rats fed a low protein (LP) diet have elevated serum NEFA levels (Latorraca et al. Reference Latorraca, Reis, Carneiro, Mello, Velloso, Saad and Boschero1998) and several studies have shown that long-term exposure to high NEFA levels contributes to the inhibition of glucose-induced insulin secretion (Zhou & Grill, Reference Zhou and Grill1994). The detrimental effects of elevated NEFA levels on β-cell function have been demonstrated in several animal models of diabetes, including Fa/Fa and db/db mice (Alstrup et al. Reference Alstrup, Brock and Hermansen2004), cell lineages (Ritz-Laser et al. Reference Ritz-Laser, Meda, Constant, Klages, Charollais, Morales, Magnan, Kartoza and Philippe1999) and in the fasting state (Zhou et al. Reference Zhou, Priestman, Randle and Grill1996). The mechanisms involved include the stimulation of fatty acid oxidation with consequent inhibition of glucose metabolism (Randle et al. Reference Randle, Kerbey and Espina1988), decreased pancreatic duodenal homeobox-1 (PDX-1) mRNA and protein levels and reduced binding activity to the cognate cis-regulatory elements of the insulin gene promoter (Gremlich et al. Reference Gremlich, Bonny, Waeber and Thorens1997).
PDX-1 plays an important role in the development of the pancreas and in islet cell ontogeny (Jonsson et al. Reference Jonsson, Carlsson, Edlund and Edlund1994; Guz et al. Reference Guz, Montminy, Stein, Leonard, Garner, Wright and Teitelman1995) and is an essential component of the mechanisms by which glucose controls insulin promoter activity and endogenous insulin mRNA levels. The DNA-binding activity of PDX-1 is modulated by glucose via a pathway involving stress-activated protein kinase 2 (SAPK2) (Macfarlane et al. Reference Macfarlane, Smith, James, Clifton, Doza, Cohen and Docherty1997). SAPK2, also known as RK/p38, is a member of an expanding family of kinases related to mitogen-activated protein kinase and is activated in response to stimuli such as heat, osmotic shock, UV light and DNA-damaging reagents, as well as by proinflammatory cytokines produced under stress (Cohen, Reference Cohen1997). The activation of SAPK2 leads to the phosphorylation of an inactive, 31 kDa, cytoplasmic form of PDX-1 that is transformed into a 46 kDa active form and translocated to the nucleus (Macfarlane et al. Reference Macfarlane, McKinnon, Felton-Edkins, Cragg, James and Docherty1999). Currently, it is unclear whether NEFA alters the SAPK2 pathway.
We have shown elsewhere (Arantes et al. Reference Arantes, Teixeira, Reis, Latorraca, Leite, Carneiro, Yamada and Boschero2002) that a 6 % protein diet during gestation and lactation reduced PDX-1 protein expression but did not modify the mRNA expression in pancreatic islets from weaned rats. Since high concentrations of NEFA in the culture medium also reduced PDX-1 protein expression in control islets (Gremlich et al. Reference Gremlich, Bonny, Waeber and Thorens1997), we decided to evaluate the effect of palmitate on glucose-induced insulin secretion and glucose metabolism; PDX-1, insulin and p38/SAPK2 expression in islets derived from LP rats.
Experimental methods
Animals
The animal experiments were approved by the institutional Committee for Ethics in Animal Experimentation (UNICAMP). Virgin female Wistar rats (80–90 d old) were obtained from the breeding colony at UNICAMP. Mating was done by housing females with males overnight and pregnancy was confirmed by examining vaginal smears for the presence of sperm. Pregnant females were separated at random and fed an isoenergetic diet containing 6 % protein (LP diet) or 17 % protein (control diet) from day 1 of pregnancy until the end of lactation. The protein in the LP diet was replaced by the same amount of carbohydrate as described elsewhere (Reis et al. Reference Reis, Carneiro, Mello, Boschero, Saad and Velloso1997) (Table 1). Rats that were 60 d old were assigned to two groups: 1) a control group consisting of rats born to and suckled by dams fed a control diet during pregnancy, lactation and after weaning; 2) a LP group consisting of the offspring of dams fed a LP diet during pregnancy, lactation and after weaning. During the experimental period, rats consumed their respective diets ad libitum and had free access to water. The rats were housed on a 12 h light–dark cycle at 24°C. At the end of the experimental period, the rats were killed by decapitation.
Table 1 Composition (g/kg) of the control and low protein diets*
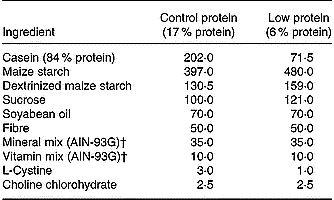
* For details of diets and procedures, see this page.
† For further details, see Reeves et al. (Reference Reeves, Nielsen and Fahey1993).
Islet isolation, culture and insulin secretion
The pancreas was removed and digested with collagenase as described elsewhere (Boschero et al. Reference Boschero, Szpak-Glasman, Carneiro, Bordin, Paul, Rojas and Atwater1995). In the first series of experiments, isolated islets were cultured for 48 h in RPMI 1640 medium containing 5·6 mmol/l glucose, due to normoglycaemic feature of LP rats, and supplemented with 5 % fetal bovine serum, penicillin (100 U/ml) and streptomycin (100 μg/ml) (Anderson, Reference Anderson1978), with the addition of 0·6 mmol/l palmitate, due to serum NEFA concentration observed in LP rats in an atmosphere of 5 % CO2 at 37°C. Albumin-bound palmitate (1:4,v/v) was prepared by stirring the fatty acid Na+ salt at 45°C with defatted bovine serum albumin (Sigma Fraction V; Sigma-Aldrich, St. Louis, MO, USA). After adjustment to pH 7·4, the solution was filtered through a 0·22 μm filter and the fatty acid concentration was measured using a NEFA C kit (Wako Chemicals, GmbH, Richmond, VA, USA). The medium was renewed every 24 h to maintain a constant concentration of fatty acid. After 48 h, groups of five islets each were incubated for 90 min at 37°C in Krebs-bicarbonate buffer containing glucose (2·8 mmol/l or 16·7 mmol/l) balanced with a mixture of 95 % O2–5 % CO2, pH 7·4. The incubation medium contained 115 mmol/l NaCl, 5 mmol/l KCl, 24 mmol/l NaHCO3, 1 mmol/l CaCl2, 1 mmol/l MgCl2 and bovine serum albumin (3 g/l). The insulin released was measured by RIA (Scott et al. Reference Scott, Atwater and Rojas1981).
Glucose metabolism
Glucose oxidation was measured in isolated islets as previously described (Malaisse et al. Reference Malaisse, Sener and Mahy1974). Briefly, groups of thirty islets, cultured for 48 h with glucose (5·6 mmol/l) in the absence or presence of palmitate (0·6 mmol/l) were placed in wells containing Krebs-bicarbonate buffered medium (50 μl) supplemented with trace amounts of D-[U-14C] glucose (370 000 Bq/ml) plus non-radioactive glucose at a final concentration of 2·8 mmol/l or 16·7 mmol/l. The wells were suspended in 20 ml scintillation vials that were gassed with 5 % O2 and 95 % CO2 and capped airtight with rubber membranes. The vials were then shaken continuously for 2 h at 37°C in a water bath. After incubation, 0·1 ml 0·2 N HCl and 0·2 ml hyamine hydroxide were injected through the rubber cap into the glass cup containing the incubation medium and into the counting vial, respectively. After 1 h at 4°C, scintillation fluid (3 ml) was added to the hyamine and the radioactivity was counted. The rate of glucose oxidation was expressed as pmol/islet.2 h.
Western blot
After islets were cultured for 48 h with glucose (5·6 mmol/l) in the absence or presence of palmitate (0·6 mmol/l), a pool of at least 1000 clean islets from each experimental group was homogenized by sonication (15 s) in an anti-protease cocktail (10 mmol/l imidazole, pH 8·0; 4 mmol/l EDTA; 1 mmol/l ethylene glycol bis-2-aminoethyl ether-N,N′,N′,n′-tetraacetic acid; 0·5 mg/l pepstatin A; 2 mg/l aprotinin; 2·5 mg/l leupeptin; 30 mg/l trypsin inhibitor; 200 μmol/l DL-dithiothreitol; 200 μmol/l phenylmethylsulfonyl fluoride). After sonication, an amount of extract was collected and the total protein content was determined using a dye-binding protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Samples of crude membrane preparations (50 μg protein) from each experimental group were incubated for 5 min at 80°C with 5 × concentrated Laemmli sample buffer (1 mmol/l sodium phosphate, pH 7·8, 0·1 % bromophenol blue, 50 % glycerol, 10 % SDS, 2 % mercaptoethanol) (4:1, v/v) and then run on 10 % polyacrylamide gels. Electrotransfer of proteins to nitrocellulose membranes (Bio-Rad Laboratories) was done for 1 h at 120 V (constant) in buffer containing methanol and SDS. After checking the efficiency of the transfer by Ponceau S staining, the membranes were blocked with dry skimmed milk (50 g/l) in Tris-Tween buffered saline (10 mmol/l Tris, 150 mmol/l NaCl, 0·5 % Tween 20) overnight at 4°C. PDX-1 and p38/SAPK2 were detected in the membrane after 2 h incubation at room temperature with a rabbit polyclonal antibody against PDX-1 and p38/SAPK2 (diluted 1:2500 in Tris-Tween buffered saline plus dry skimmed milk, 30 g/l). Detection was done using enhanced chemiluminescence (SuperSignal West Pico; Pierce Biotechnology Inc., Rockford, IL, USA) after incubation with a horseradish peroxidase-conjugated secondary antibody. Band intensities were quantified by optical densitometry (Scion Image, Frederick, MD, USA) of the developed autoradiogram.
mRNA expression
Total RNA from 500 islets, isolated and separated as described for Western blot analysis, was extracted using TriZol reagent (Life Technologies, Auckland, New Zealand). For PCR analysis, RNA (1 μg) was reverse-transcribed using oligo (DT) primers. The resulting cDNA was amplified by PCR using oligonucleotides complementary to sequences in the PDX-1 gene (5′-CCGAATGGAACCGAGACTGG-3′ and 5′-AGGTGGTGGCTTTGGCAATG-3′), insulin gene (5′-TTGCAGTAGTTCTCCAGTT-3′ and 5′-ATTGTTCCAACATGGCCCTGT-3′) and β-actin gene (5′-CTGCTGGCTGCTTTGCTCAC-3′ and 5′-ACGGCATAGACAGGAAGTGGG-3′), with the latter as the internal control. The PCR was done in a 25 μl reaction volume containing 1 μl cDNA, 0·05 mmol/l each cold dNTP (dATP, dCTP, dGTP, dTTP), 0·37 mmol/l MgCl2, 0·25 × PCR buffer, appropriate oligonucleotides primers (0·1 μmol/l) and 1 U Taq polymerase (Life Technologies). The PCR amplification conditions for PDX-1 were as follows: 3 min at 94°C followed by 29 cycles (30 s each) at 94°, 59° and 72°C, and for insulin gene 3 min at 94°C followed by 23 cycles (30 s each) at 94°, 57° and 72°C, and for β-actin gene (internal control), 2 min at 94°C followed by 25 cycles (30 s each) at 94°, 58° and 72°C. The PCR products were separated on a 1·5 % agarose gel in Tris-borate-EDTA buffer 1 × and stained with ethidium bromide. All assays included a negative control. The absence of contamination was confirmed by reverse transcription-negative RNA samples. The relative band intensities were determined by densitometry and the ratio of PDX-1 and insulin to β-actin gene expression was calculated for each sample.
Statistical analysis
The results were expressed as means with their standard errors. Student's two-tailed unpaired t test was used to compare biochemical parameters and body weight in the control and LP groups. Levene's test for the homogeneity of variances was initially used to check the normality of the data before testing with a parametric two-way ANOVA (nutritional status and the presence of palmitate or glucose concentration and the presence of palmitate). When necessary, these analyses were complemented by the least squares difference test to determine the significance of individual differences. To correct for variance heterogeneity or normality (Sokal & Rohlf, Reference Sokal and Rohlf1995), serum insulin and insulin secretion in response to glucose were log-transformed. A P-value < 0·05 indicated a significant difference. All statistical analyses were done using a statistical software package (Statsoft, Tulsa, OK, USA).
Results
The main values for body weight, serum total protein, serum glucose, NEFA and insulin concentrations are shown in Table 2. At the end of the experimental period, body weight, serum total protein and insulin concentrations were significantly lower (P < 0·05), whereas the serum NEFA level was significantly higher (P < 0·05) in the LP group compared with the control group; there was no difference in the serum glucose levels of the two groups.
Table 2 Body weight, serum total protein, glucose and insulin concentrations of young adult rats maintained on control or low-protein (LP) diets during fetal life, lactation and after weaning† (Mean values with their standard errors)
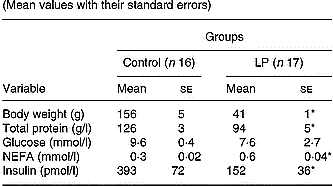
Mean values were significantly different compared with the control group (unpaired t test): *P < 0·05.
† For details of diets and procedures, see pp. 1007-1008.
After 48 h culture in a medium containing 5·6 mm-glucose with or without palmitate, the islets were challenged with 2·8 or 16·7 mm-glucose for a supplemental period of 90 min. At 2·8 mmol/l glucose, the insulin secretion was similar in the control (55 (se 9·2) pmol/islet.90 min) and LP (43 (se 5·2) pmol/islet.90 min) groups. The presence of palmitate in the culture medium did not change the insulin release in LP islets (41·4 (se 4) pmol/islet.90 min) but raised the secretion 2·3-fold in the control islets (128·2 (se 0·4) pmol/islet.90 min) (Fig. 1(A)). At 16·7 mm-glucose, the insulin secretion in the control islets was 11-fold higher than that obtained at 2·8 mm-glucose (598 (se 4) pmol/islet.90 min) (Fig. 1(B)), but only 3·1-fold higher in LP islets (134 (se 11) pmol/islets.90 min). Control islets cultured in the presence of palmitate and subsequently stimulated with 16·7 mm-glucose secreted 1002 (se 42) pmol/islet.90 min, 2-fold higher than that observed for the LP islets 488 (se 79) pmol/islet.90 min (Fig. 1(B)).
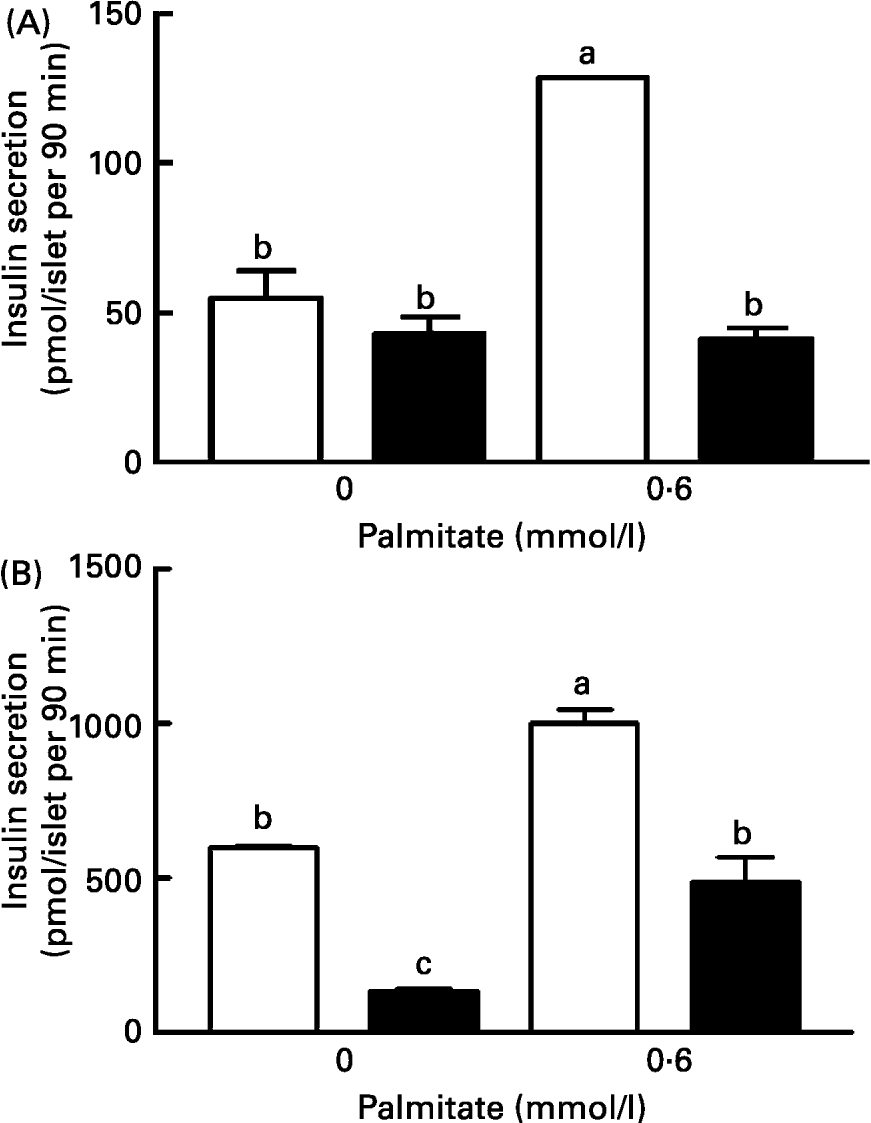
Fig. 1 Effect of palmitate on insulin secretion by islets from control (□) and low protein (LP; ■) groups in response to 2·8 mmol/l (A) or 16·7 mmol/l (B) glucose. Both groups of islets were cultured for 48 h with the indicated concentration of palmitate. The columns are the mean values with their standard errors for six batches of islets in each group. a,b Mean values within a column with unlike superscript letters were statistically different (P < 0·05). For details of diets and procedures, see pp. 1007-1008 .
Glucose metabolism in the presence of 2·8 mmol/l glucose, with or without palmitate, was influenced only by the nutritional status (F1,17 14·8, P < 0·01), i.e. islets from LP rats metabolized less glucose than islets from control rats (Fig. 2(A)). Raising the glucose concentration of the medium from 2·8 to 16·7 mmol/l significantly increased the rate of glucose metabolism in both groups, in the absence or presence of palmitate. In 16·7 mmol/l glucose, two-way ANOVA revealed a significant effect of nutritional status (F1,20 104·34; P = 0·00) and palmitate concentration (F1,20 71·47; P = 0·00), as well as interaction between these factors (F1,20 9·0; P = 0·007) (Fig. 2(B)). The glucose oxidation in islets incubated in the presence of 16·7 mmol/l glucose was 66·24 (se 6·98) pmol/islet.2 h and 33 (se 6·3) pmol/islet.2 h in control and LP islets, respectively (Fig. 2(B)). The presence of palmitate in the culture medium reduced the 14CO2 production in both groups of islets, but the magnitude of this effect was higher in LP than in control islets (11·0 (se 1·73) pmol/islet.2 h and 39·0 (se 2·8) pmol/islet.2 h, respectively) (Fig. 2(A)(B)). Hence, the effect of palmitate on glucose oxidation was also enhanced by protein restriction.
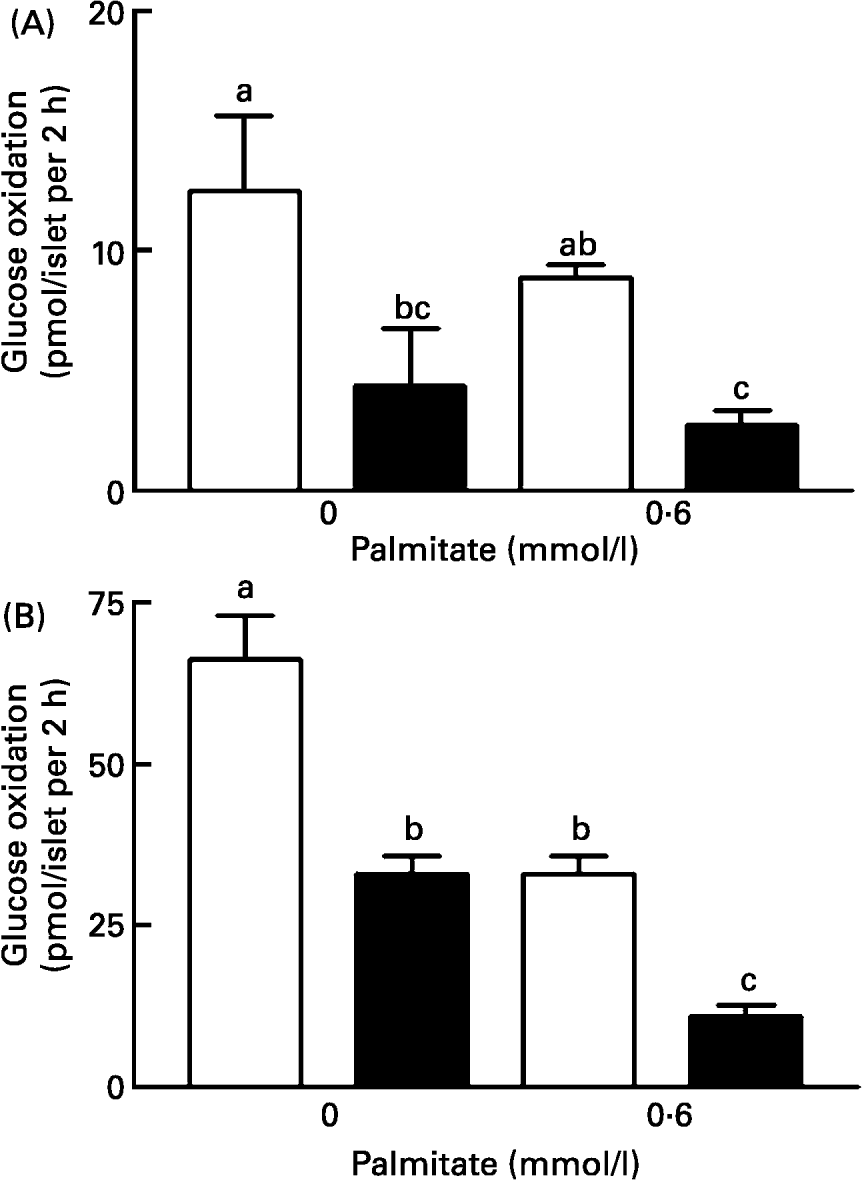
Fig. 2 Effect of palmitate on glucose oxidation by islets from control (□) and low protein (LP; ■) rats. Islets were cultured for 48 h in RPMI 1640 medium with the indicated concentration of palmitate. The rate of glucose oxidation was estimated by measuring the 14CO2 production in groups of 30 islets for 2 h at 37°C in the presence of a trace amount of D-[U-14] glucose. The final glucose concentration was 2·8 mmol/l (A) or 16·7 mmol/l (B). The columns are the mean values with their standard errors for six batches of islets in each group. a,b,c Mean values within a column with unlike superscript letters were statistically significant (P < 0·05). For details of diets and procedures, see pp. 1007-1008.
The PDX-1 and insulin mRNA levels were influenced only by palmitate (F1,14 25·8, P = 0·0002 and F1,10 7·64, P = 0·02, respectively). Islets cultured in medium containing palmitate showed higher PDX-1 (Fig. 3(A)) and insulin (Fig. 3(B)) mRNA expression than islets cultured without palmitate, regardless of the nutritional status. p38/SAPK2 protein expression was modified by palmitate (F1,8 199·5, P = 0·000) and by interaction between the nutritional status and palmitate (F1,8 9·08, P = 0·01). LP and control islets maintained in culture medium without palmitate had similar levels of p38/SAPK2 expression (3394 (se 374) and 5829 (se 750), respectively) and the addition of palmitate increased the p38/SAPK2 expression by the same magnitude in both groups (17 774 (se 1350) and 15 151 (se 1313), respectively) (Fig. 4(A)). PDX-1 expression was influenced by the nutritional status (F1,8 49·2, P = 0·0001) and by palmitate (F1,8 68·2, P = 0·000) such that PDX-1 expression in islets from LP rats was lower than in islets from control rats. In both groups, PDX-1 expression was higher in islets maintained with palmitate than in those cultured in medium containing only glucose (Fig. 4(B)).

Fig. 3 Pancreatic duodenal homeobox 1 (PDX-1 (A)) and insulin (B) mRNA levels in pancreatic islets from control (□) and low protein (LP; ■) rats. Total RNA was extracted from approximately 500 islets for each group and reverse-transcribed. Equal amounts of cDNA were subjected to PCR using primers complementary to the 5′ and 3′ ends of the PDX-1, insulin and actin genes. The mRNA concentrations of PDX-1 and insulin were expressed relative to actin mRNA. The columns are the mean values with their standard errors of three independent experiments. a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05). For details of diets and procedures, see pp. 1007-1008

Fig. 4 p38/stress activated protein kinase 2 (SAPK2 (A)) and pancreatic duodenal homeobox 1 (PDX-1 (B)) protein content in islets from control (□) and low protein (LP; ■) rats. Each lane contained 50 μg protein extracted from isolated islets cultured for 48 h in RPMI 1640 medium with the indicated concentration of palmitate. PDX-1 and p38 were detected by immunoblotting with polyclonal anti-PDX-1 and anti-p38 antibodies, respectively. The columns are the mean values with their standard errors for three independent experiments. a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05). For details of diets and procedures, see pp. 1007-1008
Discussion
In the present study, LP rats showed typical features of protein malnutrition, including a low body weight, low serum insulin and total protein concentrations, a high NEFA level and normoglycaemia, as previously reported for this same animal model (Latorraca et al. Reference Latorraca, Reis, Carneiro, Mello, Velloso, Saad and Boschero1998).
In LP and control islets cultured in medium containing 5·6 mmol/l glucose for 48 h, the PDX-1 protein expression and insulin secretion in response to glucose were similar to those seen in fresh isolated islets (Arantes et al. Reference Arantes, Teixeira, Reis, Latorraca, Leite, Carneiro, Yamada and Boschero2002), indicating that culture per se did not alter the parameters analysed here. In contrast, and somewhat unexpectedly, we verified that palmitate enhanced PDX-1 mRNA and protein expression in LP and control islets. Since the effect of palmitate on islet physiology is apparently glucose-dependent (Gremlich et al. Reference Gremlich, Bonny, Waeber and Thorens1997), an explanation for this seemingly contradictory result could be the different glucose concentrations used in the culture medium. Moreover, although Gremlich et al. (Reference Gremlich, Bonny, Waeber and Thorens1997) have shown an inhibitory effect of palmitate on PDX-1 mRNA expression in islets cultured in the presence of 5·6 mmol/l glucose, the inhibitory effect on protein expression was evaluated only in the presence of 30 mmol/l glucose.
p38/SAPK2 is a protein involved in the phosphorylation and activation of PDX-1 that in turn regulates the expression of various genes (Gremlich et al. Reference Gremlich, Bonny, Waeber and Thorens1997). LP diets have been reported not to modify p38/SAPK2 expression but reduced the PDX-1 levels (Martin et al. Reference Martin, Fernandez, Pascual-Leone, Escriva and Alvarez2004). As shown here, protein restriction also did not alter p38/SAPK2 protein levels, whereas the addition of NEFA to the medium significantly increased the expression of this protein in both groups. The increase in p38/SAPK2 expression in the presence of NEFA seen here provides important new information about the role of NEFA in regulating P38/SAPK2 expression. Considering the lower PDX-1 protein expression in LP islets compared with control islets, and the increase in insulin mRNA in both groups in the presence of palmitate, we believe that the higher expression of p38/SAPK2 enhanced the activation of PDX-1 that consequently increased the insulin mRNA levels.
Although still incompletely understood, there is a relationship between the coupling factors that link glucose metabolism to insulin gene expression (Alarcon et al. Reference Alarcon, Wicksteed, Prentki, Corkey and Rhodes2002). However, as shown here, glucose metabolism in the absence or presence of elevated NEFA levels was unrelated to the alterations seen in PDX-1 and insulin mRNA levels in both groups.
Glucose oxidation is influenced by the chronic exposure of β-cells to NEFA. Almost all studies reported in the literature indicate that elevated NEFA levels reduce glucose metabolism in islets (Sako & Grill, Reference Sako and Grill1990; Zhou et al. Reference Zhou, Priestman, Randle and Grill1996; Assimacopoulos-Jeannet et al. Reference Assimacopoulos-Jeannet, Thumelin, Roche, Esser, McGarry and Prentik1997). Consequently, high concentrations of NEFA may shift the metabolism of lipids and glucose into a relatively more pronounced NEFA oxidation, which could explain the impaired insulin secretion in response to glucose (Alstrup et al. Reference Alstrup, Brock and Hermansen2004). In contrast, we observed an increase in glucose-induced insulin secretion, despite the lower glucose metabolism in the presence of elevated NEFA in both groups. Based on this observation, we suggest that in LP islets and in the presence of high glucose, NEFA may be preferentially used for insulin secretion. Considering that exogenous NEFA can acutely potentiate glucose-stimulated insulin secretion, possibly by providing additional acyl groups for long-chain acyl-CoA formation or complex lipid synthesis (Corkey et al. Reference Corkey, Deeney, Yaney, Tornhein and Prentki2000), and since LP rats are chronically exposed to elevated serum NEFA levels, it is possible that the intracellular level of acyl-CoA may be elevated in the islets of these rats and could activate enzymes, such as protein kinase C (Alcazar et al. Reference Alcazar, Qiu-yue, Gine and Tamarit-Rodriguez1997) and synaptosome-associated protein 25 (Zraika et al. Reference Zraika, Dunlop, Proietto and Andrikopoulos2004), that are involved in the insulin secretory and exocytotic machinery.
In conclusion, the present results indicate that the elevated NEFA levels found in protein restriction models may exert benefits upon insulin secretion. The decreased glucose metabolism in the presence of palmitic acid indicates a shift from glucose to NEFA as the oxidative fuel. Finally, the increase in insulin mRNA expression provoked by NEFA may be linked to an increase in p38/SAPK2 and PDX-1 protein expression.
Acknowledgements
The authors thank L.D. Teixeira for technical assistance, S. Hyslop and C.A. Magalhães for language editing and C. H. Fregadolli for statistical analysis. This work was partly supported by the Brazilian foundations CNPq, CAPES/PQI, FAPESP, and CNPq/PRONEX.








