INTRODUCTION
Although the prevalence rate of pulmonary tuberculosis (PTB) has declined significantly in western countries in the past few decades, PTB remains one of the most common infectious diseases in developing countries [Reference Dye1]. Diabetic patients are considered a high-risk population [Reference Alisjahbana2] and many studies have shown a 1·5–7·8 times higher prevalence of PTB among diabetics than non-diabetics [Reference Mugusi3–Reference Stevenson6]. With increasing life expectancy, there is also a current global increase in diabetic patients [Reference Wild7]. Thus, PTB in diabetic patients will become an increasingly important problem.
A high index of suspicion and prompt investigation of diabetic patients with clinical presentation of PTB may allow earlier diagnosis and treatment. Nonetheless, some previous reports have described an atypical presentation of PTB in diabetic patients [Reference Perez-Guzman8–Reference Shaikh10]. When these differences are ignored, establishing a diagnosis may be delayed, leading to increased morbidity and mortality.
On the other hand, there is scarce information about the influence of type 2 diabetes mellitus (DM) on the treatment outcome of culture-proven PTB patients. The present study aimed to determine, in a developed Asian city with a sizable study sample and a high prevalence of PTB, if type 2 DM alters manifestations and treatment outcome of PTB.
METHODS
Subjects and study design
This study was conducted at the Kaohsiung Municipal Hsiao-Kang Hospital, a 500-bed, university-affiliated teaching hospital that serves as a tertiary referral centre and primary-care facility in southern Taiwan. From the retrospective review, 217 consecutive adult patients with PTB from 1 January 2003 to 31 December 2006 were selected and 21 patients were excluded because either the chest radiograph (CXR) done at the first presentation was missing or there was no clear data on bacteriology and history of DM, as radiographic and bacteriological findings were the main parameters that this study investigated in patients with and without DM. The study protocol was reviewed and approved by the hospital's human experiment and ethics committee.
Cases were identified from the hospital coding system and microbiology department records, and included both outpatients and in-patients. In this institution, the main criteria for hospital admission of tuberculous patients included sepsis, haemoptysis, respiratory failure, advanced malnutrition, and pleural effusion. However, about two-thirds of hospitalized tuberculous patients were admitted for treatment or diagnosis confirmation even in the absence of the above-mentioned criteria. Patients were excluded if one of the following was present: aged <18 years, non-Taiwanese, and already diagnosed with tuberculosis (TB) in another hospital. Diagnosis of PTB was based on at least one sputum culture being positive for Mycobacterium tuberculosis, i.e. ‘definite’ cases, as defined by Rieder et al. [Reference Rieder11]. Sputum samples were obtained by spontaneous morning expectoration.
Patients were included in the PTB-DM group if they had a known history of DM and had been receiving insulin and/or an oral hypoglycaemic agent, or were diagnosed as having DM during this hospitalization with subsequent confirmation by two or more fasting plasma glucose levels >126 mg/dl on a different day in an outpatient setting. Patients who did not have DM in the same period were chosen as the control group (PTB group). The characteristics of the 74 type 2 DM patients (PTB-DM group) were compared to those of the 143 non-diabetic patients (PTB group).
Clinical data collection
All of the patients had a medical chart, microbiology results, and standard posterior–anterior CXR. Although this was a retrospective study, the patients were interviewed by the same trained nurse-case manager using a structured questionnaire and the completed questionnaires were then reviewed by the physician investigator. Demographic information included age and sex. Risk factors for PTB infection were recorded, including previous TB disease, infectious TB contact history, alcoholism, illegal drug use, long-term glucosteroid use, immunosuppressive drug use, and other comorbidities associated with TB, such as DM, end-stage renal disease, cancer, human immunodeficiency virus infection, silicosis, and gastrectomy history.
Initial presenting symptoms prior to hospital consultation included the presence of cough, expectoration, fever, weight loss, dyspnoea, anorexia, haemoptysis, chest pain, fatigue, and night sweats, which were considered positive if these symptoms were present for ⩾2 weeks. Less than this was coded as negative for the analysis. Weight loss was defined as positive if it was >10% of body weight within the last 6 months. Fever was defined as a body axillary temperature above 37·5°C. Haemoptysis was recorded as positive even if it occurred only once.
All of the sputum smears were concentrated and stained with Ziehl–Neelsen stain by trained technicians. Each sputum sample was prepared in Löwenstein–Jensen culture medium and Middlebrook 7H11 selective agar, and maintained for at least 8 weeks to detect the presence of growth.
Initial standard posterior–anterior CXR taken at the time of the patient's first hospital visit was independently reviewed by two external chest physicians, both of whom were unaware of the patient's clinical data. Differences were then resolved by consensus. CXR results were categorized by the involved field and pattern. An upper lung field lesion was defined as the presence of any lesion above an imaginary line across the hilum. Radiographic presentation was categorized as normal, consolidation, cavity, or pleural effusion [Reference Tuddenham12]. ‘Normal’ pattern was defined as the absence of any abnormal lesion on CXR. ‘Consolidation’ pattern was defined as an essentially homogenous opacity in the lung characterized by little or no loss of volume, effacement of blood vessel shadows, and sometimes by the presence of an air bronchogram. ‘Cavitary’ pattern was defined as a lucent area within the lung that may or may not contain a fluid level and that is surrounded by a wall, usually of varied thickness. ‘Pleural effusion’ was defined as a uniform opacity extending upwards from the costophrenic angle in an erect film. Ultrasound was later used to confirm pleural effusion.
TB treatment consisted of a standard regimen of daily rifampicin, isoniazid, pyrazinamid, and ethambutol for 2 months and rifampicin, isoniazid, and ethambutol for another 4 months or daily rifampicin, isoniazid, and ethambutol for 9 months. Treatment outcomes after 1 year from the initiation of anti-TB treatment were extracted from the records. They were analysed and defined according to World Health Organization recommendations [13]. ‘Cure’ was defined as a patient who was sputum smear-negative in the last month of treatment and on at least one previous occasion. ‘Treatment completed’ was defined as a patient who had completed treatment but who did not meet the criteria to be classified as a cure or a failure. ‘Treatment failure’ was defined as a patient who was sputum smear-positive at 5 months or later during treatment. ‘Died’ was defined as a patient who died for any reason during the treatment course. ‘Default’ was a patient whose treatment was interrupted for two consecutive months or more, and ‘Transfer out’ was a patient who was transferred to another recording and reporting unit, with unknown outcome. A favourable outcome was defined as including cure and treatment completed. Any other outcome was classified as unfavourable. In subgroup analysis, ‘PTB-related death’ was defined as a patient who died due to PTB during the treatment course.
Statistical analysis
Univariate comparisons between the PTB-DM and PTB groups were performed using the χ2 test except when expected values of <5 required using Fisher's exact test for categorical variables and Student's t test for continuous variables where appropriate. All of the tests of significance were two sided. A P<0·05 was considered statistically significant. Data in the text and illustrations correspond to mean±s.d. (continuous variables) or to frequencies and percentages (non-continuous variables).
To identify variables that might affect treatment outcome, comparison of the groups with favourable and unfavourable outcome was made using univariate analysis and multiple logistic regression analysis. An attempt was made to include all key factors from demographic characteristics, bacteriology results, coexisting medical diseases, initial presenting symptoms, and initial presenting CXR findings. After the initial univariate analysis, the variables were put into the regression model to identify those independently associated with unfavourable outcome. A backward stepwise approach was used in the regression analysis, with the probability of F to enter being ⩽0·05 and the probability of F to remove being ⩾0·10. All of the analyses were performed using a statistical software program (version 12.0, SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic characteristics and sputum acid-fast bacilli (AFB) stain results
A total of 217 culture-positive PTB patients were enrolled in the study. There were no type 1 DM patients in this study. The demographic characteristics and sputum AFB stain results of the PTB-DM and PTB groups are presented in Table 1. There was no statistically significant difference between the two groups regarding age, sex, previous TB disease, infectious TB contact history, alcoholism, end-stage renal disease, and cancer. No patient had the following risk factors: illegal drug use, long-term glucosteroid or immunosuppressive drug use, human immunodeficiency virus infection, silicosis, and gastrectomy history. In the PTB-DM group, 68·9% of patients were AFB positive on sputum smear compared to 53·8% of the PTB group (OR 1·90, 95% CI 1·051–3·435).
Table 1. Demographic characteristics and sputum acid-fast bacilli stain results of the two study groups

PTB-DM, Pulmonary tuberculosis patients with type 2 diabetes; PTB, pulmonary tuberculosis patients without associated diabetes; s.d., standard deviation; TB, tuberculosis.
Data are presented as number of patients (%) unless otherwise indicated.
Initial presenting symptoms
The initial presenting symptoms of the PTB-DM and PTB groups are described in Table 2. The PTB-DM group had higher frequencies of fever (OR 2·2, 95% CI 1·233–4·011) and haemoptysis (OR 2·6, 95% CI 1·238–5·297) than the PTB group. There were no statistically significant differences in terms of cough, expectoration, weight loss, dyspnoea, anorexia, chest pain, fatigue, and night sweats.
Table 2. Initial presenting symptoms of the two study groups

PTB-DM, Pulmonary tuberculosis patients with type 2 diabetes; PTB, pulmonary tuberculosis patients without associated diabetes.
Data are presented as number of patients (%).
Initial presenting CXR findings
Initial presenting CXR findings of the PTB-DM and PTB groups are shown in Table 3. Isolated lower lung field lesions were significantly more common in the PTB-DM group than the PTB group (OR 2·04, 95% CI 1·027–4·042). The PTB-DM group also had significantly higher frequencies of consolidation (OR 2·23, 95% CI 1·040–4·798) and cavity (OR 1·91, 95% CI 1·072–3·412) in terms of lung lesions. There were no statistically significant differences between the two groups regarding radiographic features, including the prevalence of normal and pleural effusion.
Table 3. Initial presenting chest radiograph findings of the two study groups
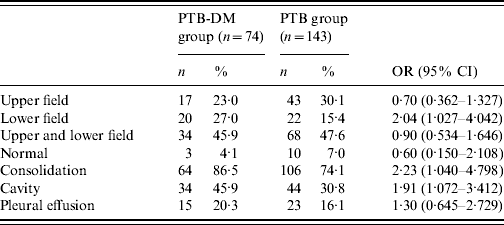
PTB-DM, Pulmonary tuberculosis patients with type 2 diabetes; PTB, pulmonary tuberculosis patients without associated diabetes.
Data are presented as number of patients (%).
Treatment outcome after 1 year from the initiation of anti-TB treatment
Treatment outcome after 1 year from the initiation of anti-TB treatment is shown in Table 4. Mortality for PTB-DM patients was 17·6%, in sharp contrast to 7·7% for PTB patients (OR 2·56, 95% CI 1·084–6·034). Univariate analysis shows that type 2 DM (OR 2·9, 95% CI 1·456–5·928), age ⩾65 years (OR 8·5, 95% CI 3·970–18·352), and extensive radiographic disease that was defined as radiographic lesions involving both upper and lower lung field (OR 3·2, 95% CI 1·551–6·802) were all associated with an unfavourable outcome. On multiple logistic regression analysis after adjusting for age and sex, type 2 DM (OR 5·5, 95% CI 2·273–13·452), age ⩾65 years (OR 10·8, 95% CI 4·402–26·251), and extensive radiographic disease (OR 2·40, 95% CI 1·027–5·617) remained as independent and significant risk factors (Table 5). In subgroup analysis, PTB-related death was significantly more common in the PTB-DM group than the PTB group (12·2% vs. 4·2%; OR 3·16, 95% CI 1·080–9·257). On multiple logistic regression analysis after adjusting for age and sex, type 2 DM (OR 7·6, 95% CI 1·976–29·083) still remained as an independent and significant risk factor for PTB-related death.
Table 4. Treatment outcome of the two study groups after 1 year from the initiation of anti-tuberculosis treatment
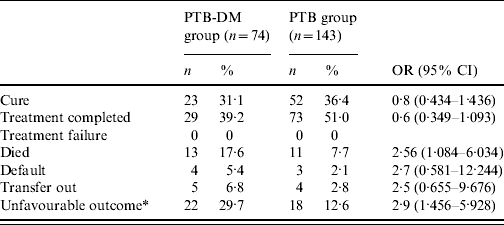
PTB-DM, Pulmonary tuberculosis patients with type 2 diabetes; PTB, pulmonary tuberculosis patients without associated diabetes.
Data are presented as number of patients (%).
* A favourable outcome was defined as including cure and treatment completed. Any other outcome was classified as unfavourable.
Table 5. Independent predictive factors of unfavourable outcomeFootnote * for pulmonary tuberculosis on multiple logistic regression analysis after adjusting for age and sex
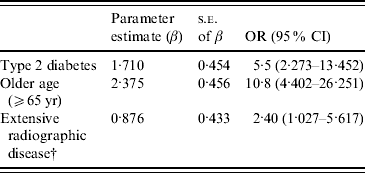
* A favourable outcome was defined as including cure and treatment completed. Any other outcome was classified as unfavourable.
† Extensive radiographic disease was defined as radiographic lesions involving both upper and lower lung field.
DISCUSSION
The increased incidence of DM among PTB patients is well known [Reference Mugusi3–Reference Stevenson6], but less is known about its possible effects on the manifestations and treatment outcome of PTB. Recent studies show that 10–30% of patients with PTB may also have DM [Reference Alisjahbana2–Reference Kim4, Reference Restrepo14–Reference Alisjahbana18]. In this study, 34% of the total PTB patients had co-existing type 2 DM.
Recently, a number of studies compared disease presentation between diabetic and non-diabetic PTB patients. Studies in Saudi Arabia [Reference Singla16], Malaysia [Reference Nissapatorn19], and Turkey [Reference Bacakoglu20] did not find major differences in presenting symptoms. Similar to our findings, a recent large retrospective study involving the Texas–Mexico border region revealed a higher rate of fever and haemoptysis among diabetic PTB patients [Reference Restrepo14]. Whether the symptomatic differences of PTB patients with DM in the present study represent physiological or cultural differences (or both) remains unclear.
Comparative studies of PTB images in diabetics have yielded conflicting results. The impact of DM on the radiological presentation of PTB is important because misinterpretations may delay appropriate diagnostic tests and treatment, thus risking dissemination of M. tuberculosis to others. Some studies on the radiographic findings of PTB patients with DM report atypical localization patterns, namely an increased incidence of lower lung field involvement [Reference Perez-Guzman8–Reference Shaikh10], which is confirmed in the present study. Other reports do not confirm these observations and find no difference in terms of the radiographic involvement of the lung fields [Reference Bacakoglu20, Reference al-Wabel21]. Possible explanations for these discrepancies are demographic characteristics and patient selection process.
Our study shows a significantly higher frequency of consolidation and cavitary lung lesions on CXR in the PTB-DM group. Some previous studies have also reported cavitary lesions as more common among diabetic patients [Reference Perez-Guzman8–Reference Shaikh10, Reference Restrepo14, Reference Wang, Lee and Hsueh22]. Moreover, our sputum bacteriology results reveal that diabetics have a higher prevalence of AFB smear-positive cases compared to non-diabetic PTB patients. Our study also shows an interesting finding – increasing cavitation together with increased smear positivity in the diabetic group – which is the same as a previous study [Reference Hendy and Stableforth23]. Cavitary disease is associated with a larger population of bacilli.
Immunosuppression induced by DM may be responsible for the atypical images and higher bacillary load in PTB patients with DM [Reference Weaver24]. It is known that DM causes a decrement in lymphocyte activity and a diminution in the number of monocytes and macrophages, with abnormalities in their chemotactic and phagocytic activities [Reference Koziel and Koziel25, Reference Glass26]. Moreover, DM also causes dysfunction of polymorphonuclear leukocytes, with a reduction in their bactericidal activity [Reference Repine, Clawson and Goetz27].
There are conflicting reports regarding the influence of associated DM on the treatment outcome of TB patients. One study has reported no effect of DM on the treatment outcome of TB patients [Reference Singla16], while another has reported a negative effect [Reference Alisjahbana18]. In the present study, the mortality rate for all patients at 12 months was 11·0%, accounting for 60% (24/40) of the patients with unfavourable outcome. There was also a sharp contrast in mortality rates (17·6% vs. 7·7%; OR 2·56, 95% CI 1·084–6·034) and PTB-related death (12·2% vs. 4·2%; OR 3·16, 95% CI 1·080–9·257) between PTB-DM and PTB patients. As more than one third of patients were diabetics with PTB, the higher mortality rate may have contributed to the overall low treatment completion rate.
Altered pharmacokinetics of anti-TB drugs may explain the adverse effect of DM on the treatment outcome of TB patients. One recent study has reported low plasma concentrations of rifampicin in diabetic patients with TB [Reference Nijland28]. Several mechanisms have been postulated to explain the altered pharmacokinetics of anti-TB drugs in TB patients with DM. The absorption, distribution, metabolism, and excretion of drugs could all be changed in TB patients with DM [Reference Gwilt, Nahhas and Tracewell29]. Lower plasma concentrations of anti-TB drugs have been associated with clinical failure and acquired drug resistance [Reference Weiner30]. If these findings are confirmed, higher fixed dosages of rifampicin may be warranted for TB patients with DM. If available, physicians may consider the assessment of plasma concentrations of rifampicin in patients with DM in order to individualize dosing.
The present study reveals on univariate analysis that type 2 DM, age ⩾65 years, and extensive radiographic disease were all associated with an unfavourable outcome. Moreover, even after adjustment for age and sex, logistic regression analysis also showed that type 2 DM was an independent and significant risk factor associated with an unfavourable outcome. This suggests that clinicians must pay more attention to PTB patients with associated DM. A previous study has shown that the advent of effective anti-TB and anti-diabetic treatments has led to a decrease in the death rate of TB in patients with DM [Reference Bagdade31].
Our study had some methodological limitations. Twenty-one patients were excluded in the retrospective study because either the CXR that was done at the first presentation was missing or there was no clear data on bacteriology and history of DM. Part of the findings might result from missing patient records unrelated to DM status. Therefore, future prospective studies should formally evaluate the differences.
In summary, this study has shown that PTB patients with associated type 2 DM had higher frequencies of fever, haemoptysis, positive AFB sputum smears, and consolidation, cavity, and lower lung field lesions on CXR, and mortality rate. Furthermore, PTB-DM patients also have higher frequencies of unfavourable outcome. PTB should be included in the differential diagnosis when diabetic patients present with unusual findings, thus avoiding postponing the diagnosis and the start of treatment, and reducing the dissemination of M. tuberculosis to others and the mortality rate for PTB-DM patients.
DECLARATION OF INTEREST
None.






