Introduction
In the last few years, the interest for the development of feed additives has significantly increased, with the essential oils (EOs) emerging as a promising alternative for the substitution of antibiotics in animal production (Kishawy et al., Reference Kishawy, Amer, Abd El-Hack, Saadeldin and Swelum2019; Mahgoub et al., Reference Mahgoub, El-Hack, Saadeldin, Hussein, Swelum and Alagawany2019). This interest is based on the biological properties of the EOs, such as antimicrobial, antioxidant, anti-inflammatory and immunomodulatory activities (Donsì and Ferrari, Reference Donsì and Ferrari2016; Han et al., Reference Han, Parker and Dorsett2017; Lee et al., Reference Lee, Kim, Kim, Jeong, Oh, Cho and Lee2020; Su et al., Reference Su, Wang, Zhou, Wu, Chen, Yu, Huang, Luo, Mao, Zheng, Yu, Luo and He2021).
EOs are volatile and aromatic compounds extracted from plants, many of which show a broad spectrum of antimicrobial activity, affecting Gram-positive as well as Gram-negative bacteria (Su et al., Reference Su, Wang, Zhou, Wu, Chen, Yu, Huang, Luo, Mao, Zheng, Yu, Luo and He2021). EOs antimicrobial efficacy is intrinsically related to two important characteristics: their lipophilic character and the ability to penetrate in the membranes of bacterial cells, due to this lipophilic property (Bona et al., Reference Bona, Pickler, Miglino, Kuritza, Vasconcelos and Santin2012; Chouhan et al., Reference Chouhan, Sharma and Guleria2017; Abd El-Hack et al., Reference Abd El-Hack, El-Saadony, Saad, Salem, Ashry, Abo Ghanima, Shukry, Swelum, Taha, El-Tahan, AbuQamar and El-Tarabily2022). When bacteria are exposed to EOs, they experience an increase in the permeability of their membranes, resulting in the cell lysis due to the release of cellular content (Dorman and Deans, Reference Dorman and Deans2000; Bona et al., Reference Bona, Pickler, Miglino, Kuritza, Vasconcelos and Santin2012; Su et al., Reference Su, Wang, Zhou, Wu, Chen, Yu, Huang, Luo, Mao, Zheng, Yu, Luo and He2021). Also, this increase in permeability allows other active compounds present in EOs to penetrate the cells and bind to specific proteins, triggering a supplementary inhibitory action (Chouhan et al., Reference Chouhan, Sharma and Guleria2017).
Depending on the composition of EOs or their combinations, we can observe a broad diversity of biological effects that go beyond the antibacterial activity. These effects include the reduction of oxidative stress in critical situations, leading to a reduction in the energy demand that is required for the antioxidant functions (Windisch et al., Reference Windisch, Schedle, Plitzner and Kroismayr2008; Mohebodini et al., Reference Mohebodini, Jazi, Ashayerizadeh, Toghyani and Tellez-Isaias2021). Moreover, it is important to highlight the ability of some EOs to stimulate the secretion of digestive enzymes and endocrine hormones, resulting in further promotion of motility that is enhanced in the gastrointestinal system. This, in turn, contributes for the optimization of the processes of digestion and absorption of nutrients (Wade et al., Reference Wade, Manwar, Kuralkar, Waghmare, Ingle and Hajare2018; Su et al., Reference Su, Wang, Zhou, Wu, Chen, Yu, Huang, Luo, Mao, Zheng, Yu, Luo and He2021), important to improve poultry performance. EOs have other benefits described in literature, such as antiviral, anti-helminthic and coccidiostatic activities (Basmacioğlu Malayoğlu et al., Reference Basmacioğlu Malayoğlu, Baysal, Misirlioğlu, Polat, Yilmaz and Turan2010).
Due to the diversity of bioactive compounds that are present in the EOs, and to the influence that biological factors can exert on their composition and combinations, as well as the diverse results related to the type of plant, harvest location and conditions; production methods, including types of extraction, distillation and stability; and storage conditions, such as light, temperature and storage time (Huyghebaert et al., Reference Huyghebaert, Ducatelle and Van Immerseel2011), conflicting results are observed in the use of EOs in broilers. Many authors suggested positive effects (Barbarestani et al., Reference Barbarestani, Jazi, Mohebodini, Ashayerizadeh, Shabani and Toghyani2020; Su et al., Reference Su, Wang, Zhou, Wu, Chen, Yu, Huang, Luo, Mao, Zheng, Yu, Luo and He2021) in poultry performance, while others could not identify such effects and, in some cases, they even showed negative effects (Akbarian et al., Reference Akbarian, Golian, Kermanshahi, De Smet and Michiels2015; Irawan et al., Reference Irawan, Hidayat, Jayanegara and Ratriyanto2021). Based on the foregoing, this study aimed to evaluate the effect of EOs supplementation in broiler diets, and their effects on the animal development and gut morphometry, based on a systematic review with meta-analysis.
Materials and methods
Bibliographic research
This systematic review was conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses – PRISMA (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald, McGuinness, Stewart, Thomas, Tricco, Welch, Whiting and Moher2021). Until March 2022, an electronic search was conducted in the PubMed, ScienceDirect and SciELO databases using the following keywords in English: broilers, chickens, oil, thymol, performance, blood (blood was used as one of the keywords because the initial objective was to evaluate, in addition to performance and intestinal morphometry, the blood profile as well; however, after data tabulation, it was observed that there were not enough data involving this parameter to perform statistical analysis) and morphology.
These keywords were used in various combinations. For the ScienceDirect platform, one combination was used: (broilers OR chickens) and (‘oil’) and (‘thymol’) and (‘performance’) and (‘blood’) and (‘intestinal morphology’). Initially, the same combination ((broilers OR chickens) and (‘oil’) and (‘thymol’) and (‘performance’) and (‘blood’) and (‘intestinal morphology’)) was used on the PubMed and SciELO platforms; however, the search result was zero. For this reason, three other search combinations were used to expand the database. The keyword combinations used on the PubMed and SciELO platforms were: (broilers OR chickens) and (‘oil’) and (‘thymol’) and (‘performance’); (broilers OR chickens) and (‘thymol’) and (‘intestinal morphology’); and (broilers OR chickens) and (‘thymol’) and (‘blood’).
Eighty-eight articles were found, and after filtering by article titles, 26 articles were pre-selected for data tabulation and extraction. After a review of the obtained data, it was discussed among the researchers the need for a new search in the databases, which was conducted in December 2022. In this second search, the keywords set ((broilers OR chickens) and (‘essential oil’) and (‘performance’) and (‘blood’) and (‘intestinal morphology’)) was used. The difference in the keyword set aimed to obtain new articles to expand the database. Only articles involving research with broilers being supplemented with EOs were selected.
The screening carried out in the systematic review was through the exclusion of titles that were not aligned with the researchers’ objectives, those that did not qualify as experimental studies, and those that were carried out in vitro, when they did not provide results in the type of quantitative data, as well as the papers that were duplicated between the databases. Moreover, the papers that approached EOs and other compounds, and the ones in which the animals were subjected to sanitation challenges.
Criteria for selecting papers and elaboration of databases
There were selected studies that included a control diet without supplementation of EOs, and a diet with the addition of EOs. The selected papers exhibit significant variation in the composition of bioactives, as well as their concentrations. Therefore, to assess the use of EOs, only the effects with or without supplementation were considered, similar to the approach taken by Moreira et al. (Reference Moreira, Perez Palencia, Moita, Caputo, Saraiva, Andretta and de Abreu2020) when evaluating different amino acid blends.
No restrictions were imposed regarding the poultry's sex, strain, geographical latitude, season of the year, year the study was done or language used in the publishing of papers. In situations discrepancies between the documents were identified, all criteria were submitted to a detailed review and debated among researchers.
For the meta-analysis, information related to the performance of the animals was compiled (by including weight gain – WG, feed intake – FI and feed conversion – FC) and to the gut morphometry (covering measures such as villus height and crypt depth). This information was extracted from the tables present in the results section of each paper and organized into spreadsheets in Microsoft Excel (Arifin, Reference Arifin2016). Four distinct databases were created, one to evaluate the performance of the animals and the other to analyse the gut morphometry (duodenum, jejunum and ileum).
Evaluation of papers quality
After the selection criteria were applied, there was the evaluation of the quality of the papers, by taking into consideration the following criteria for the allocation of scores (Palencia et al., Reference Palencia, Lemes, Garbossa, Abreu, Pereira and Zangeronimo2018; Moreira et al., Reference Moreira, Perez Palencia, Moita, Caputo, Saraiva, Andretta and de Abreu2020): (A) randomization: papers that described a randomized study were assigned a score of 2 points, while those that did not mention randomization, or where randomization was not clearly described in the text, were assigned 0 points; (B) detailing of density and creation: papers that mentioned the dimensions of the cages or pens for the calculation of density were allocated with 2 points, while the ones that did not mention this information obtained 0 points; (C) reference to the type of experimental unit (cage, pen box or stall): papers that mentioned the type of experimental unit revived 2 points, while those that did not mention it received 0 points; (D) reference to initial and final temperatures: papers that mentioned initial and final temperatures received 2 points, while those that did not received 0 points; (E) reference to lighting programme used: papers that mentioned the amount of lighting provided received 2 points, while those that did not mention received 0 points; (F) rearing broilers and mixed or single-sex: papers that related single-sex studies received 2 points, while those that related mixed-sex received 1 point; (G) nutritional phases: papers that mentioned three nutritional phases were assigned with 2 points, those with two phases got 1 point, and the ones that did not mention the nutritional phases or only cited the strain manual got 0 points; (H) definition of strain: papers that included the definition of strain received 1 point, while those that did not were not assigned.
Each paper was classified based on the total score obtained after the sum of the scores assigned to each evaluated variable. This classification was used as a qualitative weighting criterion for the studies selected for this research. The quality criteria are necessary to evaluate the state of the art of the research line related to the objective in the article, considering the possible confounding factors in the analysis and conclusion of this work.
Statistical analysis
For the processing of statistical analysis, the data were tabbed by using electronic spreadsheets from Arifin (Reference Arifin2016). The standard error of the mean (SEM) has been presented in studies. SEM involves a general estimate without distinguishing the group. Thus, to estimate the standard deviation (S) the relationship ${\rm SEM} = S/\sqrt n$![]() was used, with n being the number of repetitions in each group (McGrath et al., Reference McGrath, Katzenschlager, Zimmer, Seitel, Steele and Benedetti2023). The ‘effect size’ was determined by the mean difference between control treatment and the treatment with the inclusion of EOs, with confidence intervals of 95%. Heterogeneity was evaluated through the index of inconsistency (I 2) and Cochran's Q test (Davoodi et al., Reference Davoodi, Ehsani, Vaez Torshizi and Masoudi2022).
was used, with n being the number of repetitions in each group (McGrath et al., Reference McGrath, Katzenschlager, Zimmer, Seitel, Steele and Benedetti2023). The ‘effect size’ was determined by the mean difference between control treatment and the treatment with the inclusion of EOs, with confidence intervals of 95%. Heterogeneity was evaluated through the index of inconsistency (I 2) and Cochran's Q test (Davoodi et al., Reference Davoodi, Ehsani, Vaez Torshizi and Masoudi2022).
The I 2 statistics is a crucial measure in the meta-analysis in order to evaluate the aggregate studies. Derived from Cochran's Q test, and taking into consideration the number of involved studies, its P value was compared to the significance level of 5%, in order to determine, or not, heterogeneity. Moreover, the following classification of the I 2 statistics was used: values close to 0% show lack of heterogeneity, close to 25%, low heterogeneity, about 50%, moderate heterogeneity, and 75%, high heterogeneity among the studies. When heterogeneity is indicated, the model of random effect is the indicated one if compared to the model of fixed effect.
Upon finding a significant difference between the oil application and the control, a regression adjustment was performed using a mixed model (Irawan et al., Reference Irawan, Hidayat, Jayanegara and Ratriyanto2021). The model structure included a random effect associated with the variable Study, allowing for variation in both the intercept and the slope concerning Dose. Additionally, two fixed-effect models were considered: one with a linear effect of Dose and the other with both a linear and quadratic effect of this variable. These models were compared using the likelihood ratio test. The model was adjusted using the lme function from the nlme package (Pinheiro and Bates, Reference Pinheiro and Bates2006) in R software. All the statistical analysis was held at software R (R Core Team, 2023). Meta-analysis was done at the metalibrary (Balduzzi et al., Reference Balduzzi, Rücker and Schwarzer2019).
Results
Systematic review
After searches in the three databases (PubMed, ScienceDirect and SciELO), it was observed that 78.40% of the papers were found at ScienceDirect. After the search, papers were excluded based on the pre-established criteria as follows: 5.48% of the papers were excluded because they were duplicate among the databases, 52.74% because of the title, 13.30% were reviews, 1.37% were in vitro studies, 2.05% of the studies did not clearly present the values of the analysed parameters, 6.16% were experiments in which substances other than EOs were evaluated and, finally, 18.49% were excluded because they were studies in which the animals were challenged, somehow, leaving 9.88% of the studies for the elaboration of the systematic review and meta-analysis (Table 1). The PRISMA flow diagram describes the stages of the study selection process and reasons for exclusion (Fig. 1).
Table 1. Papers screening


Figure 1. Modified PRISMA flow diagram (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald, McGuinness, Stewart, Thomas, Tricco, Welch, Whiting and Moher2021) with the systematic review search strategy and study selection.
In the evaluation of the quality of the papers, the following percentage were observed of studies that were allocated a score of 2, according to measured parameters: (A) randomization (56.25%); (B) density or dimensions of cage or box (50.00%); (C) experimental unit (87.50%); (D) initial and final temperature (81.25%); (E) light provided (93.75%); (F) sexed (75.00%); (G) three nutritional phases (37.50); (H) defined strain (93.75%) (Table 2).
Table 2. Evaluation of papers’ quality according to pre-established criteria

(A) Randomization: a randomized study scored 2 points, a non-randomized study (or when randomization was not clearly described in the text) scored 0 points; (B) studies that mentioned (density or) dimensions of cage or box for the calculation of density were allocated with 2 points, and when they did not, they scored 0 points; (C) studies that referred the type of experimental unit (cage, box or stall) were allocated with 2 points, and when they did not, they scored 0 points; (D) studies that mentioned initial and final temperature were allocated with 2 points, and when they did not, they scored 0 points; (E) studies that referred to the amount of provided light were allocated with 2 points, and when they did not, or only cited the lineage manual, they scored 0 points; (F) sexed studies were allocated with 2 points, and studies with mixed sexing got 1 point; (G) studies with three nutritional phases were allocated with 2 points, with two phases, they scored 1 point, and when they did not, or only cited the lineage manual, they scored 0 points; (H) studies with the definition of lineage scored 1 point, the study with no reference to the lineage did not score any points. For all the parameters that had not been mentioned, they were allocated 0 points.
The 16 selected papers met the criteria of eligibility for the parameters to be evaluated, such as: effect of supplementation of EOs on the development at 42 days (WG, FI and FC) and gut morphometry (villus height, crypt depth and ratio between villus height and crypt depth) of the duodenum, jejunum and ileum.
Among the performance parameters with the treatment of EOs, it is weight gain (WG), where 14 studies reached the result and 57.00% of measurements were significant; for feed conversion (FC), the result was more expressive, from which the 13 studies that measured this parameter, 71.00% obtained significant result with the treatment of EOs. For feed intake (FI), the result was unimpressive, once, from the 13 studies that measured this parameter, only 7.60% reached a significant result with the treatment of EOs.
Regarding the gut morphometry, villus height was the parameter that best responded to EOs and in eight studies that measured this parameter in the duodenum, 50.00% differed from control treatment; in jejunum, out of the nine studies that measured this parameter, 33.00% showed significant result; in the ileum, the results were unimpressive, with only 11% of the nine studies that measured this parameter with significant result, with diets treated with EOs.
Among the 16 selected papers, it was possible to observe that Ross 308 strain represented 43.75% of the genetics found in studies, while the second largest participation of the strain was Arbor Acres with 31.25%. About the poultry sex, 68.75% were male flocks, 18.75% were mixed flocks and 12.50% of the studies did not describe the poultry sex. The mean inclusion of EOs, or its combinations was of 411 g/T feed. The protected EOs were present in 31.25% of papers. The treatment lasted from 1 to 42 days, for 62.50% of the analysed papers. Regarding the ages for the performance parameters measured, 81.25% were up to 42 days, and the same age for the observation of morphology in 56.25%, in a single collection or associated to one more data collection, varying from 21 to 28 days. Finally, the bioactive carvacrol, thymol and cinnamaldehyde were present in 56.25, 50.00 and 31.25%, respectively (Table 3).
Table 3. General abstract of periodicals
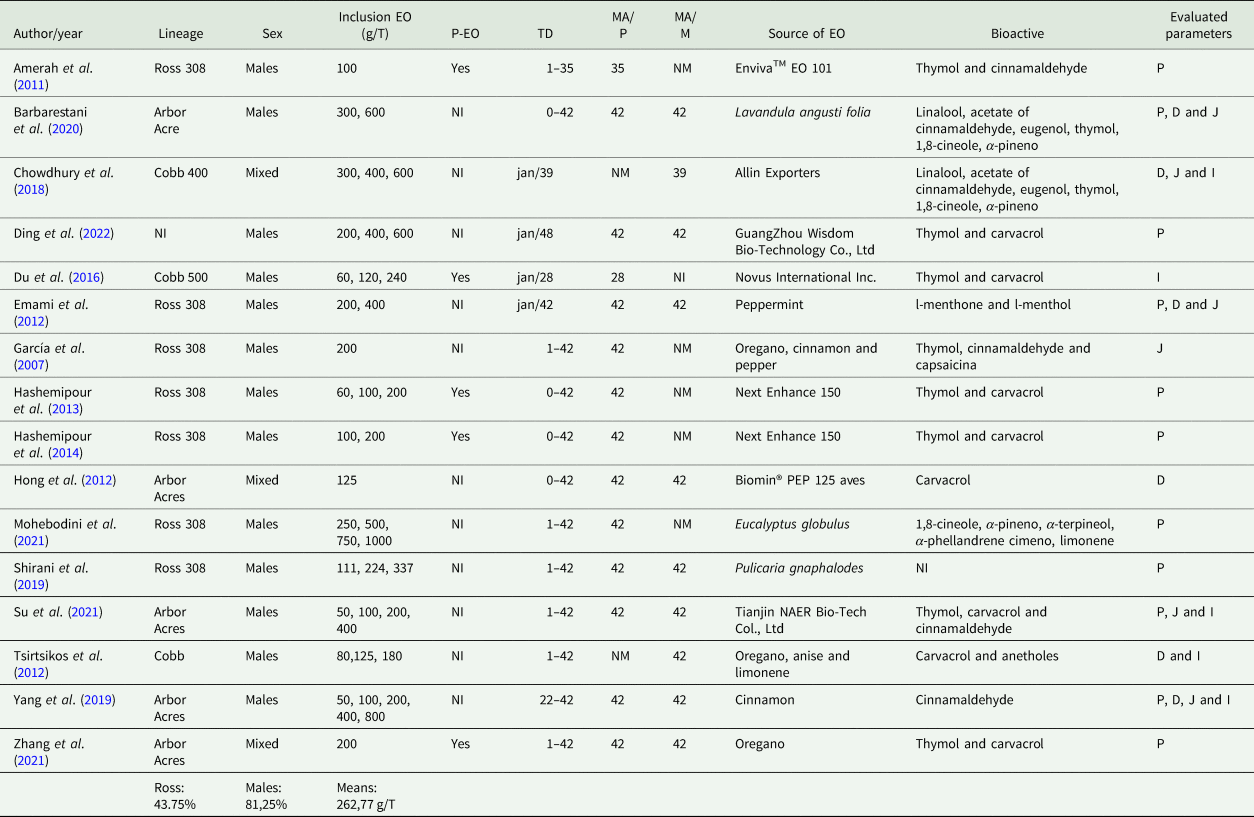
NI, non-identified; NM, not measured; EO, essential oil; P-EO, protected essential oil; TD, treatment duration; MA-P, measured age for performance; MA-M, morphology measured age; P, performance; D, duodenum; J, jejunum; I, ileum.
Meta-analysis
In the duodenum, P values in Cochran's Q test for villus height, crypt depth and villus height:crypt depth ratio were 0.002, 0.030 and 0.760, respectively. The values for I 2 statistics for these parameters were 73, 61 and 0%, respectively. For villus height, the model of random effects is the most appropriate; additionally, the value of the ratio between the means was 39.44 (P = 0.335), and, then, there is no effect of supplementation of EOs. For crypt depth, the model of random effects is the most appropriate, and, also, the value of the mean difference ratio was −5.61 (P = 0.451), so, there is no effect of supplementation of EOs. For the villus height:crypt depth ratio, the model of fixed effects is the best one, the mean difference was −0.20 (P = 0.094), which suggests that there is no difference between supplementing or not with EO (Fig. 2).

Figure 2. Forest plot of villus height (VC), crypt depth (CD) and the relationship between them (V:C) in the duodenum of broiler chickens as a function of dietary supplementation with essential oils.
The P values of Cochran's Q test for villus height, crypt depth and villus height:crypt depth ratio in jejunum were 0.560, 0.001 and 0.009, respectively. The I 2 statistical values for these parameters were 0, 84, 67%, respectively. For villus height, the model of fixed effects is the most appropriate one, the ratio mean difference was −28.15 (P = 0.031), suggesting that there is difference between supplementing or not with EOs. For crypt depth, the model of random effects is the best one, and, also, it suggests that the ratio mean difference was 10.81 (P = 0.142), and, then, there is no effect of supplementation with EOs. For the villus height:crypt depth ratio, the model of random effects is the most appropriate one, and the ratio mean difference was −0.45 (P = 0.243), which suggests that there is no difference between supplementing or not with EOs (Fig. 3).

Figure 3. Forest plot of villus height (VC), crypt depth (CD) and the relationship between them (V:C) in the jejune of broiler chickens as a function of dietary supplementation with essential oils.
In the ileum, the P values in the Cochran's Q test for villus height, crypt depth and villus height:crypt depth ratio were 0.054, 0.029 and 0.214, respectively. The values of the I 2 statistics for these parameters were 54, 60 and 31%, respectively. For villus height, the model of fixed effects is the most appropriate one, the ratio mean difference was −43.06 (P = 0.004), which suggests that there is difference between supplementing or not with EOs. For crypt depth, the model of random effects is the most appropriate one, the mean difference rate was 5.06 (P = 0.282), so, there is no effect of supplementation with EOs. For the villus height:crypt depth ratio, the model of random effects is the most appropriate one, the mean difference was −0.84 (P = 0.001), which suggests there is difference between supplementing or not with EOs (Fig. 4).
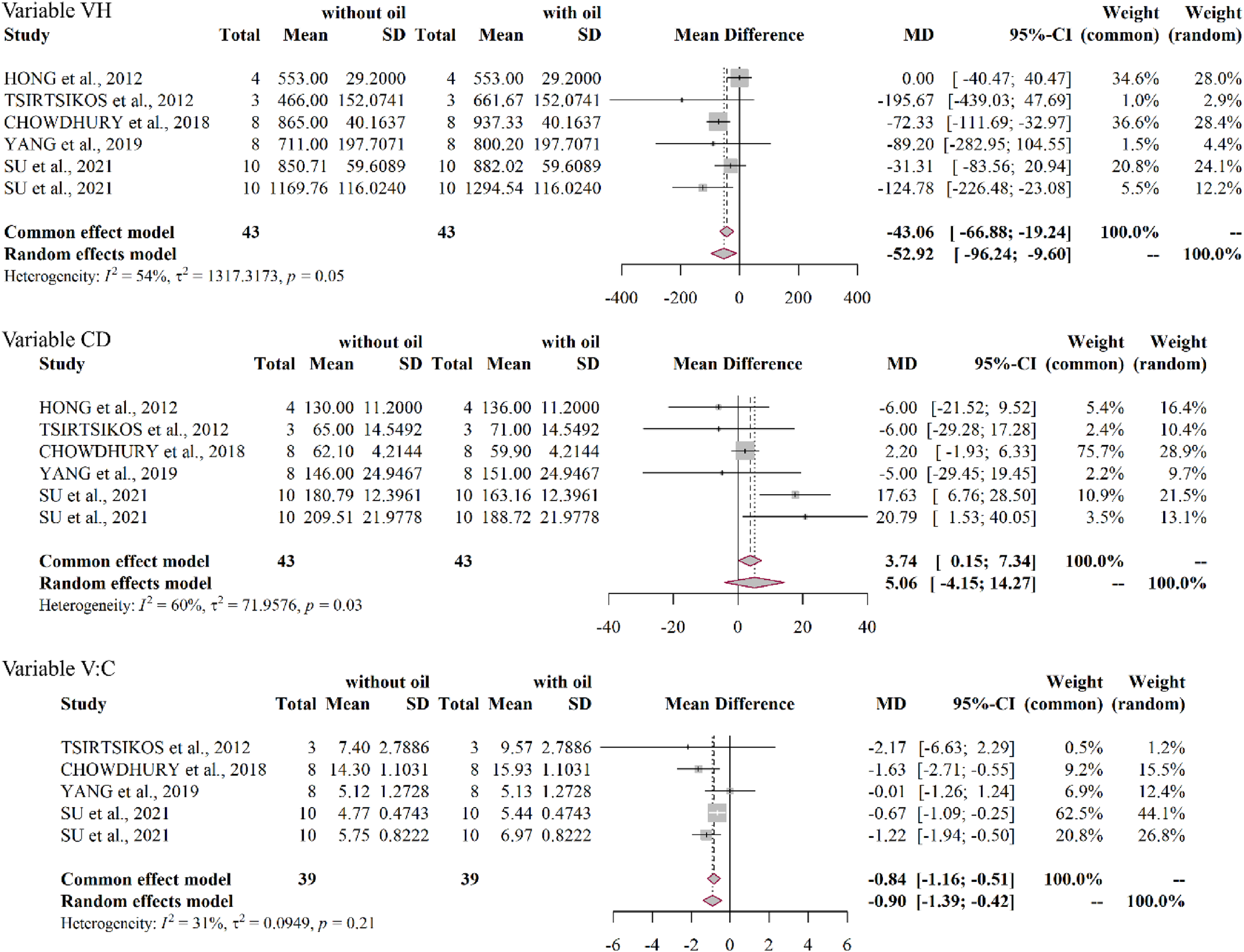
Figure 4. Forest plot of villus height (VC), crypt depth (CD) and the relationship between them (V:C) in the ileum of broiler chickens as a function of dietary supplementation with essential oils.
When the results of performance were evaluated, the P values of Cochran's Q test for final weight gain (WGP), daily weight gain (DWP), total feed intake (CF 0-42) and feed conversion (FC) were 0.767, 0.006, 0.025 and 0.001, respectively. The values of the I 2 statistics for these parameters were 0, 67, 60 and 99%, respectively. For variable WGP, the model of fixed effects is the best, the mean difference ratio was −77.40 (P = 0.001), which suggests there are differences between supplementing or not with EOs. For DWP, the model of random effects is the most appropriate one, the value of the mean difference was −1.35 (P = 0.001), so, there is an effect of supplementation with EOs. For CF 0-42, the model of random effects is the most appropriate one, the mean difference ratio was 22.42 (P = 0.337), which suggests there is no difference between supplementing or not with EOs. For FC, the model of random effects is the most appropriate one, the mean difference ratio was 0057 (P = 0.129), which suggests there is no difference between supplementing or not with EOs (Fig. 5).

Figure 5. Forest plot of weight gain per phase (WGP), daily weight gain (DWP), feed consumption per phase (CF) and feed conversion (FC) as a function of the use of essential oils in the diet of broiler chickens.
Meta-regression
The likelihood ratio test indicated, in all adjustments, that the linear Dose model is the most appropriate. The results of the mixed-model estimates showed varying impacts of doses on the response variables. For the VH variables (ileum and jejunum) and CD (ileum), there was no significant dose effect (P > 0.05). However, for performance variables, WGP showed an intercept of 2379.194 (SE = 114.0602) and a significant slope (P < 0.05) of 0.144 (SE = 0.051), with an R 2 of 0.986. Similarly, performance measured by DWP had an intercept of 57.201 (SE = 3.233) and a significant slope (P < 0.05) of 0.004 (SE = 0.001), with an R 2 of 0.990 (Table 4).
Table 4. Regression equations on the effect of essential oils dose (g/T of diet) on production villus height (VH), crypt depth (CD), weight gain per phase (WGP) and daily weight gain (DWP) of broiler chickens
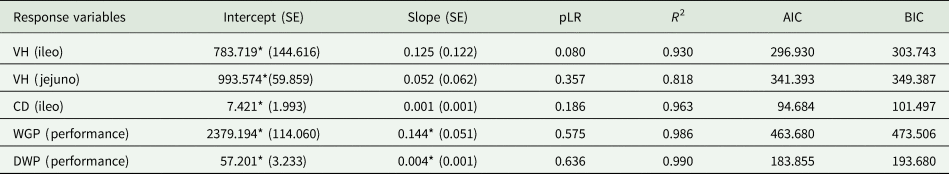
*Significant by t-test at 5%; pLR P value of the likelihood ratio test for comparison between the linear and quadratic model; R 2 represents the variance explained by the entire model, including both fixed and random effects (Nakagawa et al., Reference Nakagawa, Johnson and Schielzeth2017); AIC, Akaike information criterion; BIC, Bayesian information criterion; thy, thymol; car, carvacrol; cin, cinnamaldehyde; men, menthol; cap, capsaicina; cine, 1,8-cineole; pin, α-pineno; ter, α-terpineol; phe, α-phellandrene; cim, cimeno; lim, limonene.
Discussion
All the data on gut morphometry were collected from studies that measured this parameter after the poultry under treatment, at 21 days; this is because the broilers’ gut reaches its maximum performance during the first 20 or 30 days of life; when there is a period of maturation that involves morphological adaptations that are relevant for the poultry (Maiorka, Reference Maiorka2004).
The integrity of the cells that constitute the gut mucosa is one of the main factors for better absorption of nutrients, and, therefore, keep a healthy organism (Adedokun and Olojede, Reference Adedokun and Olojede2019). Thus, the gut immune system is its own epithelium, which is also responsible for the poultry development and growth. This gut barrier is formed by epithelial cells that are linked through joints, and provide impermeability to this layer of cells. In the area for the absorption of nutrients, the presence of villus and microvillus allows the maximization of the absorption, increasing the surface of epithelial layer (Celi et al., Reference Celi, Cowieson, Fru-Nji, Steinert, Kluenter and Verlhac2017). The villus and crypts are two important components from the small intestine, and its geometry provides an indicator of the absorption ability (Heydarian et al., Reference Heydarian, Ebrahimnezhad, Meimandipour, Hosseini and Banabazi2020). The renewal of gut epithelium reflects the dynamic balance between the production of enterocytes in the crypts and its subsequent peeling of villus; therefore, villus height and crypt depth are available criteria to evaluate gut health and function (Su et al., Reference Su, Zhou, Wang, Chen, Chen, Li and He2018). The villus height:crypt depth ratio (villus: crypt) is an indicator of the digestive ability of the small intestine. According to Luquetti (Reference Luquetti2005), a lower villus:crypt ratio means harmed villus and increased proliferative activity in the crypts, aiming to restore the epithelial form and function. On the other hand, the increase in this ratio corresponds to an increase in the nutrient's digestion and absorption (Montagne et al., Reference Montagne, Pluske and Hampson2003), due to a bigger surface area.
In many studies, the effects of EOs were demonstrated in feed intake, nutrients metabolism, digestive secretions and growth (Krishan and Narang, Reference Krishan and Narang2014; Peng et al., Reference Peng, Li, Li, Duan and Wu2016; Mehdi et al., Reference Mehdi, Létourneau-Montminy, Gaucher, Chorfi, Suresh, Rouissi, Brar, Côté, Ramirez and Godbout2018), as well as the effects on cultivable pathogens (Zhai et al., Reference Zhai, Liu, Wang, Wu and Kluenter2018). However, other studies (Lee et al., Reference Lee, Kim, Kim, Jeong, Oh, Cho and Lee2020; Mohebodini et al., Reference Mohebodini, Jazi, Ashayerizadeh, Toghyani and Tellez-Isaias2021) have shown that the response to feed supplementation of EOs in broilers improves the performance and feed efficiency, which has not been consistent, due to the dosage, source, type of EOs, diet and handling (Cross et al., Reference Cross, McDevitt, Hillman and Acamovic2007).
The results show that diets supplemented with EOs had a significant effect, with better development of the ileum and jejunum. Therefore, the poultry that had been supplemented with EOs gained more weight, when compared to the poultry that had only been fed with a basal diet. Similar results for the morphometry of jejunum were also observed in the studies of Chowdhury et al. (Reference Chowdhury, Mandal, Patra, Kumar, Samanta, Pradhan and Samanta2018); Barbarestani et al. (Reference Barbarestani, Jazi, Mohebodini, Ashayerizadeh, Shabani and Toghyani2020); Zhang et al. (Reference Zhang, Peng, Liu, Ma, Zhang, Guo, Xue and Zhao2021); Su et al. (Reference Su, Zhou, Wang, Chen, Chen, Li and He2020) and Ding et al. (Reference Ding, Hu, Yao, He, Chen, Wu, Wu, Zhang, He and Song2022). On the other hand, in the ileum, there were similar results observed to the study by Chowdhury et al. (Reference Chowdhury, Mandal, Patra, Kumar, Samanta, Pradhan and Samanta2018). In the duodenum, there was no significant result with the supplementation of EOs, which can be attributed to the way the supplementation is carried out as in general, EOs are encapsulated to guarantee the efficacy of their compounds, which depend on stability, bioactivity and bio-availability of the active ingredients in the food matrix (Holkem et al., Reference Holkem, Codevilla and Menezes2015), and this is due to the volatility and ease of oxidation, which tend to suffer before the presence of light, air, humidity and high temperatures (Aburto et al., Reference Aburto, Tavares and Martucci1998).
This microcapsule consists of a layer of the encapsulated agent, being, in general, constituted by polymeric material, which acts as a protective film, isolating the active substance, hindering its inadequate exposure. This membrane is torn under specific stimulus, releasing the substance in the place or in the ideal moment (do Carmo et al., Reference do Carmo, Fernandes and Borges2015), and in this case, the acting of EOs as a bioactive seems to freely happen in the jejunum and ileum, because the release of these compounds happens from the duodenum and, likewise, it is possible to infer that the results happened in the segments where the bioactive has longer time of action.
Beyond the microencapsulation, many factors may have influenced the results of meta-analysis, as a type of phytotherapy supplementation (dry herbs or plant extracts, e.g.). The concentration of active herbs in the plants can vary according to the used vegetative part, season, vegetative cycle, type of the soil where it was grown and the technique used for extraction (Windisch et al., Reference Windisch, Schedle, Plitzner and Kroismayr2008). Therefore, according to Jamroz et al. (Reference Jamroz, Wiliczkiewicz, Wertelecki, Orda and Skorupińska2005) the standardization of EOs is difficult (as it can be observed in table 5 that there are diverse sources of EOs, with different bioactives), as well as the standardization of their antimicrobial, antioxidant, immunomodulation activities and anti-inflammatory action.
Some studies show that the antimicrobial activity of EOs can induce a more balanced microbiota, because of the increase of the concentration of Lactobacillus ssp. and the decrease of coliforms and E. coli in broilers (Cetin et al., Reference Cetin, Yibar, Yesilbag, Cetin and Cengiz2016; Liu et al., Reference Liu, Yang, Xin, Chen, Yang, Duan and Yang2017; Giannenas et al., Reference Giannenas, Bonos, Skoufos, Tzora, Stylianaki, Lazari, Tsinas, Christaki and Florou-Paneri2018). The stimulatory effects of phytogenic additives in the gut secretion of mucus can prevent the adherence of pathogens to the mucosa gut (Jamroz et al., Reference Jamroz, Wertelecki, Houszka and Kamel2006). On the other hand, beneficial bacteria such as the lactic ones can stimulate the increase of calceiform cells that are involved in the secretion of mucin. The layer of gut mucus plays a fundamental role in the hindering of the adherence of pathogens to gut epithelial cells, which, consequently, reduces the incidence of their toxic effects (Baurhoo et al., Reference Baurhoo, Ferket and Zhao2009; Kim and Ho, Reference Kim and Ho2010) and provides means for the increase of gut villus. In addition, the antimicrobial activity can be corroborated by the findings of Trombetta et al. (Reference Trombetta, Castelli, Sarpietro, Venuti, Cristani, Daniele, Saija, Mazzanti and Bisignano2005) and Devi et al. (Reference Devi, Nisha, Sakthivel and Pandian2010), who affirmed that the group carbonyl of the Cinnamaldehyde is linked to the proteins, hindering the action of the enzyme and creating pores in the cellular membrane, and thymol and eugenol induce their antimicrobial action through a disorder of lipid fraction in the plasmatic membrane of the microorganisms, resulting in changes in the permeability of the membrane, and the extravasation of intracellular materials, till the possible cellular death.
Another property of EOs that may explain the results in this study is this antioxidant action, because, during the digestive processes, oxygen radicals are released, and they hinder the gut mucosa. The EOs protect the villi from oxidative damage. Nevertheless, bioactive substances that stimulate the activity of oxidative enzymes avoid damages to villus (Chowdhury et al., Reference Chowdhury, Mandal, Patra, Kumar, Samanta, Pradhan and Samanta2018). The proximity of the mucosa surface and the gut content can motivate the oxidative stress which is caused by the digestive process (Windisch et al., Reference Windisch, Schedle, Plitzner and Kroismayr2008). In this sense, phytogenic additives can positively affect the activity of antioxidant enzymes which, in turn, reduces the production of reactive species of oxygen, known as inflammatory factors in tissues and cells, by causing gut atrophy and disorder of the gut epithelial barrier (Moretti et al., Reference Moretti, Mrakic-Sposta, Roncoroni, Vezzoli, Dellanoce, Monguzzi, Branchi, Ferretti, Lombardo, Doneda, Scricciolo and Elli2018). Excessive oxidative stress can cause gut inflammation and, even, cellular apoptosis in the tissue, following the dysfunctions (Xue et al., Reference Xue, Shen, Hu, Hu, Kumar, Yang and Wen2020). Therefore, the antimicrobial and antioxidant properties of EOs can stimulate a healthy microbiota and consequently, the improvement in the immunological system of the gut mucosa, which has the duty of eliminating potential pathogens, keeping a relation which is mutually beneficial with the commensal microbiota (Liu et al., Reference Liu, Gao, Shi, Cong, Chen, Zhang, Shi, Cao, Wang, Zhang, Ji, Jing and Feng2020); the consequence can be a better availability of nutrients, resulting in efficiency in the feed conversion and weight gain.
In summary, this work represents significant advances in the use of EOs in broiler chickens. However, it is important to note that applying the article selection criteria revealed some limitations in the database for meta-analysis and meta-regression. In the meta-analysis, considering the presence or absence of EOs in the broiler diet, it was not possible to include the different doses reported in the papers within the statistical model. In the meta-regression, when considering the doses used in the studies, there is no standardization of the composition of the bioactives or their proportions within the compound. At present, the state of the art in this field requires a larger number of publications to address these limiting factors, although this study provides potential indications of which bioactives and concentrations may be studied or utilized.
Conclusion
The use of EOs in broiler diets has been proven, through this study of systematic review with meta-analysis, to be a supplementary tool to act in the improvement of feed efficiency and in the integrity of gut mucosa.
Acknowledgements
The National Council for Scientific and Technological Development (CNPq), the National Institute of Animal Science and Technology (INCT-CA/CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Foundation for Research Support of Rio Grande do Norte (FAPERN).
Author contributions
Roberto Rocha, Pedro Fidelis and Andreia Massuquetto collected the data for this study; Sérgio Turra, Cláudia Lopes, Elias Medeiros and Rennan Moreira conducted the statistical analyses; Roberto Rocha, Camilla Silva and Marcelle Araújo wrote the initial draft of this manuscript; Rennan Moreira and Roberto Rocha collaborated in interpreting the results; Camilla Silva and Rennan Moreira finalized the manuscript. Both authors have read and approved the finalized manuscript.
Funding statement
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
None.
Ethical standards
Not applicable.












