IntroductionFootnote **
The Cambrian fauna has long been recognized as distinct from the later Paleozoic fauna (Conway Morris, Reference Conway Morris1989), notably thanks to soft-bodied elements recovered from Burgess Shale-type biotas in the former (Gaines et al., Reference Gaines, Briggs and Yuanlong2008; Gaines, Reference Gaines2014). However, more recent fossil discoveries from Konservat Lagerstätten around the world have demonstrated the persistence of select members of the Cambrian fauna into the mid to late Paleozoic. Important examples include diverse organisms from the early Ordovician (Tremadocian–Floian) Fezouata Lagerstätte (Van Roy et al., Reference Van Roy, Orr, Botting, Muir, Vinther, Lefebvre, el Hariri and Briggs2010), the Silurian (Pridolian) Herefordshire Lagerstätte (Siveter et al., Reference Siveter, Briggs, Siveter and Sutton2020), and the early Devonian (Pragian to Emsian) Hunsrück Slate (Rust et al., Reference Rust, Bergmann, Bartels, Schoenemann, Sedlmeier and Kühl2016), among others. Nonetheless, the record of Burgess Shale-type faunas in post-Cambrian deposits remains sparse and patchy, not least because of the decrease in abundance of Lagerstätten from open marine environments after the Cambrian (Orr, Reference Orr2014; Muscente et al., Reference Muscente2017). This presents a major challenge for understanding the geographic distribution and ultimate decline of biotas of Cambrian origin through the Paleozoic.
One of the most distinctive classes belonging to the Cambrian fauna is Marrellomorpha, rare arthropods with unmineralized cuticle. Their distribution is restricted to Cambrian–Devonian Konservat Lagerstätten, but even here they occur only sporadically (Aris et al., Reference Aris, Corronca, Quinteros and Pardo2017). Where they do occur, however, they range from among the most abundant species (Whittington, Reference Whittington1971; Caron and Jackson, Reference Caron and Jackson2008; Kühl and Rust, Reference Kühl and Rust2010) to an exceedingly rare element of the fauna (Liu, Reference Liu2013). Two orders have been recognized within Marrellomorpha: Marrellida and Acercostraca (Rak et al., Reference Rak, Ortega-Hernández and Legg2013). Members of the former are characterized by cephalic shields with two or three pairs of extremely elongate spinous projections and two to three pairs of uniramous cephalic appendages, while the latter possess large ovoid carapaces that cover the entire dorsal side of the animal and up to five pairs of cephalic appendages, the posteriormost one with a distinct filiform endopod. Both are united in possessing uniramous antennules and a multisegmented trunk bearing biramous appendages with exopods ornamented with medially directed setae (Rak et al., Reference Rak, Ortega-Hernández and Legg2013). To date, four species of marrellids (Whittington, Reference Whittington1971; Kühl and Rust, Reference Kühl and Rust2010; Rak et al., Reference Rak, Ortega-Hernández and Legg2013; Aris et al., Reference Aris, Corronca, Quinteros and Pardo2017) and three acercostracans (Siveter et al., Reference Siveter, Fortey, Sutton, Briggs and Siveter2007; Kühl et al., Reference Kühl, Bergström and Rust2008; Legg, Reference Legg2016a) have been formally described, and at least one additional undescribed species of marrellid has been figured (Van Roy et al., Reference Van Roy, Orr, Botting, Muir, Vinther, Lefebvre, el Hariri and Briggs2010). In addition to these, the smaller and less well-known Skania and Primicaris have been suggested to have acercostracan affinities (Legg, Reference Legg2015, Reference Legg2016a) while other putative marrellids are represented by isolated fragments (Haug et al., Reference Haug, Castellani, Haug, Waloszek and Maas2012; Legg, Reference Legg2016b). Marrella splendens Walcott, Reference Walcott1912 from the Cambrian remains the most abundant and well-known marrellomorph (Whittington, Reference Whittington1971; García-Bellido and Collins, Reference García-Bellido and Collins2006).
Despite the relative wealth of morphoanatomical information, the phylogenetic affinities of marrellomorphs have been problematic. In line with traditional views (Whittington, Reference Whittington1971; Kühl et al., Reference Kühl, Bergström and Rust2008), some phylogenetic analyses in the past decade have found marrellomorphs to be arachnomorphs, allied closely with artiopodans (Aria and Caron, Reference Aria and Caron2017b; Moysiuk and Caron, Reference Moysiuk and Caron2019) or, more unexpectedly, pycnogonids (Vannier et al., Reference Vannier, Aria, Taylor and Caron2018). Alternatively, marrellomorphs have been considered either stem mandibulates (Legg et al., Reference Legg, Sutton and Edgecombe2013; Legg, Reference Legg2015) or stem “crustaceans” (Siveter et al., Reference Siveter, Fortey, Sutton, Briggs and Siveter2007; Ortega-Hernández et al., Reference Ortega-Hernández, Legg and Braddy2013) because of suggested homology of the carapace in acercostracans with that of certain early mandibulates and the presence of tergopleural rings and multisegmented exopods with medially directed lamellar setae, at least in marrellids (see also Haug et al., Reference Haug, Maas and Waloszek2009). Finally, positions in the euarthropod stem group have also been suggested (Haug et al., Reference Haug, Castellani, Haug, Waloszek and Maas2012; Aria and Caron, Reference Aria and Caron2017a). The monophyly of Marrellomorpha has been favored in the most recent literature although this has also been questioned (Siveter et al., Reference Siveter, Fortey, Sutton, Briggs and Siveter2007). These broad discrepancies pose a challenge for rooting the marrellomorph tree.
Here, for the first time, we describe the occurrence of soft-bodied fossils, including a specimen representing a new genus and species of marrellomorph arthropod, from shallow marine deposits of the Late Ordovician (early Katian) Kirkfield Formation (Paton and Brett, Reference Paton and Brett2020) of southeastern Ontario. Referred to as the Brechin Lagerstätte, this locality has already been made famous by its exceptionally preserved echinoderm-dominated assemblages of biomineralizing organisms (Brett and Liddell, Reference Brett and Liddell1978; Cole et al., Reference Cole, Ausich, Wright and Koniecki2018, Reference Cole, Wright, Ausich and Koniecki2020). This finding represents a rare example of soft-tissue preservation in an Ordovician open shelf environment in association with a typical “shelly” marine biota. The marrellomorph is particularly significant as the first post-Cambrian representative found in Laurentia, and we take this finding as an opportunity to reevaluate marrellomorph systematics.
Geological setting
The marrellomorph specimen and other soft-bodied elements, including algae and potential arthropod carapaces, were collected from a study site exposing units of the Upper Member of the Kirkfield Formation in Tomlinson Quarry near Brechin, Ontario, Canada. This formation makes up the middle portion of the Simcoe Group, which records the widespread development of the Trenton Carbonate Platform in the epeiric seas along the southeastern margin of Laurentia (Brookfield and Brett, Reference Brookfield and Brett1988; Paton and Brett, Reference Paton and Brett2020). The Sandbian–Katian boundary is identified in the Lower Member of the Kirkfield Formation, with the remainder of the formation being deposited in the earliest Katian (Paton and Brett, Reference Paton and Brett2020). The Kirkfield Formation is dominated by carbonate facies, representing offshore shoal to shallow marine environments (around tens of meters of water); however, thin intercalations of K-bentonites and storm-deposited silty shales record occasional input of volcanic ash and other terrigenous material from the nearby Taconic Mountains (Brookfield and Brett, Reference Brookfield and Brett1988).
The study area (approximately 44°35.496′N, 79°5.626′W) consists of biohermal communities established on hardground surfaces within a complex of sedimentary structures that follows a paleocurrent-aligned submarine ridge situated along a northeast to southwest line of strike, visible in outcrop. The ridge formed a subtle positive topographic feature at the northern margin of the biohermal zone and exerted strong controls over the formation and subsequent development of a range of structures, notably the complex hardground surfaces that formed the substrate for the diverse echinoderm–bryozoan-dominated fauna (Paton et al., Reference Paton, Brett and Kampouris2019). The basal hardground (Unit a) and tiered hardground mounds (Units b and c) were repeatedly buried by up to 11 discrete obrution beds that are nonuniformly present across the study area, depending on local variations in depth and distance from the marginal ridge where all beds thin, pinch out, and grade into each other (Fig. 1.1–1.3). The local stratigraphy has been examined comprehensively (Paton et al., Reference Paton, Brett and Kampouris2019; Paton and Brett, Reference Paton and Brett2020), and the diverse echinoderm faunas have been the focus of several recent publications (e.g., Cole et al., Reference Cole, Ausich, Wright and Koniecki2018, Reference Cole, Wright and Ausich2019, Reference Cole, Wright, Ausich and Koniecki2020; Wright et al., Reference Wright, Cole and Ausich2020).

Figure 1. Brechin Lagerstätte locality information. (1) Stratigraphic column in the study area, modified after Paton et al. (Reference Paton, Brett and Kampouris2019). (2) Photographs of the site, looking east, showing the beds in which soft-tissue preservation has been observed and their relationship to the basal hardground and mound/basin structures, with hammer for scale. (3) Topographic map of the main study plot, with the location of ROMIP 66233 marked with an X; high relief mounds (prefix M) are numbered and shaded dark while surrounding basins are shaded lighter. (4) Generic richness subdivided by major fossil group in Bed 3 from the study plot; see supplementary text for details.
Of primary interest here are two shale horizons, Beds 1 and 3 (Fig. 1.1, 1.2), which preserve remains of nonmineralized fauna and flora. Bed 1 is soft brown-grey organic-rich shale, present only in the deepest mound basins, that covered robust echinoderm–bryozoan bioherms at their peak development and diversity. Preservation of nonmineralized elements is common, but they are fragmentary. Deposition of Bed 2, cross-bedded siltstone up to 15 cm thick, subsequently buried much of the hardground surface except for protruding mounds from units b and c, around which firmground communities locally reestablished. No soft-bodied elements are found in this bed. Bed 3, from which the marrellomorph was retrieved, consists of up to 10 cm of soft-grey shale grading to a hard brown-grey silty shale due to coarser material accumulating closer to the marginal ridge. This represents a major burial event that wiped out the recovering bioherms, resulting in crinoids being uprooted, decapitated, and buried with their stems oriented to the south. Large (100–300 μm) clusters of pyrite crystals are common. Soft-tissue preservation has been encountered thus far only in Tomlinson Quarry, but Bed 3 is laterally extensive at least to Carden Quarry (see Paton and Brett, Reference Paton and Brett2020). Other shale layers are present (Beds 5, 7, 9), but none has thus far yielded soft-bodied fossils.
Cooccurring with the marrellomorph in Bed 3 are macroalgae, carapaces, and other indeterminate remains of nonbiomineralizing organisms, suggesting that conditions were conducive to soft-tissue preservation (Fig. 2). Bed 3 also hosts a diverse shelly marine fauna, including various trilobites, echinoderms, bryozoans, mollusks, conulariids, and receptaculitids, as well as trace fossils (Fig. 1.4; Supplementary Text, Supplementary Table 1). Fossils are abundant only around mound-adjacent basins where Bed 3 reaches its thickest.

Figure 2. Representative biota from Brechin Lagerstätte. (1) ROMIP 66259, branching macroalga associated with the crinoid Reteocrinus, recovered from Bed 3. (2) ROMIP 66258, partial arthropod carapace, recovered from Bed 3. (3) ROMIP 65095, large slab covered in mineralized fauna, recovered from Bed 1 near Mound 37. (1, 2) Scale bars = 10 mm; (3) scale bar = 50 mm.
Materials and methods
In the following, we detail methods involved in field data collection, elemental mapping, and phylogenetic analyses.
Field collection
A collected surface area of ~85 by 30 meters on a platform near the top of Tomlinson Quarry was available for excavation and study (Fig. 1.3). At least 20% of the soft and friable Bed 3 shale was lost due to wastage during excavation. The fossil abundance data reported in this paper reflects collections made from the remaining portions of Bed 3 in the study site, with greatest collecting intensity in the thickest mound-adjacent fossil-rich areas. Following the unexpected discovery of the marrellomorph in a fossil-poor section of shale between mounds, ~700 m2 of surrounding shale was excavated more systematically. Taxon abundances are reported in Supplementary Table 1 but must be interpreted cautiously considering the uneven sampling approach.
Elemental mapping
The composition of the marrellomorph was investigated with elemental mapping, performed with an environmental scanning electron microscope (FEI Quanta 200 FEG) equipped with an energy scanning spectroscopy (EDS) X-ray detector and octane plus silicon drift detector at the University of Windsor Great Lakes Institute for Environmental Research, Canada. Imaging analyses were conducted with the following operating conditions: fixed average working distance of 10.3 mm (minor variation across specimen due to differences in topography) for basic imaging and EDS, 12 kV beam accelerating voltage, 252 μA beam current at source, 70 Pa chamber pressure (low vacuum), 30 μm aperture for imaging, and 40 μm aperture for EDS.
Phylogenetic methods
The objective of our phylogenetic analysis was to build upon previous work on marrellomorphs (Rak et al., Reference Rak, Ortega-Hernández and Legg2013; Legg, Reference Legg2015, Reference Legg2016a; Aris et al., Reference Aris, Corronca, Quinteros and Pardo2017) but also to critically review all relevant characters, compare additional phylogenetic methods, and test the influence of competing outgroup hypotheses. Rather than using a major panarthropod phylogeny, (e.g., Legg, Reference Legg2016a), we favored a marrellomorph-centric data set that would allow us to test the sensitivity of patterns of character evolution and ingroup relationships under different outgroup hypotheses. We therefore chose Aris et al. (Reference Aris, Corronca, Quinteros and Pardo2017) as our primary dataset of reference.
Our new phylogenetic matrix consists of 19 taxa and 44 characters and includes substantial corrections and modifications and new outgroups based on recent advances. More specifically, we removed noninformative and redundant characters as well as any whose definitions we considered to be insufficiently specific or poorly justified (see Supplementary Text). Since the position of Marrellomorpha among arthropods has been controversial, we employed multiple outgroups and used topological constraints to investigate how differing outgroup relationships might affect the ingroup. We selected Kylinxia (stem Euarthropoda; Zeng et al., Reference Zeng, Zhao, Niu, Zhu and Huang2020), Waptia (total group Mandibulata; Vannier et al., Reference Maddison and Maddison2018), Tokummia (total group Mandibulata; Aria and Caron, Reference Aria and Caron2017a), Mollisonia (stem Euchelicerata; Aria and Caron, Reference Aria and Caron2019), Eoredlichia (Artiopoda; Hou et al., Reference Hou, Clarkson, Yang, Zhang, Wu and Yuan2008), Haliestes (Pycnogonida; Siveter et al., Reference Siveter, Sutton, Briggs and Siveter2004), and Palaeoisopus (Pycnogonida; Bergström et al., Reference Bergström, Stürmer and Winter1980) as well-known representatives of major outgroups. The trees were rooted on Kylinxia. We also included the problematic taxon Aquilonifer (Briggs et al., Reference Briggs, Siveter, Siveter, Sutton and Legg2016) as in one previous study this was found to be possibly allied to marrellomorphs (Vannier et al., Reference Vannier, Aria, Taylor and Caron2018). We specifically excluded some larval crustaceomorph taxa previously used as outgroups due to the known problems of incorporating different developmental stages in the same phylogeny (Sharma et al., Reference Sharma, Clouse and Wheeler2017).
Our main analyses made use of the inapplicable state-corrected Parsimony approach (Brazeau et al., Reference Brazeau, Guillerme and Smith2019) available in the R 4.1.0 (R Core Team, 2020) package TreeSearch 0.4.3.9010 (Smith, Reference Smith2018), which relies on MorphyLib (Brazeau et al., Reference Brazeau, Smith and Guillerme2017). Additional functions were supplied from packages ape 5.5 (Paradis et al., Reference Paradis, Claude and Strimmer2004), phangorn 2.7.0 (Schliep, Reference Schliep2011), TreeTools 1.4.5 (Smith, Reference Smith2019a), strap 1.4 (Bell and Lloyd, Reference Bell and Lloyd2015), and extraDistr 1.9.1 (Wolodzko, Reference Wolodzko2020). R code is available in supplementary files. For each analysis, we used 100 independent searches starting from random trees, with 3 × 6 initial TBR iterations followed by 12 rounds of Parsimony ratchet with six TBR iterations each, and finishing with 3 × 6 more TBR iterations. The maximum number of hits was set to 100. Analyses were performed under equal and implied weights, sampling concavity constants for the latter from a discrete gamma distribution with shape and size parameters equal to 3 with an additive constant of 2, chosen to sample the range of recommended values (Smith, Reference Smith2019b). In addition to these unconstrained analyses, we ran two equal-weights analyses with topological constraints to enforce the monophyly of Chelicerata exclusive of marrellomorphs and a sister group relationship between mandibulates and marrellomorphs. Jackknife resampling was performed 1,000 times to estimate clade support, using 2 × 6 starting iterations of TBR followed by five ratchet iterations, with six TBR iterations each, finishing with 3 × 6 TBR iterations per round. The maximum number of hits was set to 20.
We also performed standard Fitch Parsimony analysis in TNT 1.5 (Goloboff and Catalano, Reference Goloboff and Catalano2016) using 1,000 searches from random starting trees. We used five ratchet iterations and five rounds of tree fusing up to 50 hits of the best tree length, otherwise maintaining default xmult search settings. Results under implied weights (concavity = 3 and 10) resulted in identical consensus trees, so only the equal-weights result is shown. Jackknifing was performed 1,000 times using xmult settings with 100 replicates each and five ratchet iterations.
Finally, we performed Bayesian analysis in MrBayes 3.2.6 (Ronquist et al., Reference Ronquist, Van Der Mark, Teslenko, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) using an Mkv + gamma model (Lewis, Reference Lewis2001). Four chains starting from random trees were run for 3 × 106 generations, sampling every 1,000 generations, with a burn-in fraction of 20%. Convergence was verified in Tracer 1.7.1 (Rambaut et al., Reference Rambaut, Surchard, Xie and Drummond2014). We also performed two analyses with the same topological constraints detailed in the preceding.
To investigate character optimization over the resulting trees, we used Parsimony ancestral state reconstruction in Mesquite 3.40 (Maddison and Maddison, Reference Maddison and Maddison2018).
Repository and institutional abbreviation
All specimens figured in this study are reposited in the Royal Ontario Museum Invertebrate Palaeontology (ROMIP) collections.
Systematic paleontology
Phylum Euarthropoda Lankester, Reference Lankester1904
Class Marrellomorpha Beurlen, Reference Beurlen1930
Order Marrellida [nom. correct. Størmer, Reference Størmer and Moore1959, pro Marrellina Raymond, Reference Raymond1920]
Diagnosis
Euarthropod with cephalic shield extending into at least two pairs of extremely elongated lateral spines. Some cephalic spines bearing secondary spines. First and second cephalic appendages uniramous. Trunk subequal to or shorter than cephalic spines, composed of more than 25 segments. Each trunk segment consisting of a short tergopleural ring and a pair of biramous appendages, with endopods bearing blunt subtriangular endites and highly subdivided exopods (~15 or more podomeres), each emitting elongated lamellae. Terminal segment a small undifferentiated plate with no distinct telson or caudal rami.
Remarks
Emended from Raymond (Reference Raymond1920). Størmer (Reference Størmer and Moore1959) later corrected the name but did not revise the diagnosis. Raymond's order included only the genus Marrella and the short original diagnosis was: “Form trilobite-like, pleural lobes reduced, endobases absent from coxopodites of body, pygidium a small plate.” We here revise the diagnosis considering more recent discoveries and our phylogenetic character optimizations.
Family Mimetasteridae Birenheide, Reference Birenheide1971
Type genus
Mimetaster Gürich, Reference Gürich1931.
Other genera
Tomlinsonus new genus.
Undescribed Moroccan marrellid
Specimens of this taxon have been referred to as Furca mauritanica (nomen nudum), as informally described by Van Roy (Reference Van Roy2006), although they have been figured in several publications (e.g., Van Roy et al., Reference Van Roy, Briggs and Gaines2015). Specimens in the ROMIP collection show the diagnostic traits of Mimetasteridae (see Phylogenetic results). Differences between the shield shape of the Moroccan taxon and Furca bohemica Fritsch, Reference Fritsch and Barrande1908 are sufficient to justify considering the former a new genus, but a formal description is beyond the scope of this paper.
Other putatively included genera
Furca Fritsch, Reference Fritsch and Barrande1908: F. bohemica, as most recently described by Rak et al. (Reference Rak, Ortega-Hernández and Legg2013), has a mimetasterid-like shield, with anterolateral spines and elongate secondary spines on the anterior and posterior edges of both the anterolateral and posterolateral spines. However, other body parts for Furca are currently unknown, and thus, we regard the assignment of Furca to Mimetasteridae as tentative, contra Rak et al. (Reference Rak, Ortega-Hernández and Legg2013).
‘Mimetaster’ florestaensis Aris et al., Reference Aris, Corronca, Quinteros and Pardo2017 was considered as a species of Mimetaster because of its phylogenetic position. As with F. bohemica, the shield of ‘M.’ florestaensis shows diagnostic traits of Mimetasteridae, but the absence of the trunk and appendages prevents a confident assessment of its affinity. Furthermore, we suggest that ‘M.’ florestaensis presents important differences from M. hexagonalis Gürich, Reference Gürich1931, including shape of the cephalic shield, shape and orientation of the cephalic spines, and shape of the secondary spines. The two also differ widely in age (Ordovician versus Devonian). ‘M.’ florestaensis may be more appropriately assigned to Furca or a new genus, but this is also beyond the scope of this paper.
Diagnosis
Marrellids with anterolateral spines protruding from the shield, each carrying a row of secondary spines on both anterior and posterior margins. Mediolateral and posterolateral spines also bearing marginal secondary spines. Secondary spines elongate, digitiform. Shield extends ventrally into a large, ellipsoidal hypostome. Cephalon includes three appendage-bearing segments. Second pair of appendages stenopodous, uniramous, greatly enlarged compared with other limbs, subdivided into around nine podomeres. Terminal podomere elongated, flattened, sometimes may be subdivided. Subterminal podomere bearing a spine. Third pair of appendages stenopodous, uniramous. Trunk with 30 or more appendage-bearing segments.
Remarks
Emended from Birenheide (Reference Birenheide1971). Mimetasteridae was originally diagnosed as for the type genus, by monotypy. Following new discoveries and phylogenetic analyses, the family has been expanded to include other taxa (Rak et al., Reference Rak, Ortega-Hernández and Legg2013; Aris et al., Reference Aris, Corronca, Quinteros and Pardo2017) but has not been formally emended up to now. We here revise the diagnosis considering phylogenetic character optimizations.
Genus Tomlinsonus new genus
Type species
T. dimitrii n. sp., by monotypy.
Diagnosis
As for type species, by monotypy.
Etymology
In reference to Tomlinson Quarry, where the holotype was discovered.
Holotype
ROMIP 66233, part and counterpart, a nearly complete head and partial trunk remains preserved in ventral view, representing the only known specimen.

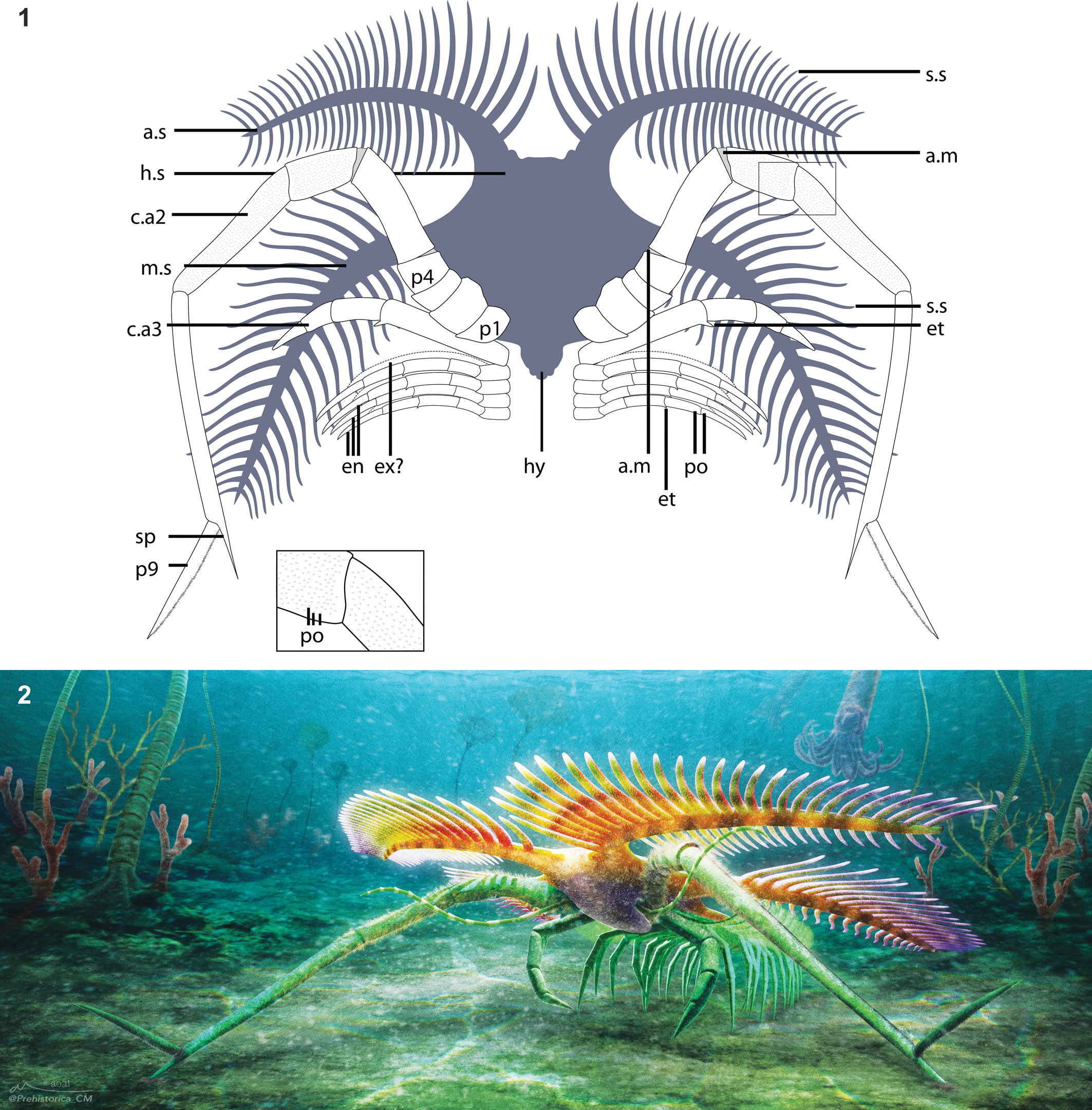
Figure 3. Overview of Tomlinsonus dimitrii, holotype, ROMIP 66233. (1) Composite image of part and counterpart. (2) Part. (3) Counterpart. Scale bars = 10 mm. Photos taken with specimen submerged in alcohol and polarized lighting. a.s = anterolateral spine; c.aX = cephalic appendage X; en = endopod of trunk appendage; hs = head shield; hy = hypostome; m.s = mediolateral spine; pX = podomere number X; s.s = secondary spine; sp = spine on p8 of hypertrophied limb.

Figure 4. Closeup views of Tomlinsonus dimitrii, holotype, ROMIP 66233. (1) Line drawing of specimen (part–counterpart composite), with boxed areas representing numbered closeups in following panels. (2) Proximal part of hypertrophied appendage and other appendages on counterpart. (3) Appendages and mediolateral spine on part. (4) Closeup of well-preserved cuticle with pores on podomere 7. (5) Distal end of hypertrophied appendage on counterpart. (2, 3, 5) Scale bars = 4 mm; (4) scale bar = 1 mm. a.m = possible arthrodial membrane; et = endite; po = pores; se? = possible serrated margin; other abbreviations as in Figure 3.

Figure 5. Elemental maps of the seventh podomere of a hypertrophied appendage of Tomlinsonus dimitrii. (1) Boxed region showing the location of the maps on the part. (2) Photograph of the mapped region with arrows indicating fragments of well-preserved cuticle; note the large crystals occupying central void on left side. (3) Carbon map with arrows pointing to cuticle fragments. (4) Aluminum map. (5) Silicon map. (6) Calcium map; note enrichment in crystals on the left. Scale bars = 1 mm.
Diagnosis
Mimetasterid with subhexagonal central shield bordered by narrow, backward-curving spines. Broad rounded notch between anterolateral and mediolateral spines. Elongate, digitiform secondary spines present on anterolateral and mediolateral spines, on both anterior and posterior margins, oriented at a slight acute angle to primary spines. Second pair of appendages extremely elongate with subterminal podomere bearing a spine about 30% as long as the terminal podomere. Terminal podomere flattened, elongated, and undivided with a blunt tip.
Occurrence
Kirkfield Formation, Upper Member (Katian; Corynoides americanus Graptolite Biozone; Amorphognathus tvaerensis Conodont Biozone); Bed 3 of Tomlinson Quarry, southeastern Ontario, Canada.
Description
The holotype consists of a partially visible cephalic shield, a pair of hypertrophied cephalic appendages, one smaller cephalic appendage, and traces of at least four minute trunk appendages (Figs. 3–4). The shale split irregularly through the specimen, but preparation of both part and counterpart revealed details of the cephalic spines and appendages, respectively.
The cephalic shield (Fig. 3) measures about 13.5 mm long to the attachment of the hypertrophied appendages and about 9.6 mm wide at the point of maximal constriction between the anterolateral and mediolateral spines. A slightly darker, posteriorly tapering, fusiform medial region, extending nearly from the anterior margin of the shield to slightly beyond the attachment site of the hypertrophied appendages, may represent the hypostome. The anterolateral spines project forward from the central shield at an angle of ~40° to the midline, but they curve along the proximal third of their length to project laterally, nearly orthogonal to the sagittal axis. They measure approximately 3.4 mm wide at the base, tapering gradually over their length of about 31.6 mm, measured anteriorly. Approximately 27 digitiform secondary spines are visible on the anterior margins of the anterolateral spines. Partly preserved spines on the posterior margin indicate that there may have been a similar number of spines present (Figs. 3.1, 4.1). The secondary spines appear to be longest proximally, ranging from about 7.6 mm, but poorer preservation distally prevents confident measurement.
Posterior to the anterolateral spines, the shield widens to about 14.4 mm, producing an overall subtrapezoidal shape. From here, the mediolateral spines project approximately orthogonal to the midline (Figs. 3, 4.3). They measure about 3.6 mm wide at the base and at least 38.2 mm along the anterior margin. The mediolateral spines curve sharply for the first third of their length, achieving an angle of ~15° posterolateral to the midline and then projecting roughly straight. Secondary spines also ornament the mediolateral spine, with ~30 along the anterior face and at least ~16 distally on the posterior face. The margins of the cephalic shield are not visible posterior to the mediolateral spines, so the presence of posterolateral spines, as found in other marrellids (Rak et al., Reference Rak, Ortega-Hernández and Legg2013), cannot be confirmed.
The first pair of visible appendages are the hypertrophied pair (Figs. 3, 4). They consist of nine podomeres (P1–9) and are about 70.0 mm in total length, extending well beyond the cephalic spines even when partially bent. Small patches of cuticle are well preserved and show evidence of surface ornamentation consisting of small, rounded, closely spaced pores, ~50–70 μm in diameter (Fig. 4.4). The hypertrophied appendages attach behind and inward of the mediolateral spines. From here, one curves anteriorly and outward relative to the body, while the other bends inward over the head before turning outward. The attachment podomere (P1) is small (2.6 mm), ovoid, and bears no evidence of spines or a gnathobase. P2 is stout and subrectangular in profile, about 2.9 by 3.7 mm. P3 is extremely short and crescentic. P4 is rounded and tapers distally toward P5. P5 is elongate, and the distal end is inclined at about 25°, producing a strong bend in the appendage. P6 is longer and subrectangular in profile, about 7.5 by 3.4 mm. Small fields of carbon between P4, 5, and 6 may represent arthrodial membranes. P7 is about 11.2 by 2.2 mm. On the right appendage, it appears to be curved along its length, but this is likely an artefact of localized deformation of the adjacent sediment. P8 is much longer and narrower than the preceding podomeres, at 16.5 by 1.3 mm, and is also preserved in a strongly bent orientation relative to P7. The distal inner margin of P8 projects into a straight spine, 2.9 mm long, parallel to the podomere long axis. P9 is an elongate, straight, flattened spine, possibly with marginal serrations, about 9.2 mm in length. P9 articulates in an outward direction, opposite to preceding podomeres. On the left appendage, the terminal podomere appears to twist along its length (Fig. 4.5), but this may be due to deformation.
Several incomplete appendages are also present (Fig. 4.2, 4.3). One moderately sized appendage on the right side, 1.0 mm wide, preserves four elongate subrectangular podomeres and protrudes from near the base of the hypertrophied appendage. Its proximal and distal ends are incomplete, but it seems likely it inserted just posterior to the hypertrophied appendage. One podomere bears a small, blunt, triangular endite, distally. At least three or four filamentous endopods, 0.5 mm wide, with traces of up to five elongate subrectangular podomeres are also visible on the right side. The proximalmost podomeres are relatively short and lacking any outgrowths. These are succeeded by at least four more elongate podomeres, each showing traces of a small, distal, spinose, blunt endite. We find no evidence of exopods. At least one more equivalent appendage from the left side appears to be preserved folded over the midline while the base of another is visible posterior to the base of the hypertrophied appendage. These filamentous appendages clearly attach posterior to the two larger head appendages and are interpreted as belonging to the trunk region.
Etymology
In recognition of Dimitri G. Kampouris, who emigrated from Egypt to Sudbury, Ontario, as a hard rock miner and whose support and encouragement were necessary to carry out the study of the Brechin Lagerstätte.
Taphonomy
The single marrellomorph specimen is dorsoventrally compressed but retains some dimensionality. Patchy brown layers of carbonaceous cuticle, sometimes retaining ornamentation, are preserved surrounding a central sediment-filled void (Fig. 5). This is reminiscent of the mode of preservation of, for example, eurypterid cuticles at other mid-Paleozoic Lagerstätten (Gupta and Briggs, Reference Gupta, Briggs, Allison and Bottjer2011). In parts of the appendages, the central void also contains blocky crystals enriched in calcium and carbon (Fig. 5), presumably representing calcium carbonate, which must have precipitated within the enclosed environment formed by the cuticular remains before collapse. This is intriguingly similar to the otherwise unique mode of preservation of trilobite appendages exhibited at the Walcott Rust Lagerstätte (Brett et al., Reference Brett, Whiteley, Allison and Yochelson1999); however, in the marrellomorph, this is evidently secondary to the carbonaceous mode of cuticle preservation. We observe no enrichment in magnesium, iron, or sulfur. Algae and carapaces cooccurring with the marrellomorph are preserved as brown to blackish traces and presumably exhibit an equivalent, primarily carbonaceous, mode of preservation.
Remarks
T. dimitrii is the first marrellomorph to be formally described from the post-Cambrian of Laurentia and represents the second-youngest occurrence of Marrellida globally.
Phylogenetic results
We present the unconstrained inapplicable-corrected Parsimony result as our main tree (Fig. 7) with other trees available in Supplementary Text (Supplementary Figs. 1–3). Here we discuss the major similarities and differences between approaches.

Figure 7. Phylogeny of marrellomorphs. Topology from equal weights analysis using inapplicable state-corrected parsimony. Node ages, intended for visual purposes, were generated in the R package strap using “equal” dating and a root length of 15, set to be roughly consistent with the earliest arthropod fossil record. See supplementary text for alternative topologies.
In unconstrained Parsimony topologies, marrellomorphs are found within Arachnomorpha (Fig. 7; Supplementary Figs. 1, 2). Several potential synapomorphies allying marrellomorphs with other arachnomorphs are a head shield with a doublure and lateral spines, a ventral hypostome, well-developed endopods on all cephalic appendages, and the absence of caudal rami. The probable presence in Xylokorys of gnathobasic basipods (Siveter et al., Reference Siveter, Fortey, Sutton, Briggs and Siveter2007) also constitutes a connection as these have been argued to be an important arachnomorph synapomorphy (Aria and Caron, Reference Aria and Caron2017b). A mandibulate affinity of marrellomorphs is not supported by any of our consensus topologies, except where constrained, although a marrellid–mandibulate relationship is represented in a minority of unconstrained Bayesian posterior trees (Supplementary Fig. 3; 0.30 probability). We note, however, that the unconstrained Maximum Clade Credibility tree (MCC) also recovers the presumably spurious result that pycnogonids are more closely related to mandibulates than to Mollisonia, presumably a result of limited outgroup taxon sampling. When the monophyly of Chelicerata is enforced, the MCC instead suggests weak support for Arachnomorpha. When a mandibulate + marrellomorph clade is constrained, the Bayesian approach produces a grouping of mandibulates and marrellids to the exclusion of acercostracans.
In addition, we find poor or ambiguous support for the monophyly of Marrellomorpha, with marrellids and acercostracans frequently separated (as in Parsimony and unconstrained Bayesian MCC) or in a polytomy with other taxa (as in Bayesian majority rule consensus). Our unconstrained topologies surprisingly favor the paraphyly of Marrellomorpha with respect to pycnogonids, the latter most closely related to marrellids. These taxa are united, albeit with low support, by the loss of lateral compound eyes (excepting possibly in Mimetaster); differentiation of at least the second, and often third (excepting Marrella), cephalic appendages; the presence of cephalic endopods with more than seven podomeres (excepting Marrella); and possibly the loss of cephalic exopods. Previously proposed apomorphies of Marrellomorpha are the presence of a frontal rim on the head, which homologizes the general structure of the acercostracan carapace with the cephalic spines of the marrellids (Legg, Reference Legg2016a), trunk multisegmentation, and a multipodomerous trunk exopod (Rak et al., Reference Rak, Ortega-Hernández and Legg2013). It should be noted that the last of these is in fact unknown in acercostracans. Our parsimony topologies favor either convergence in the first two characters in acercostracans and marrellids or their loss in pycnogonids. The problematic Aquilonifer always resolves close to pycnogonids, regardless of constraints or treatment of inapplicability, but experimental removal of this taxon from the matrix does not impact other aspects of the topology (results not shown).
A clade of Xylokorys, Vachonisia, and Enosiaspis—Vachonisiidae sensu Legg (Reference Legg2016a)—is recovered in all trees, while a more inclusive acercostracan clade additionally containing Skania and Primicaris is typically recovered with lower support and is uncertain depending on outgroup constraints. While Skania and Primicaris appear commonly associated with acercostracans, the only reliable synapomorphies for this grouping are the relative enlargement of the trunk exopods and possibly the cephalic carapace.
Marrellida is well supported in all Parsimony analyses (Fig. 7; Supplementary Figs. 1,2) but receives relatively low posterior probability under a Bayesian approach. The presence of mediolateral and posterolateral spines bearing secondary spines are diagnostic for marrellids. Similarly, a clade of all marrellids excluding Marrella, Mimetasteridae sensu Rak et al. (Reference Rak, Ortega-Hernández and Legg2013), is recovered in all Parsimony analyses (supported by the presence of anterolateral spines) but not in the Bayesian majority rule consensuses (Supplementary Fig. 3). The Bayesian result appears to be due to alternative placements of Furca bohemica and ‘Mimetaster’ florestaensis as basally diverging marrellids in some posterior trees (e.g., MCC trees). The missing appendicular data for these taxa are presumably responsible for this instability. F. bohemica and ‘M.’ florestaensis are favored as sister taxa with weak support regardless of outgroup constraints. Presence of short anterolateral spines is the synapomorphy for these taxa. Tomlinsonus dimitrii n. gen. n. sp. is always found sister to Mimetaster hexagonalis, united by a similar subhexagonal cephalic shield and the conspicuous hypertrophied appendage.
Discussion
The discovery of Tomlinsonus has implications for understanding the evolution, mode of life, biogeography, and diversity of Marrellomorpha, among the most problematic of Paleozoic arthropods. Our findings also have more general significance as a surprising case of soft-tissue preservation from an Ordovician open shelf environment.
Significance for marrellomorph ecology and evolution
Tomlinsonus constitutes the second-youngest occurrence of a marrellid in the fossil record, helping to bridge the stratigraphic gap between earlier marrellids and the Devonian Mimetaster. Occurrence of a marrellomorph, a classic constituent of the Cambrian biota, in Ontario also demonstrates for the first time the persistence of this clade in Laurentia after the Cambrian. Along with reports from Ordovician deposits in Morocco (Van Roy et al., Reference Van Roy, Orr, Botting, Muir, Vinther, Lefebvre, el Hariri and Briggs2010), Wales (Legg, Reference Legg2016b), Czech Republic (Rak et al., Reference Rak, Ortega-Hernández and Legg2013), and Argentina (Aris et al., Reference Aris, Corronca, Quinteros and Pardo2017) and from the Devonian of Germany (Kühl and Rust, Reference Kühl and Rust2010), this discovery demonstrates the broad post-Cambrian distribution of marrellomorphs and emphasizes their peak in generic diversity in the Ordovician.
The most remarkable aspect of the morphology of Tomlinsonus is the pair of hypertrophied appendages. These are virtually identical in form to the post-antennular appendages in Mimetaster hexagonalis (Fig. 8.3; Kühl and Rust, Reference Kühl and Rust2010) although they show the number and morphology of proximal and distal podomeres with greater clarity. A similar, though shorter, pair of appendages also appears to be present in the undescribed marrellid from the Fezouata Formation (Fig. 8.2). Mimetasterid second appendages differ in structure from the equivalent pair in Marrella, which are flattened, fringed laterally with rows of setae, and have been compared to the oar-like appendages of nektonic aquatic insects (Fig. 8.1; García-Bellido and Collins, Reference García-Bellido and Collins2006). While the pores visible on the cuticle of Tomlinsonus may represent setal attachment sites, their even distribution across the podomeres is suggestive of a sensory rather than locomotory function (Garm and Watling, Reference Garm, Watling, Watling and Thiel2013).

Figure 8. Comparative images of marrellids from other deposits. (1) ROMIP 61142, Marrella splendens, from the Burgess Shale. (2) ROMIP 63766, undescribed marrellid from the Fezouata Formation. (3) ROMIP 49452, Mimetaster hexagonalis, from the Hunsrück Slate. Scale bars = 2 mm. a.s = anterolateral spine; c.aX = cephalic appendage X; en = endopod of trunk appendage; ex = exopod; hy = hypostome; m.s = mediolateral spine; p.s = posterolateral spine; s.s = secondary spine; tr = trunk.
Despite the high number of podomeres, the postantennular appendage in mimetasterids is composed of mainly two elongated sections ending in a long distal podomere articulating outward. The proximal section, composed of short podomeres 1–5 probably served to lift the body and facilitate lateral movement, while the longer podomeres (P6–8) in the distal section elevated the body above the substrate. The arthrodial membrane between P5 and P6 may have allowed for a high range of rotation of the distal axis. This type of appendage is reminiscent of that of some pycnogonids, as previously noted (Kühl and Rust, Reference Kühl and Rust2010), and may have similarly functioned as pushing or pulling anchors enabling long strides and possibly direction changes, while the posterior appendages were used for forward propulsion (Schram and Hedgpeth, Reference Schram and Hedgpeth1978). The pulling strategy in pycnogonids is dependent mostly on the presence of terminal claws. In at least Tomlinsonus, the terminal podomere appears to be a simple spine, but the additional spine on the subterminal podomere could have penetrated the substrate, providing traction. In this case, the outward-articulating, flattened terminal podomere may have been able to rest in plantigrade fashion on the substrate, distributing weight over a larger area. The situation may be convergent with the tarsal “feet” of many terrestrial arthropods (Kühl and Rust, Reference Kühl and Rust2010), although in Tomlinsonus this would more likely represent an adaptation to life on a soft marine substrate. Alternatively, the entire distal podomere could have been buried in the substrate, providing an anchor while the body shifts position, as takes place in the feeding process of some pycnogonids (Manton, Reference Manton1978). These similarities aside, the precise mode of locomotion in Tomlinsonus and other mimetasterids must have been somewhat different from that of other arthropods discussed given the presence of only a single pair of hypertrophied appendages.
Previous findings of marrellomorphs as stem group mandibulates relied on proposed homologies with Cambrian “crustaceomorph” larvae. Questions about the validity of these homology statements (Kühl et al., Reference Kühl, Bergström and Rust2008; Haug et al., Reference Haug, Castellani, Haug, Waloszek and Maas2012) combined with the problems associated with coding different developmental stages in phylogenetic analyses render these results ambiguous. At least our Parsimony analyses with exclusively adult taxa favor an arachnomorph affinity. We caution that the small fraction of outgroup taxa and relevant characters sampled in our dataset are insufficient to provide robust support, as also reflected by our more ambiguous Bayesian results. More outgroup taxa will ultimately be needed to resolve marrellomorph affinities; however, we suspect that new fossil discoveries will also be essential given the high levels of conflicting phylogenetic signal that characterize this group.
The unexpected paraphyly of Marrellomorpha with respect to pycnogonids recovered in some of our analyses warrants discussion. Support for this relationship is relatively low, but the result is convergent with previous findings using a very different data matrix (Vannier et al., Reference Vannier, Aria, Taylor and Caron2018). Despite obvious differences in gross morphology, some characteristics of marrellids, in particular, such as the loss of lateral compound eyes and the few-segmented head with uniramous agnathal appendages bearing more than seven podomeres, find commonality with pycnogonids. This hypothesis could have the radical implication that pycnogonid chelifores evolved convergently with euchelicerate chelicerae from a uniramous antennule. This is further suggested by the recovery of Aquilonifer as the sister taxon to pycnogonids in several trees. Nonetheless, considering the bizarre morphologies of both pycnogonids and marrellomorphs, this result should be viewed cautiously. Given the relatively weak support for marrellomorph paraphyly, we retain Marrellomorpha for taxonomic purposes in this paper. We note that the internal relationships of marrellids and acercostracans remain stable when chelicerate monophyly (exclusive of marrellomorphs) is enforced.
Our analyses suggest revisions of the internal relationships of Marrellida. While ‘Mimetaster’ florestaensis was previously found together in a polytomy with M. hexagonalis and the Fezouata marrellid, our analyses find some evidence for a closer affinity of the former with Furca bohemica. The original assignment to the genus Mimetaster was based entirely on the phylogenetic result, supported by a single vaguely defined character (“elongate anterior cephalic spines”). Provisionally, we suggest that reassignment to the genus Furca or to a new genus would be more in keeping with the evidence, acknowledging the present limitations imposed by incomplete preservation.
While Tomlinsonus dimitrii n. gen. n. sp. is phylogenetically closest to M. hexagonalis, it is morphologically and temporally distinct enough to warrant assignment to a new genus. It is also distinct from the Ordovician Dyrnwynia conollyi Legg, Reference Legg2016b, which is known from a single putative mediolateral cephalic spine apparently lacking any secondary spines on the posterior margin (Legg, Reference Legg2016b). T. dimitrii, by contrast, bears secondary spines on both margins of the mediolateral spine. Unfortunately, D. conollyi was too incomplete and problematic to be included in our phylogeny.
Soft-tissue preservation on a Late Ordovician open shelf
The Brechin area is already famous for its exceptionally preserved record of echinoderms, which in some beds were smothered rapidly in situ (Brett and Liddell, Reference Brett and Liddell1978; Cole et al., Reference Cole, Wright, Ausich and Koniecki2020). This paper constitutes the first report of soft-tissue preservation in the formation and the oldest such record in Ontario. While already meeting the broad criteria for consideration as a Konservat Lagerstätte (Seilacher et al., Reference Seilacher, Reif and Westphal1985), the preservation of soft tissues at Brechin cements this status in a more exclusive sense. More generally, this shows that the preservation of nonmineralized tissues is possible on the widespread Late Ordovician carbonate platforms of Laurentia.
Soft-bodied macrofossils remain rare at Brechin, with only six identifiable specimens recovered. While a considerable area of Bed 3 was examined, a systematic search for soft-bodied fossils was conducted only in a more limited area. Given also that nonmineralized fossils are difficult to discriminate against the matrix under outdoor lighting conditions, identification is challenging in the field. We think that more intensive sampling from intermound areas has great potential to reveal other soft-bodied organisms.
In contrast to the many marine shelf Lagerstätten of the Cambrian, most Laurentian Ordovician Lagerstätten such as Winneshiek (Briggs et al., Reference Briggs, Liu, Mckay and Witzke2018), William Lake and Airport Cove (Young et al., Reference Young, Rudkin, Dobrzanski, Robson, Cuggy, Demski and Thompson2012), Big Hill (Lamsdell et al., Reference Lamsdell, LoDuca, Gunderson, Meyer and Briggs2016), and Kagawong (Stott et al., Reference Stott, Tetlie, Braddy, Nowlan, Glasser and Devereux2005), represent hostile, restricted, marginal marine settings with a low diversity of hardy taxa. Eurypterids, xiphosurans, phyllocarids, medusae, and unmineralized tubes tend to dominate these soft-bodied faunas. Far fewer Ordovician Lagerstätten have been reported from more distal marine shelf environments (Orr, Reference Orr2014; Van Roy et al., Reference Van Roy, Briggs and Gaines2015). Beecher's Trilobite Beds and related sites in New York are likely the most significant of those in Laurentia, exquisitely preserving the soft tissues of trilobites, ostracods, echinoderms, and several soft-bodied taxa yet to be described (Farrell et al., Reference Farrell, Martin, Hagadorn, Whiteley and Briggs2009). Here, the environment has been interpreted as generally dysoxic, with the fossil assemblage representing a specialized biota adapted to these conditions (Farrell et al., Reference Farrell, Briggs and Gaines2011). Cat Head, Manitoba, represents a similar case, preserving a diversity of sponges, macroalgae, and possible hydrozoans alongside typical Ordovician shelly biotas, but showing signs that the basin in which it resided may have been circulation restricted (Young et al., Reference Young, Rudkin, Dobrzanski, Robson, Cuggy, Demski and Thompson2012). Another well-known marine shelf Lagerstätte, the Walcott Rust Quarry in New York, shows soft-tissue preservation largely confined to trilobite appendages (Brett et al., Reference Brett, Whiteley, Allison and Yochelson1999). Finally, a few other examples of soft-tissue preservation in deep basinal environments demonstrate potential for further discoveries, but presently sampling has yielded only a low diversity of problematica (Macgabhann and Murray, Reference Macgabhann and Murray2010; Meyer et al., Reference Meyer, Ganis, Wittmer, Zalasiewicz and De Baets2018; Peel et al., Reference Peel, Willman and Pedersen2019). Thus, while all these sites provide critical insights, their biotas are not easily comparable to those of Cambrian Lagerstätten from open marine shelf environments.
Outside of Laurentia, the Fezouata biota of Morocco provides the best view of an Ordovician open shelf biota. Here, shelly and soft-bodied organisms characteristic of both Cambrian (e.g., radiodonts, lobopodians, marrellomorphs, nektaspids, paleoscolecids) and Paleozoic (e.g., horseshoe crabs, eurypterids, phyllocarids, various echinoderms, mollusks, and bryozoans) faunas occur together (Van Roy et al., Reference Van Roy, Briggs and Gaines2015). A few other sparser assemblages may provide comparable windows in other regions (e.g., Muir et al., Reference Muir, Ng, Li, Zhang and Lin2014; Balinski and Sun, Reference Balinski and Sun2015; Botting et al., Reference Botting, Muir, Jordan and Upton2015; Hearing et al., Reference Hearing, Legg, Botting, Muir, McDermott, Faulkner, Taylor and Brasier2016; Aris et al., Reference Aris, Corronca, Quinteros and Pardo2017; Kimmig et al., Reference Kimmig, Couto, Leibach and Lieberman2019). The occurrence of Tomlinsonus in shallow marine deposits in Ontario, preserved alongside diverse echinoderms, trilobites, brachiopods, and bryozoans, provides a tentative connection with these other sites and indicates that marrellomorphs were likely typical members of Ordovician marine shelf communities. Being Katian in age, and thus younger than the aforementioned assemblages, this occurrence also lends further support to the notion that this type of fauna may have persisted broadly until at least the end-Ordovician extinction.
The Brechin Lagerstätte thus represents one of very few examples of soft-tissue preservation from an Ordovician open marine shelf and an important window into this environmental setting in Laurentia. While few examples of soft-tissue preservation have been collected to date, the lateral extent and repetitive nature of obrution events in the upper Kirkfield Formation offers a tantalizing hint that further exploration may yield more insights into the origin, biogeography, and longevity of distinctive soft-bodied fauna. This will provide an important complement to Silurian Lagerstätten of Ontario, which have already yielded rare elements of the Cambrian fauna, such as naraoiids and lobopodians, alongside more typical Paleozoic taxa (Caron et al., Reference Caron, Rudkin and Milliken2004; von Bitter et al., Reference von Bitter, Purnell, Tetreault and Stott2007). More broadly, our findings illustrate the potential for discovering cryptic cases of soft-tissue preservation among well-studied “shelly” biotas of the mid-Paleozoic.
Author contributions
J.M. wrote an initial draft of the paper. A.I.-L. created the reconstruction and locality diagrams, drafted the phylogenetic character list and taxonomic acts, and undertook dataset coding and analysis collaboratively with J.M. G.E.K. planned and performed the field collections, supplied data on stratigraphy and biotic cooccurrences, and donated the soft-bodied fossils published in this paper to the ROM. J.-B.C. prepared and photographed the marrellomorph specimen and created the fossil figures. All authors contributed to the discussion of results and writing of the paper.
Funding
External funding comes primarily from a National Science and Engineering Research Council (NSERC) Discovery grant (no. 341944) to J.-B.C. and scholarships awarded to J.M. and A.I.-L. through the University of Toronto, Department of Ecology and Evolution: NSERC Vanier Canada Graduate Scholarship to J.M., and “la Caixa” Foundation (ID 100010434) under agreement (LCF/BQ/AA18/11680039) to A.I.-L. We also acknowledge the Dorothy Strelsin Foundation (ROM) for the purchase of a Leica M205C stereomicroscope used in this research.
Acknowledgments
The Tomlinson Group Aggregate Division has provided access to the study area to G.E.K. since 2014. We thank R. Tomlinson and S. Berquist in particular for their interest and support. We thank M. Akrami for assistance in the ROMIP collections, S. Lackie for elemental maps, C. McCall for artistic reconstruction, M. Smith for assistance with the R package TreeSearch and for enabling constraint functionality, D. de Carle for discussions on phylogenetic approaches, C. Aria for comments on nomenclature, D. Rudkin for information on Lagerstätten of Ontario, and R. Lerosey-Aubril for helpfully providing a reference. We also appreciated constructive remarks from S. Zamora, J. Kimmig, and an anonymous reviewer. TNT is freely available thanks to the Willi Hennig Society.
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.dfn2z353m
Supplementary materials are available from Zenodo: https://doi.org/10.5281/zenodo.5851832