Ruminant-derived foods (meat and milk) are the main source of conjugated linoleic acid in the human diet(Reference Lawson, Moss and Givens1), with evidence in animal models that the predominant isomer, cis-9, trans-11, exhibits anticarcinogenic and anti-atherogenic properties(Reference Wahle, Heys and Rotondo2). In milk, cis-9, trans-11-conjugated linoleic acid (rumenic acid; RA) is derived both from ruminal metabolism of 18 : 2n-6 and further absorption and uptake by the mammary gland, and from endogenous Δ9-desaturation of trans-11-18 : 1 (vaccenic acid; VA), an intermediate of linoleic (18 : 2n-6) and linolenic (18 : 3n-3) acid metabolism in the rumen(Reference Bauman and Griinari3). In addition, oleic acid is the main unsaturated fatty acid found in milk TAG and is considered to exert positive effects on human health(Reference Parodi4). Like RA from VA, most oleic acid is synthesised from endogenous Δ9-desaturation of 18 : 0. For these reasons, the regulation of the Δ9-desaturase system in the ruminant mammary gland may modulate the milk fat content of these fatty acids.
In bovines, the few studies that have estimated the endogenous desaturation of VA to RA employed three different methods. Two of them are indirect, involving either an inhibition of the desaturase system(Reference Griinari, Corl and Lacy5), or a quantification of the duodenal and milk flows of VA and RA(Reference Shingfield, Ahvenjarvi and Toivonen6, Reference Glasser, Ferlay and Doreau7). Recently, Mosley et al. (Reference Mosley, Shafii and Moate8) developed and used a third method using tracer methodology to measure the in vivo conversion of VA to RA in the mammary gland. This study showed that the mammary gland is the major site of Δ9-desaturation of VA in the lactating cow. However, no data are available in goats on in vivo conversion of VA to RA, although a close relationship between milk RA and VA has been established(Reference Chilliard, Ferlay and Rouel9).
Nutrition is the main environmental factor regulating milk fat synthesis and fatty acid composition in ruminants(Reference Jensen10), with caprine response differing from bovine in terms of milk fat synthesis and, to a lesser extent, milk fatty acid composition(Reference Chilliard, Ferlay and Rouel9, Reference Chilliard, Glasser and Ferlay11). A nutritional strategy to increase VA supply to the mammary gland is to feed animals with either plant oil or marine oil. In cows, feeding a lipid source rich in linoleic acid along with fish oil very effectively increased concentrations and yields of milk RA and VA(Reference AbuGhazaleh, Schingoethe and Hippen12). Also, sunflower-seed oil in combination with fish oil was more effective than sunflower-seed oil alone in increasing milk concentrations of VA and RA(Reference Qiu, Eastridge and Firkins13). Similar results were observed in goats, comparing fish oil in combination with sunflower-seed oil with fish oil alone(Reference Gagliostro, Rodriguez and Pellegrini14). These effects are a consequence of higher ruminal VA intermediate production due to the inhibition by very-long-chain fatty acids from fish oil of the final biohydrogenation step of dietary PUFA to 18 : 0. In addition, in cows, interaction between either plant or marine oils and high levels of starch in the diet increased the trans-10-18 : 1 pathway(Reference Shingfield and Griinari15) and induced milk fat depression. However, in goats such diets increased milk trans-10-18 : 1 without inducing milk fat depression(Reference Chilliard, Glasser and Ferlay11, Reference Bernard, Shingfield and Rouel16).
To address these issues, the first aim of the present study was to compare two dietary treatments in a 2 × 2 cross-over design with addition of sunflower-seed oil (SO) alone or in combination with fish oil plus additional starch (SFO) on milk yield and composition. The second aim was to investigate the metabolism of VA in the lactating goat by measuring the conversion of VA to RA using a chemical tracer strategy allowing an in vivo measurement of the Δ9-desaturation of VA. This measurement is based on the hypothesis that in goats the mammary gland is the major site of conversion of VA to RA as demonstrated in cows, even though we cannot exclude the possibility of a limited conversion in other tissues. The data of VA conversion to RA can be related to the expression of genes encoding for Δ9-desaturase (or stearoyl-CoA desaturase; SCD), SCD1 and the recently described SCD5 (Reference Lengi and Corl17), and to the milk fatty acid normalised Δ9-desaturation ratios, thus allowing an evaluation of the indirect variables used to estimate the mammary Δ9-desaturation. The third aim was to evaluate the effect of the two dietary treatments (SO and SFO) on the Δ9-desaturation of VA in vivo and on the fraction of milk RA originating from VA. The second and third aims were only achieved during the last experimental period. We hypothesised that milk RA and VA in goats fed sunflower-seed oil combined with fish oil would be higher than with sunflower-seed oil alone and that the interaction of oils with starch in the diet would increase the ruminal trans-10-18 : 1 pathway, which would consequently lead to a simultaneous increase in trans-11- and trans-10-18 : 1. The subsequent hypothesis was that SO in combination with fish oil plus additional starch would negatively affect the mammary Δ9-desaturase activity through an increase in the availability of either trans-10 fatty acids or long-chain PUFA of fish oil, which have both been associated in cows with a decrease in Δ9-desaturase activity(Reference Bauman and Griinari3, Reference Ahnadi, Beswick and Delbecchi18).
Materials and methods
Animals, management and experimental design
All experimental procedures were approved by the Animal Care Committee of INRA in accordance with the Use of Vertebrates for Scientific Purposes Act of 1985. Goats were housed in individual stalls, had free access to water and were milked at 07.30 and 15.30 hours.
To measure the incorporation and Δ9-desaturation of 13C-labelled VA in milk lipids, we first determined in a preliminary study the effect of the form and dose of injection of [1-13C]VA on the kinetics of milk [13C]VA enrichment.
In this preliminary experiment, two multiparous non-pregnant Alpine goats (322 (sd 7) d in milk) received a diet of lucerne hay distributed ad libitum and a mixture of concentrate (composed of 65 % maize, 15 % soyabean meal and 20 % dehydrated sugarbeet pulp). The goats were fed twice per d just after milking and the amounts fed and refused were recorded daily. The goats were randomly assigned to a treatment of [1-13C]VA as either NEFA or TAG (containing 51 % [1-13C]VA and 49 % oleic acid) (CEA, Cadarache, France). Two doses of 0·25 and 0·50 g [1-13C]VA of each form (NEFA or TAG) were delivered to the same goat at 1-week intervals by jugular infusion. Doses were prepared using a modified procedure of Viswanadha et al. (Reference Viswanadha, Giesy and Hanson19). Briefly, [1-13C]VA as NEFA (pure at 99 %) or TAG was suspended in 25 ml of 20 % Intralipid (Sigma-Aldrich, St Louis, MO, USA) and brought to 30 ml with 0·9 % (v/v) saline solution. Three sonication cycles of 30 s spaced by 30 s in ice were run to ensure thorough mixing. The dose was delivered at 07.30 hours by jugular injection. The goats were completely milked 24 h and 16 h before and immediately before the injection, every 4 h for 24 h post-injection (p.i.) and then every 12 h until 72 h p.i. This preliminary study was designed to determine the appropriate delivery form of [1-13C]VA as either TAG or NEFA, and the best [1-13C]VA assay and sampling times for the subsequent nutritional study.
In the nutritional study, eight multiparous non-pregnant Alpine goats (266 (sd 7) d in lactation) were fed two diets in a 2 × 2 cross-over design with four animals per group. Each experimental period (21 d) comprised of a 14 d adaptation period followed by a 7 d sampling period. Goats were fed in two equal meals at 08.30 and 16.30 hours, just after milking. The amounts fed and refused were recorded daily. The two experimental diets consisted of a natural grassland hay-based diet distributed ad libitum and a concentrate mixture (determined according to the initial milk yield). The diet (Table 1) included a lipid supplement of 90 g sunflower-seed oil alone per d (SO; Huileries de Lapalisse SA, Lapalisse, F-03 120 France) or a combination of 60 g sunflower-seed oil and 30 g fish oil from anchovy per d (SA Daudruy Van Cauwenberghe & Fils, Dunkerque, F-59 640 France) plus additional starch from rolled barley (SFO; Table 1).
Table 1 Ingredients and chemical composition of the ingested experimental diets
(Mean values and pooled standard errors for eight samples per treatment)

SO, sunflower-seed oil; SFO, sunflower-seed oil and fish oil (2:1) plus starch from rolled barley.
* Sunflower-seed oil contained (g/kg): 14 : 0, 0·9; 16 : 0, 72·0; cis-9-16 : 1, 1·3; cis-9-18 : 1, 290·8; 18 : 2n-6, 597·1; 18 : 3n-3, 14·9; 20 : 5n-3, 1·8; 22 : 6n-3, 0·2.
† Fish oil contained (g/kg): 14 : 0, 79·0; 16 : 0, 169·5; cis-9-16 : 1, 89·3; 18 : 0, 29·8; cis-9-18 : 1, 147·9; 18 : 2n-6, 39·5; 18 : 3n-3, 16·0; 20 : 5n-3, 91·6; 22 : 6n-3, 69·0.
‡ Mineral–vitamin supplement declared as containing (g/kg): Ca, 240; P, 60; Mg, 50; Na, 15; Zn, 7; Mn, 6; α-tocopherol, 0·3; retinol, 0·2; cholecalciferol, 0·002 (Usine d'Ussel, Murat, France).
Measurements and sampling
During each sampling period, representative samples of hay and concentrates were composited daily and stored at − 20°C. We measured the chemical composition of feedstuff ingredients using standard procedures(20). Milk yields of individual goats were recorded three times a week. Samples of milk for the measurement of fat, protein and lactose were collected from each goat over four consecutive milkings starting at 08.00 hours on day 19 of each experimental period and treated with preservative (potassium bichromate; Merck, Fontenay-sous-Bois, France). Milk fat, protein and lactose were assayed by near-IR spectroscopy (CILAL, Theix, France(20)). Two samples of milk were collected (morning and afternoon milkings) on day 20 of each experimental period, and stored at − 20°C until determination of fatty acid composition on a combined sample.
On day 18 of the last experimental period (P2), a single dose of 1·5 g [1-13C]VA as NEFA (CEA, Cadarache, France), as previously described, was delivered to the goats by jugular injection at 07.30 hours just after milking. The goats were completely milked every 6 h for 24 h before the injection, every 4 h for 24 h and then every 12 h for 72 h p.i. During this sequence of milk collection, milk yield was recorded, and milk samples were collected for analysis of milk fat content for each milking (CILAL, Theix, France(20)). Samples of 3 ml milk were taken and stored at − 20°C for fatty acid composition and 13C enrichment measurements.
At the end of the experiment on day 21 of P2, the goats were slaughtered after the morning milking. Immediately before slaughtering, the goats were milked to remove milk from the mammary glands. Immediately after death, samples of mammary tissue were collected under sterile conditions, frozen in liquid N2 and maintained at − 80°C until RNA extraction.
Lipid analysis
Lipids in natural grassland hay and concentrates were extracted and transesterified as described previously(Reference Bernard, Shingfield and Rouel16). Lipids in 100 mg of lyophilised milk samples from both experiments were directly methylated by in situ transesterification with 1 ml 0·5 m-methanolic NaOCH3 at room temperature for 20 min, followed by the addition of 1 ml 14 % (v/v) boron trifluoride in methanol also at room temperature for 20 min(Reference Loor, Ferlay and Ollier21). Fatty acid methyl esters (FAME) were quantified as described previously(Reference Bernard, Shingfield and Rouel16).
For the measurement of [13C]VA enrichment, the FAME were converted to dimethyl disulfide adducts using an adaptation of the method used by Mosley et al. (Reference Mosley, Powell and Riley22). In brief, the FAME fractions ( < 1 mg) were treated with 0·35 ml dimethyl disulfide and 100 μl iodine solution (6 % iodine (w/v) in diethyl ether). The reaction mixtures were shaken in a 37°C water-bath for 1 h and diluted with diethyl ether–hexane (3 ml, 1:1, v/v). Iodine was removed by shaking with 10 % sodium thiosulfate (200 μl). The organic phase was removed and evaporated under N2 gas. The residue was dissolved in 1500 μl hexane and analysed by GC–MS (Agilent model 7890A GC system attached to an Agilent model 5975C inert XL EI/CI mass detector; Agilent Technologies France, Massy, France). A 30 m × 0·25 mm internal diameter, 0·25 μm film thickness capillary column, crosslinked 5 % diphenyl 95 % dimethyl siloxane (HP5MS) from Agilent Technologies was used for chromatographic separation. The injection volume was 1 μl in splitless mode at 250°C, with He as the carrier gas at a constant flow rate of 1 ml/min. The oven temperature program was started at 70°C, increased at a rate of 20°C/min to 195°C, then at a rate of 1°C/min to 225°C (held for 5 min), and finally at a rate of 10°C/min to 290°C (held for 5 min). The transfer line heater was set at 300°C. The mass spectrometer was operated under electron impact ionisation conditions (electron energy 70 eV, source temperature 200°C). The fragment ions m/z 245 and 246 for dimethyl disulfide derivatives were monitored using selective ion monitoring.
The enrichment of [13C]RA was measured directly from FAME(Reference Christie23) by GC–MS (Agilent model 7890A GC system attached to an Agilent model 5975C inert XL EI/CI mass detector; Agilent Technologies France) on a 100 m × 0·25 mm internal diameter fused silica capillary column (CP-Sil 88; Chrompack 7489, Middelburg, The Netherlands). Injection volume was 1 μl in splitless mode at 250°C, with He as the carrier gas, at a constant pressure of 30 pounds per square inch (206·84 kPa). The oven temperature program was started at 60°C, increased at a rate of 15°C/min to 165°C (held for 1 min), then at a rate of 2°C/min to 225°C (held for 5 min). The mass spectrometer was operated under positive chemical ionisation with ammonia reagent gas at 20 ml/min. Data were obtained in scan mode with a mass range of m/z 60 − 400.
RNA isolation and real-time RT-PCR
Total RNA was prepared from about 85 mg mammary tissues using TRIZOL Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and further purified with the SV Total RNA Isolation system (Promega, Charbonnières, France) to eliminate contaminating genomic DNA before cDNA synthesis. RNA concentration and purity were determined by spectrophotometry at 260, 280 and 320 nm (Lambda 25 UV/VIS spectrometer; Perkin Elmer Instruments, Les Ulis, France) and RNA integrity was verified using a Bioanalyser 2100 (Agilent Technologies, Massy, France). Quantitative RT-PCR was carried out as described previously(Reference Bernard, Rouel and Leroux24). Specific primers were previously described for SCD1 (Reference Bernard, Rouel and Leroux24) and SCD5 (Reference Lengi and Corl17). Abundance of SCD1 and SCD5 gene transcripts was expressed as fold change relative to SO treatment after normalisation with cyclophilin A (reference gene) to account for variations in RNA integrity, RNA quantification and cDNA synthesis.
Calculations
The tracer:tracee ratio (TTR) was calculated for VA and RA from the mass abundance of the 13C and 12C fragments using the equation TTR = 13C/12C.
For VA, TTR was calculated from GC–MS single ion monitoring data on specific mass fragments at m/z 245 and 246 for M and M+1, respectively(Reference Yamamoto, Shibahara and Nakayama25), and using a standard curve of [13C]trans-11-18 : 1. For RA, TTR was calculated from GC–MS scan data on specific mass fragments at m/z 312 and 313 for M and M+1, respectively(Reference Christie23). To account for the baseline natural isotopic abundance, the mean TTR of samples taken before the bolus was subtracted from the TTR of all samples. The enrichment (E) of the fatty acid with 13C for RA in milk was then (TTR − TTR mean before injection) × 100. The calculated E was adjusted for spectrum skewness using the correction factor 1/(1+(0·011))(Reference Wolfe26).
To calculate the amounts of [13C]VA and [13C]RA resulting from the injected dose (hereafter called post-predicted values), first total amounts of milk VA and RA secreted at each milking for 24 h p.i. were calculated, and respectively separated into g [13C]VA and [12C]VA, and [13C]RA and [12C]RA and then summed for 24 h p.i. The predicted p.i. basal secretion of [13C]VA or [13C]RA was calculated as the mean % [13C]VA or % [13C]RA value of the 24 h samples before injection multiplied by the 24 h p.i. secretion (g) of VA or RA. These values were therefore subtracted from the 13C p.i. secretion of VA or RA to determine the predicted values. They were then used to calculate the amount of VA converted to RA, and the fraction of RA originating from VA, assuming that the mammary gland is the major site of Δ9-desaturation of circulating VA to milk RA. We used the following equations:
![Percentage\,of\,VA\,converted\,to\,milk\,RA\, in\,vivo = g\,[^{13}C]RA\,post\hyphen predicted/(g\,[^{13}C]VA\,post\hyphen predicted + g\,[^{13}C]RA\,post\hyphen predicted).](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20160920223923317-0279:S0007114510000486:S0007114510000486_eqn1.gif?pub-status=live)
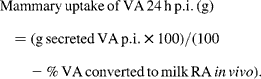

![Secreted\,RA\,in\,milk\,24\hairsp h\,p.i.\,(g) = g\,[^{13}C]RA\,secreted\,p.i. + g\,[^{12}C]RA\,secreted\,p.i.](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20160920223923317-0279:S0007114510000486:S0007114510000486_eqn5.gif?pub-status=live)
Statistical analysis
Data on DM intake, milk production and milk composition were subjected to ANOVA using the general linear models procedure of SAS (SAS Institute, Cary, NC, USA) for a 2 × 2 cross-over design with a model that included the effects of treatment, period and goat. Treatment means were compared using the least square means procedure (SAS Institute) with differences declared significant at P < 0·05. Data on in vivo [13C]VA metabolism, including the amount of VA converted to RA and the fraction of RA originating from VA, together with the mRNA abundance in mammary tissues collected after slaughter, were statistically evaluated using the non-parametric Wilcoxon U test. Treatment effects were considered significant at P < 0·05.
Results
Diet composition
The natural grassland hay was of high nutritional quality and had the following composition (g/kg DM, unless otherwise stated): DM, 861 g/kg fresh weight; organic matter, 899; crude protein, 153; acid-detergent fibre, 307; neutral-detergent fibre, 579; fatty acids, 20.
The two diets had similar organic matter and neutral-detergent fibre contents, which averaged 920 and 455 g/kg DM, respectively. Crude protein and acid-detergent fibre contents were slightly higher for SO than for SFO, while starch content was higher for SFO (124 g/kg DM) than for SO (75 g/kg DM; Table 1).
The mean forage:concentrate ratios of the diets (on a DM basis) were 68:32 and 69:31 for SO and SFO, respectively.
The major fatty acids provided by the diets were 18 : 2n-6 and cis-9-18 : 1, with 56·8 and 39·0 g 18 : 2n-6 per d, and 25·8 and 20·7 g cis-9-18 : 1 per d, respectively for SO and SFO. In addition, SFO provided 2·5 g 20 : 5n-3 and 1·9 g 22 : 6n-3 per d.
Dairy performance and milk fatty acid composition
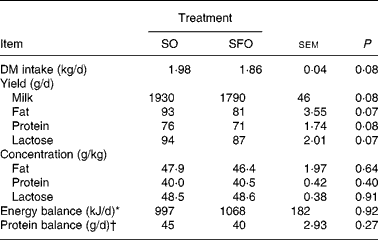
The DM intake, milk yield, milk fat, protein and lactose yields were not significantly different for SO and SFO treatments (Table 2), even though a tendency to decrease for SFO treatment (P = 0·07 to 0·08) was observed. Energy and protein balances calculated as described by Jarrige(Reference Jarrige27) were positive and similar among dietary treatments (Table 2).
Table 2 Effect of dietary sunflower-seed oil alone or in combination with fish oil plus starch on DM intake, milk yield and milk composition and energy and protein balances in goats
(Mean values and pooled standard errors for eight goats per treatment)
SO, sunflower-seed oil; SFO, sunflower-seed oil and fish oil (2:1) plus starch from rolled barley.
* Calculated according to Jarrige(Reference Jarrige27).
† Protein digestible in the intestine(Reference Jarrige27).
Compared with SO, SFO induced higher percentages of milk short- and medium-chain SFA (6 : 0 to 16 : 0), cis-9-16 : 1, trans-11, cis-15-18 : 2, 20 : 5n-3 and 22 : 6n-3 (Table 3). Conversely, SFO induced lower percentages of milk 18 : 0, cis-9-18 : 1, Σcis-18 : 1 and ΣC18 (Table 3).
Table 3 Effect of dietary sunflower-seed oil alone or in combination with fish oil plus starch on milk fatty acids composition (g/100 g fatty acids) in goats
(Mean values and pooled standard errors for eight goats per treatment)

SO, sunflower-seed oil; SFO, sunflower-seed oil and fish oil (2:1) plus starch from rolled barley; VA, vaccenic acid; RA, rumenic acid; tr, concentrations below 0·01 g/100 g fatty acids; CLA, conjugated linoleic acid.
* Included cis-9, trans-11-, cis-9, cis-11- and trans, trans-CLA.
Δ9-Desaturation
During the preliminary experiment (Fig. 1), the [13C]VA enrichments of milk fat from injection of labelled TAG and NEFA peaked respectively at 5 and 7 h and then declined over 60 h irrespective of the dose. Whatever the injected dose of [13C]VA, the maximal enrichment values were higher for the NEFA than for the TAG, with 7·3 and 10·6 % enrichment of [13C]VA, respectively, for doses 1 and 2 with NEFA compared with 1·3 and 2·3 % with TAG. After the first 24 h of milk collection, lower enrichments (close to basal values) were observed. Therefore, in the nutritional experiment, calculations were limited to the first 24 h to allow an accurate estimation of the Δ9-desaturation of VA.

Fig. 1 Enrichment of 13C in trans-11-18 : 1 (vaccenic acid; VA) in milk fat of lactating goats injected intravenously with [1-13C]VA at time zero, provided as NEFA or TAG, and at two doses (0·25 and 0·50 g). The post-injection data are plotted at the mean time point for each collection period. Each point represents one goat. (–Δ–), TAG 0·25 g; (–▲–), TAG 0·50 g; (–○–), NEFA 0·25 g; (–●–), NEFA 0·50 g.
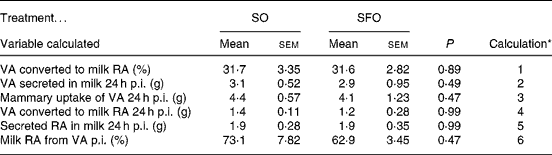
In the nutritional experiment, the [13C]VA enrichment of milk fat from the injection as NEFA peaked at 10 and 14 h p.i. for the SO and SFO treatments, respectively (Fig. 2), and then declined until 66 h p.i. Milk fat enrichments of the Δ9-desaturase product, [13C]RA, followed a pattern similar to that of the substrate, [13C]VA, peaking at 6 and 10 h p.i. for the SO and SFO treatments, respectively (Fig. 2). The percentage of mammary VA uptake desaturated to milk RA was 31·7 and 31·6 %, respectively, for the SO and SFO treatments (Table 4). From these percentages of VA desaturated to milk RA, we calculated that 73·1 and 62·9 % of milk RA came from VA desaturation for the SO and SFO treatments, respectively (Table 4). Neither the percentage of VA desaturated into milk RA nor the percentage of milk RA coming from VA desaturation was significantly different (P>0·05) between treatments.
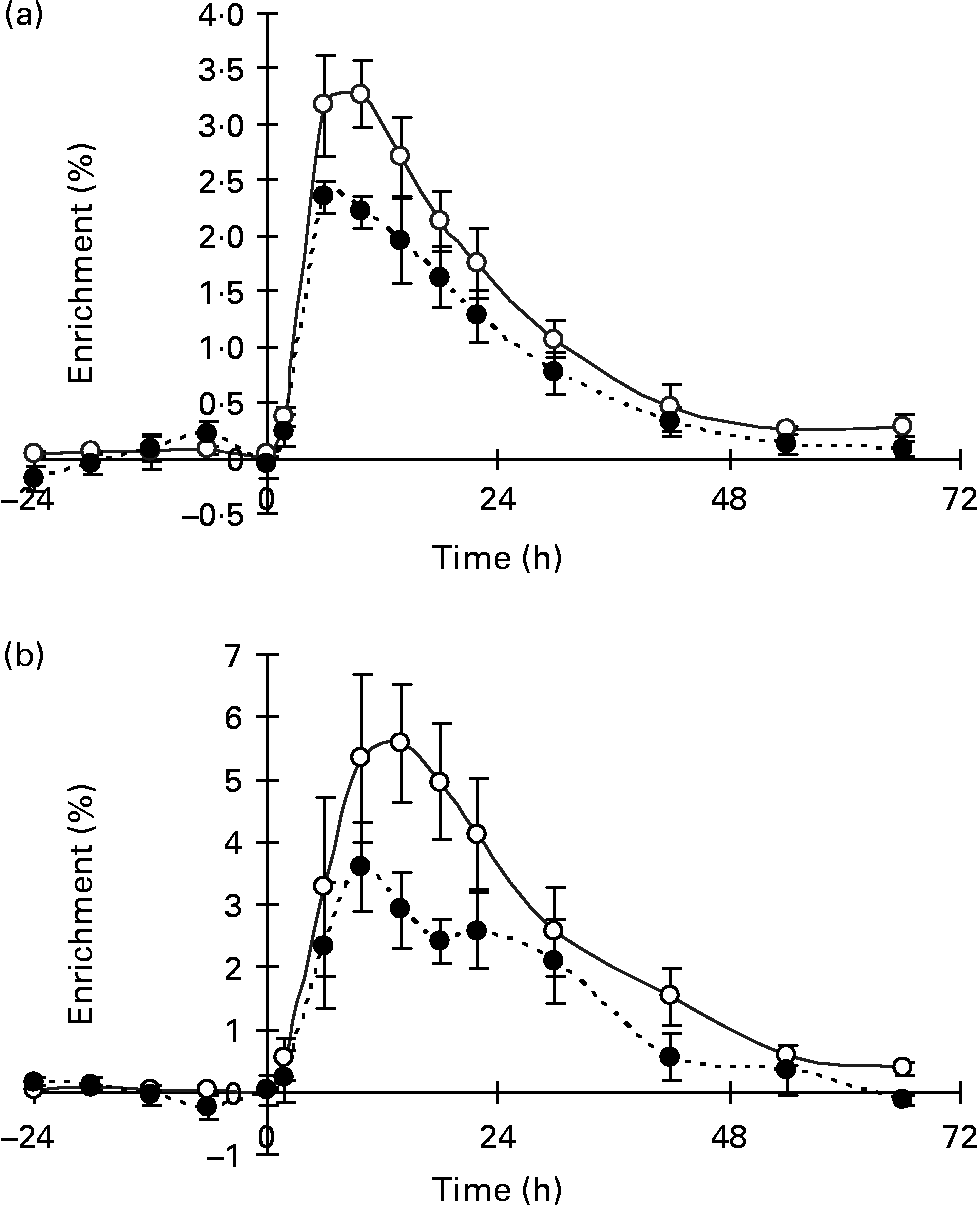
Fig. 2 Enrichment of 13C in trans-11-18 : 1 (vaccenic acid; VA; –○–) and cis-9, trans-11-18 : 2 (rumenic acid; –●–) in milk fat of lactating goats injected intravenously with [1-13C]VA at time zero, on (a) sunflower-seed oil and (b) sunflower-seed and fish oil plus starch treatments. The post-injection data are plotted at the mean time point for each collection period. Values are means for four goats, with standard errors represented by vertical bars.
Table 4 Effect of dietary sunflower-seed oil alone or in combination with fish oil plus starch on the different steps of the metabolism of vaccenic acid (VA) and of cis-9, trans-11-conjugated linoleic acid (rumenic acid; RA) in goats
(Mean values with their standard errors for four goats per treatment)
Regarding the other parameters of the Δ9-desaturation, neither the milk Δ9-desaturation ratios nor the abundance of mRNA encoding for SCD1 or SCD5 in mammary tissues (Table 5) were significantly modified by the treatments.
Table 5 Effect of dietary sunflower-seed oil alone or in combination with fish oil plus starch on mRNA relative abundance encoding for stearoyl-CoA desaturase 1 and 5 (SCD1 and SCD5) in mammary tissue and on the milk fatty acid Δ-9 desaturation ratios in goats
(Mean values with their standard errors for four goats per treatment)
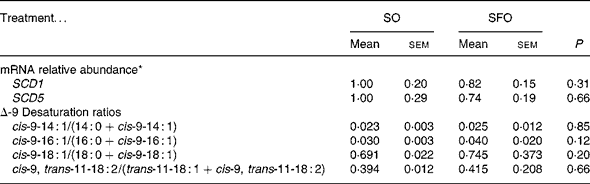
SO, sunflower-seed oil; SFO, sunflower-seed oil and fish oil (2:1) plus starch from rolled barley.
* mRNA levels expressed as fold change relative to SO treatment after normalisation with cyclophilin A mRNA.
Discussion
The present study investigated the effects of sunflower-seed oil in combination with fish oil plus starch addition compared with sunflower-seed oil alone on milk fat yield and composition in goats. Additionally, the Δ9-desaturation of VA to RA in vivo was measured using a chemical tracer strategy and was related to other variables of mammary Δ9-desaturation such as SCD1 and SCD5 gene expression and milk normalised Δ9-desaturation ratios. The effect of the dietary treatments on these variables was explored to gain further insight into the nutritional regulation of the Δ9-desaturation in goats.
Dairy performance and milk fatty acid composition
There were no significant differences in milk production or composition between the two dietary treatments (SO and SFO). In a previous study on grazing goats, Gagliostro et al. (Reference Gagliostro, Rodriguez and Pellegrini14) observed no differences between goats fed iso-starchy diets with either 30 ml fish oil per d or 30 ml fish oil plus 150 ml sunflower-seed oil per d on milk fat, protein and lactose concentrations. Elsewhere, numerous studies have been performed in dairy cows fed fish oil demonstrating a strong negative effect on milk fat yield and percentage(Reference Chilliard, Ferlay and Doreau28). Altogether, these data outline that the responses of milk yield and composition to different types of lipid supplements differ greatly between goats and cows, with an absence of decrease in milk fat content and yield in goats, conversely to cows(Reference Chilliard, Ferlay and Rouel9, Reference Chilliard, Glasser and Ferlay11). It is likely that these differences in dairy performance responses to addition of fish oil in the diet of ruminants may be partly due to species differences.
In cows, increasing the amount of starch in PUFA-rich diets, in particular with PUFA from plant oils, modified the profile of C18 : 1 and C18 : 2 intermediates formed in the rumen(Reference Griinari, Dwyer and McGuire29, Reference Piperova, Teter and Bruckental30) in favour of the trans-10 pathway, which is a factor associated with milk fat depression(Reference Bauman and Griinari3). Such effects of milk fat depression and trans-10 pathway increase were not observed in the present study on adding fish oil and increasing the level of dietary starch (SFO treatment), in line with previous studies in goats receiving starchy diets and PUFA from plant oils(Reference Chilliard, Glasser and Ferlay11, Reference Bernard, Shingfield and Rouel16). The concentration of milk trans-10-18 : 1 with SFO was low compared with values observed in cows under similar dietary conditions (0·7 in the present study v. 3·6 % of total fatty acids with a diet with 3 % DM intake added fat as 1 % of fish oil and 2 % of sunflower-seed oil(Reference Palmquist and Griinari31)), confirming the lower occurrence, in goats compared with cows, of the shift of ruminal biohydrogenation towards the trans-10 pathway(Reference Bernard, Shingfield and Rouel16). Altogether, these results show the absence of response of the ruminal biohydrogenation pathway to diets rich in starch and PUFA in the goat compared with the cow. On the other hand, an estimation of the degree of ruminal trans-18 : 1 reduction from the ratio of trans-18 : 1/(18 : 0+cis-18 : 1+trans-18 : 1)(Reference Palmquist and Griinari31) in milk (i.e. 0·20 and 0·33, respectively, for SO and SFO) is in line with fish oil acting as an inhibitor of the conversion of trans-18 : 1 to stearic acid in the rumen(Reference Chilliard, Ferlay and Doreau28). In cows these milk ratios were higher for similar dietary conditions (values of 0·33 and 0·50, respectively, with a diet with 3 % DM intake added fat as sunflower-seed oil or as 1 % of fish oil and 2 % of sunflower-seed oil(Reference Palmquist and Griinari31)), suggesting that the ruminal biohydrogenation processes are more strongly altered in cows than in goats by PUFA addition to the diet.
The goats fed fish oil and sunflower-seed oil plus additional starch had higher percentages of milk 10 : 0+12 : 0+14 : 0 (+21·4 %), which are synthesised de novo, and lower percentages of the sum of C18 ( − 21·1 %) than those fed sunflower-seed oil. This is in line with data on cows comparing diets similar to ours (for example, addition of fish oil to sunflower-seed oil v. sunflower-seed oil alone leading, respectively, to +11·6 % of milk C10 to C14 and − 11·9 % of C18(Reference Palmquist and Griinari31)). Taken together, these results suggest that the sunflower-seed oil fatty acids or their metabolites inhibit the de novo fatty acid synthesis in the mammary gland more strongly than fatty acids of fish oil or their metabolites, and/or that fish oil fatty acid modifies the metabolites of sunflower-seed oil fatty acids to less inhibitory products.
Even though milk concentrations of long-chain fatty acids 20 : 5n-3 and 22 : 6n-3 increased with SFO treatment, their concentrations remained low, with an apparent transfer rate from diet to milk fat of 4·1 and 3·6 %, respectively, in accordance with studies in cows(Reference Chilliard, Ferlay and Doreau28, Reference Palmquist and Griinari31) and goats(Reference Gagliostro, Rodriguez and Pellegrini14). These results are due to the extensive biohydrogenation of PUFA in the rumen as well as the partitioning of these fatty acids into plasma cholesteroyl esters and phospholipid fractions which are not large providers of fatty acids to the mammary gland(Reference Offer, Speake and Dixon32).
Δ9-Desaturation
We measured in vivo the Δ9-desaturation of VA to RA using a chemical tracer strategy and then related this measurement to other variables characterising the mammary Δ9-desaturase system assuming that the mammary gland in goats, like in cows(Reference Mosley, Shafii and Moate8), is the major site of fatty acid Δ9-desaturation. This assumption is supported by the absence of Δ9-desaturation of [14C]VA to [14C]RA observed in bovine liver slices(Reference Gruffat, De La Torre and Chardigny33) and by the probable low lipomobilisation (as suggested by the very low plasma NEFA concentration, 0·14 to 0·21 mm; results not shown) in late-lactating goats in positive energy balance (Table 2) in the present study.
In our preliminary study we observed higher milk fat enrichment values for VA when injected intravenously as NEFA compared with TAG (Fig. 1). In the nutritional study we thus used 13C-labelled VA as NEFA and at a greater dose than in the preliminary study to ensure a sufficient enrichment of milk VA and RA because increased VA and RA secretions were expected due to lipid addition to the diet(Reference Chilliard, Glasser and Ferlay11), to estimate in vivo conversion of VA to RA. The enrichment was longer in the nutritional study (Fig. 2) than in the preliminary study (Fig. 1), which may be explained by the addition of lipid to the diets in the nutritional study.
All the studies examining the contribution of endogenous RA synthesis have been conducted in lactating cows and involved either a chemical inhibition of the Δ9-desaturase enzyme by sterculic acid(Reference Griinari, Corl and Lacy5, Reference Corl, Baumgard and Dwyer34, Reference Kay, Mackle and Auldist35), or a quantification of the duodenal or abomasal flow of VA and RA to estimate the endogenous synthesis of RA(Reference Shingfield, Ahvenjarvi and Toivonen6, Reference Glasser, Ferlay and Doreau7, Reference Qiu, Eastridge and Firkins13, Reference Loor, Ferlay and Ollier21, Reference Piperova, Sampugna and Teter36, Reference Loor, Ferlay and Ollier37), or a tracer methodology to allow direct measurement of the conversion of VA to RA in vivo (Reference Mosley, Shafii and Moate8). These studies have provided clear evidence that endogenous desaturation of VA is the main source of milk fat RA in cows (with 64–97 % RA coming from VA), but few of them provided an estimation of VA desaturation in the mammary gland: 25·7(Reference Mosley, Shafii and Moate8), 28·9(Reference Shingfield, Ahvenjarvi and Toivonen6), 19·8(Reference Qiu, Eastridge and Firkins13) and 21 %(Reference Glasser, Ferlay and Doreau7). In the present study, direct measurements of Reference Qiu, Eastridge and Firkins13C enrichment of milk lipids following an intravenous injection of 1·5 g [13C]VA established that 31·7 and 31·6 % of VA was desaturated in vivo in goats with SO and SFO treatments, respectively. The close agreement of estimates (20–32 %) of percentages of VA converted to RA across all these studies suggests that the percentage of VA converted to RA is more closely linked to the Δ9-desaturase enzyme than to other factors that differed between these studies: methodologies, ruminant species, dietary conditions (forage:concentrate ratio, lipid supplement) and circulating fluxes of VA.
Among the milk Δ9-desaturation ratios used to estimate the in vivo mammary Δ9-desaturation(Reference Bauman and Griinari3), the variation of cis-9-14 : 1/14 : 0 was reported to be most closely correlated to the response of mammary Δ9-desaturase activity(Reference Bernard, Leroux and Chilliard38), probably due to the low level of myristic and myristoleic acids in ruminant feedstuffs. In the present experiment, the four pair normalised ratios for Δ9-desaturation did not differ between treatments. The milk RA/(VA+RA) values of 39·4 and 41·5 % for SO and SFO, respectively (Table 5), are numerically higher than the 31·7 and 31·6 % of VA conversion to RA for SO and SFO, respectively (Table 4) which represents in vivo Δ9-desaturation measurement. The absence of a dietary treatment effect on VA Δ9-desaturation estimates is also in line with the Δ9-desaturase gene expression estimated by the mRNA abundance of SCD1 and SCD5, which was similar among treatments. The SCD5 gene was recently characterised in bovines(Reference Lengi and Corl17), and no data have been published on its expression and nutritional regulation in ruminant mammary tissue. The present study shows that its expression may contribute to some extent to the mammary Δ9-desaturase activity.
Taken together, these results demonstrate that in our experimental conditions, the different variables used for studying the in vivo regulation of mammary Δ9-desaturase enzyme – gene expression (SCD1 and SCD5 mRNA), milk fatty acid Δ9-desaturation ratios, and direct measurement of the in vivo Δ9-desaturation by a chemical tracer technique – are consistent and show the absence of variation of the Δ9-desaturation between treatments.
Our initial hypothesis was that SFO treatment would negatively affect the mammary Δ9-desaturase system due to the negative effect of the very-long-chain PUFA (n-3) of fish oil(Reference Ahnadi, Beswick and Delbecchi18) and/or of specific intermediates that are synthesised via the trans-10 pathway in the rumen(Reference Bauman and Griinari3) due to interaction between PUFA and additional starch.
In cows fed protected fish oil(Reference Ahnadi, Beswick and Delbecchi18), a down-regulation of SCD1 gene expression was reported, as in goats fed formaldehyde-treated linseed(Reference Bernard, Rouel and Leroux24). The relatively low incorporation and secretion of long-chain PUFA (20 : 5n-3 and 22 : 6n-3) in milk in the SFO treatment (Table 3) could explain the lack of changes in goats' SCD1 mRNA abundance in the present experiment compared with those obtained in cows fed protected fish oil(Reference Ahnadi, Beswick and Delbecchi18) (20 : 5n-3 and 22 : 6n-3 were 0·13 and 0·09 % of total fatty acids, respectively, for SFO treatment in goats v. 0·21 and 0·13 %, respectively, in cows(Reference Ahnadi, Beswick and Delbecchi18)).
In addition, it was reported in dairy cows fed diets rich in starchy concentrate with addition of a mixture of soyabean oil and fish oil (2:1) that specific intermediates formed during the biohydrogenation of PUFA in the rumen could be involved in the down-regulation of lipogenic gene expression (in particular of SCD1) and milk fat depression(Reference Harvatine and Bauman39). One of these inhibitory intermediates was the trans-10, cis-12-conjugated linoleic acid, which also reduced milk fat Δ9-desaturase ratios in cows(Reference Baumgard, Corl and Dwyer40, Reference Peterson, Matitashvili and Bauman41) and in goats(Reference Andrade and Schmidely42–Reference Shingfield, Rouel and Chilliard44). In the present study, the absence of increase in the milk trans-10-18 : 1 concentration with SFO treatment is in line with other studies in goats(Reference Chilliard, Glasser and Ferlay11, Reference Bernard, Shingfield and Rouel16). The low concentration of this fatty acid in milk (Table 3) is an indicator of the low occurrence of the ruminal trans-10 pathway, which probably explains the absence of effect of our dietary treatments on the different variables used to estimate the in vivo regulation of mammary Δ9-desaturation.
The percentages of milk RA coming from VA desaturation were estimated to be 73 and 63 % for the SO and SFO treatments, respectively, in line with the 86 and 68 % found by measuring duodenal and milk flows in cows fed 2 % of DM intake of soyabean oil or fish oil, respectively(Reference Qiu, Eastridge and Firkins13). Thus, 27 and 37 % of the RA secreted in milk for SO and SFO, respectively, are taken up directly by the mammary gland.
Conclusions
Using 13C-labelled VA, we have shown that in goats, 32 % of VA was Δ9-desaturated into RA and that milk RA originating from VA represents 63 − 73 % of total milk RA. In addition, feeding sunflower-seed oil in combination with fish oil plus additional starch, compared with sunflower-seed oil alone, had no effect either on the percentages of VA Δ9-desaturated into RA and milk RA originating from VA or on the other variables used to estimate the Δ9-desaturase system (SCD1 and SCD5 gene expression; milk Δ9-desaturase ratios). We hypothesise that our dietary treatments were not extreme enough to induce a response of the Δ9-desaturase system in goats, which is probably due to caprine specificities that include (i) a lower occurrence of the shift of ruminal biohydrogenation towards the trans-10 pathway, thus limiting the synthesis of biohydrogenation product inhibitors of the Δ9-desaturase, and (ii) a lower sensitivity of the mammary Δ9-desaturase to long-chain PUFA, compared with cows. Further experiments with more sharply contrasting diets and with comparison of cows and goats fed the same diets would test these hypotheses.
Acknowledgements
The present study was supported by the ANR-Agence Nationale de la Recherche under the Programme National de Recherche en Alimentation et Nutrition Humaine (project ANR-05-PNRA-No.5.E.24).
The authors gratefully acknowledge the staff of the Animal Nutrition and Metabolism Unit Les Cèdres, in particular Denis Roux, Christophe Mathevon, André Combeau and Dominique Roux, for the diligent care of experimental animals, R. Jailler and his team for assisting in the slaughtering of experimental animals, the technical assistance of Jordann Domagalsky, Didier Bany, Bernard Lyan and Charlotte Joly, the advice of Pierre Juaneda and the supply of [13C]VA by Olivier Loreau (Commissariat à l'Énergie Atomique (Commission for Atomic Energy; CEA), Saclay and Cadarache, France).
All authors contributed to the preparation of the paper and agreed with the submitted manuscript content. L. B. and Y. C. designed the research and J.-M. C. was the coordinator of the Transqual project. L. B., J. M. and J. R. performed the research and J. M., L. B., P. C. and E. P.-G. analysed the samples. L. B., Y. C., F. G. and J. M. analysed the data and drafted the paper.
There are no conflicts of interest.









