The observation in higher vertebrates that the presence of luminal phospholipids (PL) enhances intestinal TAG uptakes(Reference O'Doherty, Kaksis and Kuksis1–Reference Nishimukai and Hara6) has led to the formulation of several hypotheses. For instance, the oral administration of lecithin to weanling pigs has been suggested to play a role in the emulsification of dietary fat in the lumen and hence to facilitate the uptake of fat by the intestinal cell(Reference Jones, Hancock, Harmon and Walker7). Dietary PL may furthermore enhance the export of lipid out of the cell into circulation since hydrolysed lyso PL, once taken up by the enterocyte, can be re-acylated and used for formation of the polar lipid coat of intestinal lipoproteins(Reference Tso, Kendrick, Balint and Simmonds3, Reference Beil and Grundy8). In addition, luminal PL have been reported to regulate the synthesis of apolipoproteins, crucial for the assembly of intestinal lipoproteins, as seen in vivo for apo A-I in newborn pig enterocytes(Reference Wang, Du, Lu, Sao, Hunter and Black9) and in vitro for apo B-48 in intestinal CaCo-2 cell lines(Reference Mathur, Born, Murthy and Field10). Interestingly, the latter study showed that the increased apo B-48 synthesis, accompanied by increased TAG secretion, was more pronounced following co-incubation with phosphatidylcholine (PC) than with other PL classes(Reference Mathur, Born, Murthy and Field10), indicating a specific effect of PC on intracellular lipid mobilization. Besides their effect on lipid absorption, PL administrations have also been found to affect further lipoprotein metabolism, hepatic lipid processing and to augment the biliary lipid flow, inducing alterations in the plasma lipid profile, such as lowered cholesterol(Reference Wong, Nicolosi and Low11–Reference Wilson, Meservey and Nicolosi14).
In lower vertebrates, little is known on the effect of dietary PL on circulating or postprandial plasma lipid levels. By contrast, a large number of studies have demonstrated the beneficial effects of dietary PL on growth and digestibility in juvenile and adult teleost fish(Reference Poston15–Reference Kasper and Brown18). Furthermore, at the larval stage, fish even seem to have a dietary requirement for PL in order to obtain good survival and growth during early development(Reference Geurden, Radünz-Neto and Bergot19–Reference Cahu, Zambonino-Infante and Barbosa21) with a different response according to the supplied PL class(Reference Kanazawa, Kaushik and Luquet22). In trials with first-feeding common carp larvae, PC exerted the strongest growth-promoting effect, whereas phosphatidylinositol improved survival and reduced the occurrence of larval deformities(Reference Geurden, Charlon, Marion and Bergot23, Reference Geurden, Marion, Charlon, Coutteau and Bergot24). That PC, as outlined earlier for mammals, also plays an active role in the absorption of dietary neutral lipid was evidenced by histological examination of intestines from 25-d-old carp sampled from the latter two studies. The results showed important lipid droplet accumulations in enterocytes of PC-devoid or phosphatidylinositol-fed fish, which were completely absent when fed a dietary PC source(Reference Fontagné, Geurden, Escaffre and Bergot25). This finding is thus consistent with reports in mammals that not the entry or re-esterification of the lipids in the cell, but the ability of the enterocyte to export TAG is impaired in the absence of dietary PC(Reference Tso, Kendrick, Balint and Simmonds3, Reference Mansbach, Arnold and Cox4, Reference Mathur, Born, Murthy and Field10). Similar conclusions were made following the observation of lipid droplets in the enterocytes of two species of adult salmonids fed PC-free diets(Reference Olsen, Myklebust, Kaino and Ringo26, Reference Olsen, Dragnes, Myklebust and Ringø27). Other indications of the stimulating effect of dietary PL on intestinal lipid mobilization concern the increased recovery of essential fatty acids (DHA and EPA) and of radiolabelled oleic acid in body lipids of marine fish fed PC(Reference Geurden, Bergot, Schwarz and Sorgeloos28, Reference Hadas, Koven, Sklan and Tandler29). Taken the afore-mentioned findings together, it would hence not be surprising to find an effect of a dietary PL supply, particularly of PC, on circulating and postprandial plasma lipids in fish.
The present study examined to what extent dietary PC affects the postprandial and circulating lipids in the plasma of juvenile common carp fed a semi-purified casein-based diet containing purified soyabean PC compared with an isolipidic diet without PC. We also analysed the lipid levels in the faeces of fish from both treatments in order to estimate the amount of fat absorbed when fed a diet with or without PC.
Experimental methods
Experimental animals and diets
Common carp (Cyprinus carpio) juveniles of 100 (SD 15) g initial body weight were placed in 60-litre cylindroconical tanks (fourteen fish per tank). The outlet of each tank of the recirculating system was equipped with a continuous filtering device for collecting the faeces(Reference Choubert, De La Noue and Luquet30). The water temperature was maintained at 25°C and the photoperiod was 12–12 h light–dark. Each diet was fed twice per d to visual satiation for 4 weeks to quadruplicate groups. The two semi-purified diets were based on casein and gelatine as a protein source and differed only by their lipid composition (Table 1). Diet SBO contained 12 % soyabean oil (Lesieur, France) and diet SPC contained 8 % soyabean oil and 4 % purified soyabean PC (95 % PC; Epikuron 200, Lucas Meyer GmbH, Germany). The vitamin mix supplied both diets with 2000 mg/kg choline chloride (60 % choline), fulfilling the choline requirement for carp(31). Chromic oxide (Cr2O3, 1 %) was added as an indigestible marker.
Table 1 Formulation and proximate composition of the semi-purified isolipidic diets containing soyabean oil (diet SBO) or soyabean oil plus soyabean phosphatidylcholine (diet SPC) fed to juvenile common carp for 4 weeks*

* For details of diets and procedures, see Experimental methods.
† Modified from Guelph purified salmonid diet(31). Composition (% diet): casein 40; gelatine 4; DL-methionine 0·5; L-arginine 1; starch 10·5; dextrin 8; D-glucose 5; α-cellulose 5; celite 1; sodium-alginate 1; vitamin mix 3; mineral mix 8.
Growth and apparent digestibility
The fish from each tank (four tanks per diet) were bulk-weighed and counted at the end of the trial in order to calculate the final body weight (BW) and specific growth rate ![]() of trial duration. The faeces were collected daily from each tank (four tanks per diet) during the last 2 weeks of the feeding period and frozen at − 20°C. They were freeze-dried and pooled per tank prior to analysis. Diets and faeces were analysed for DM (105°C for 24 h), ash (550°C for 12 h), lipid (Soxtherm, Gerhardt), protein (N × 6·25, Kjeldahl Nitrogen analyser 2000; Fison Instruments), gross energy (adiabatic bomb calorimetry, IKA) and chromic oxide (perchloric acid digestion, according to Bolin and colleagues(Reference Bolin, King and Klosterman32)). The apparent digestibility coefficients (ADC) were calculated using the formula:
of trial duration. The faeces were collected daily from each tank (four tanks per diet) during the last 2 weeks of the feeding period and frozen at − 20°C. They were freeze-dried and pooled per tank prior to analysis. Diets and faeces were analysed for DM (105°C for 24 h), ash (550°C for 12 h), lipid (Soxtherm, Gerhardt), protein (N × 6·25, Kjeldahl Nitrogen analyser 2000; Fison Instruments), gross energy (adiabatic bomb calorimetry, IKA) and chromic oxide (perchloric acid digestion, according to Bolin and colleagues(Reference Bolin, King and Klosterman32)). The apparent digestibility coefficients (ADC) were calculated using the formula:
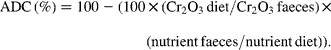
Circulating and postprandial plasma lipids
After the 4-week feeding period, the fish were starved for 48 h in order to have the basal levels of plasma lipids and fed a single meal to satiation. Five individuals from each dietary treatment were taken randomly at 4, 6, 8, 10, 12, 16, 24, 30 and 48 h after the meal for blood sampling from the caudal vein. Sampled fish were placed in a separate tank in order to avoid sampling the same fish twice. Plasma was separated from whole blood (plus 0·01 % heparin) in situ by centrifugation (1500 g, 5 min) and was frozen at − 80°C until subsequent analyses. The lipid composition of the plasma was analysed using enzymatic procedures, commercialized by Biomérieux (France) for PL, TAG and total cholesterol (free and esterified) and by Wako Chemicals (Germany) for NEFA.
Statistical analyses
The final body weight (log-transformed) and specific growth rate of the fish were subjected to a one-way analysis of covariance using the initial body weight (log-transformed) as a covariate. The apparent digestibility coefficients (asin-transformed) were analysed by a one-way ANOVA. The effects of diet and sampling time on plasma lipid levels were analysed by a two-way ANOVA, followed by one-way ANOVA in case of a significant interaction. Significant differences between means were evaluated by the Newman–Keuls test. The significance level was set at P < 0·05. The analyses were performed using the STATISTICA software program (Statsoft Inc., Tulsa, OK, USA).
Results
Growth
The survival of the carp during the 4-week feeding trial was 100 % in both dietary treatments. The SPC-fed fish had a significantly higher final body weight and specific growth rate than fish fed the SBO diet (Table 2).
Table 2 Body weight (BW), specific growth rate (SGR) and apparent digestibility coefficients of dietary components in common carp fed the experimental diets for 4 weeks*
(Values are means and standard deviations for four tanks per diet)

Diet SBO, soyabean oil; Diet SPC, soyabean oil plus soyabean phosphatidylcholine.
* For details of diets and procedures, see Experimental methods.
† ANOVA (except for final BW and SGR for which an analysis of covariance was used with initial BW as covariate).
Apparent digestibility
There was a significant increase in the apparent digestibility of total lipid (P = 0·0007, Table 2) when the carp were fed the diet supplemented with soyabean PC (96·3 %) as compared with the PC-free diet SBO (92·1 %). The positive effect of a PC addition was also seen for the apparent digestibility of energy, which was found to be slightly but significantly (P = 0·011) higher in the SPC than SBO groups. The apparent protein digestibility was elevated and did not differ between both treatments (Table 2).
Circulating baseline and postprandial plasma lipids
Following the 48 h fast, the plasma baseline levels (Fig. 1) comprised between 175 and 200 mg/dl for TAG, 130 and 140 mg/100 ml for total (free and esterified) cholesterol and between 12 and 16 mg/100 ml for NEFA. The latter baseline concentrations were unaffected by the dietary treatment (P>0·05). The plasma PL level following the 48 h fast (Fig. 1 (b)) was almost 20 % higher (P = 0·010) in the SPC-fed fish (500 mg/100 ml) than in the SBO-fed fish (420 mg/100 ml). The resulting core-to-surface TAG:PL ratio of the plasma lipids in the 48 h unfed fish were significantly lower (P = 0·023) in fish from the SPC than from the SBO treatment (0·35 and 0·47, respectively).

Fig. 1 Changes in circulating plasma lipids in juvenile carp during the 48 h following the administration of a single isolipidic meal with (diet SPC, –●–) or without (diet SBO, –○–) soyabean phosphatidylcholine (PC). (a) TAG; (b) phospholipids (PL); (c) cholesterol; (d) NEFA. Values are means with their standard errors of the mean from five fish per sampling point. Statistical differences in postprandial plasma lipid profiles related to the dietary treatment and sampling time are shown in Results. For details of diets and procedures, see Experimental methods.
Feeding a single meal led to a rapid rise in the concentration of plasma TAG (Fig. 1 (a)). This increase was apparent 4 h after the meal as shown by the 20 to 30 % increase relative to the baseline TAG level. After the meal (6 h), the relative TAG increases were 168 and 225 % in SBO-fed and SPC-fed fish, respectively. The TAG absorption peak reached its apparent maximum 8 h after the meal showing a more than 3-fold (365 %) increase relative to baseline level This corresponds with a net increase (peak minus baseline level) of 430 and 460 mg per 100 ml in fish fed diets SBO and SPC, respectively. During the next 2 h, there was a rapid drop in TAG, with levels at 10 h resembling baseline levels (Fig. 1 (a)). The two-way ANOVA performed on plasma TAG indicated a significant effect of time after meal (P < 0·0001; T8>T6>T4 = T10 = T12 = T16 = T24 = T32 = T48), but not of the diet (P = 0·12). The interaction term (diet × time) was not significant (P = 0·85).
Following the meal, the plasma PL levels first showed a slight (5 %) decrease (at 4 h), whereafter they increased up to 475 mg (SBO-fed fish, at 8 h) and 600 mg per 100 ml (SPC-fed fish, at 8–10 h), which represents a 12 and 20 % increase as compared with the respective baseline levels (Fig. 1 (b)). The net increase in plasma PL (peak minus baseline level) was 55 mg per 100 ml with diet SBO and 98 mg per 100 ml with diet SPC. In both treatments, the postprandial peak was followed by a second peak seen 30 h after the meal, which was less sharp but with slightly higher amplitude (Fig. 1 (b)). The two-way ANOVA performed on plasma PL showed a highly significant effect of the diet (P < 0·0001; SPC>SBO) and of sampling time (P = 0·0001; T30 = T24 = T10 = T8 = T12 = T16>T6 = T48>T4), without interaction (diet × time, P = 0·26).
At the moment of the postprandial TAG peak (at 8 h), the TAG:PL ratio of the total plasma lipids was 1·06 and 1·31 in SPC- and SBO-fed fish, respectively (P = 0·13, one-way ANOVA). In order to compare the plasma TAG:PL ratios of the lipids, which had entered the circulation during absorption in fish from both treatments, we used the net increases (peak minus baseline values) of TAG and PL. These ratios were significantly affected by the diet (P = 0·004, one-way ANOVA), being 7·8 in SBO-fed fish and only 4·6 in SPC-fed fish.
Postprandial fluctuations in cholesterolaemia (Fig. 1 (c)) observed at the different sampling times did not fully coincide in both treatments as indicated by the significant interaction term diet × time (two-way ANOVA, F8,72 4·25, P = 0·0003). For instance, a small peak occurred at 8 h in SPC-fed fish, which was absent in SBO-fed fish, and the second peak at 30 h in SPC-fed might correspond with the peak seen at 24 h in SBO-fed fish (P < 0·05, one-way ANOVA). The dietary effect was only significant at two sampling points (at 8 h, P = 0·013 and at 30 h, P = 0·002) indicating higher cholesterol in SPC- than SBO-fed fish (one-way ANOVA). As mentioned earlier, no differences in cholesterol levels were noted at 48 h (P = 0·63).
Postprandial plasma NEFA levels were low (Fig. 1 (d)). As for cholesterol, there was a significant interaction between diet × sampling time (two-way ANOVA, F8,72 7·33, P < 0·0001). We noted a slight increase in NEFA at 10 h in SPC-fed fish, seen 2 h later in SBO-fed fish. In both treatments, however, the highest NEFA levels were seen at 30 h and lowest levels at 4 h (P < 0·05, one-way ANOVA). At none of the sampling times did we find a dietary effect on circulating NEFA (P>0·05, one-way ANOVA).
Discussion
The observation that plasma NEFA did not significantly increase following the meal confirms early studies in teleost fish that fatty acids released in the lumen and taken up by the enterocytes are being re-esterified prior to their entry into the circulatory system(Reference Bergot and Flechon33–Reference Iijima, Aida, Mankura and Kayama35). More recent studies showed that the synthesis and re-acylation pathways of TAG and PL in teleost enterocytes do not differ substantially from those in mammals(Reference Oxley, Torstensen, Rustan and Olsen36, Reference Caballero, Gallardo, Robaina, Montero, Fernandez and Izquierdo37). In the present study, the postprandial rise in TAG was already apparent after 4 h and reached its maximum after 8 h, which is more than twice as fast as in an earlier study with carp, where the TAG peak appeared only after 20 h(Reference Iijima, Aida, Mankura and Kayama35). This difference is most probably caused by the higher temperature in the present study, known to accelerate lipid absorption in fish(Reference Wallaert and Babin38). Except for the modestly higher plasma TAG at 6 h in SPC- than SBO-fed fish, there was no noticeable acceleration of lipid absorption due to dietary SPC, in contrast with what could be expected from studies with bile-diverted rat(Reference Bennett-Clark2) or mice(Reference Werner, Havinga, Perton, Kuipers and Verkade39). Exogenous PC administration to latter rodents without biliary PL in the intestinal lumen strongly enhanced the rate of appearance of plasma TAG(Reference Bennett-Clark2, Reference Werner, Havinga, Perton, Kuipers and Verkade39) and some of this earlier work concluded that the availability of luminal lecithin is a requisite for intestinal lipid absorption(Reference O'Doherty, Kaksis and Kuksis1, Reference Tso, Kendrick, Balint and Simmonds3). Moreover, studies conducted with rat under normal physiological conditions showed that exogenous PL administration (in addition to the normal bile supply) resulted in a more efficient net uptake of the dietary lipid(Reference Nishimukai, Hara and Aoyama5, Reference Nishimukai and Hara6). Our data are consistent with the latter observations as: (i) the apparent lipid digestibility was improved in the SPC-fed carp; (ii) the maximum concentration of the postprandial TAG peak was identical in fish from both dietary treatments, despite the 33 % lower TAG level in the SPC than in SBO diet as well as the 33 % lower amount of fatty acids provided by soya PC than by soya oil. Some studies in fish(Reference Poston15, Reference Hung, Berge and Storebakken17) or young pigs(Reference Jones, Hancock, Harmon and Walker7) have attributed the improved lipid digestibility to the emulsifying properties of PL, enhancing the luminal fat digestion and uptake of micelles through the unstirred water layer and thus the entry into the cell. There is, however, ample evidence from mammalian studies that it is not the uptake of the lipolytic products by the cell, but the export from the cell that is impaired in cases of PL-deficiency(Reference Tso, Kendrick, Balint and Simmonds3, Reference Mansbach, Arnold and Cox4, Reference Mathur, Born, Murthy and Field10). This was also suggested in fish(Reference Olsen, Myklebust, Kaino and Ringo26, Reference Olsen, Dragnes, Myklebust and Ringø27), in line with our earlier histological observations of large lipid droplet accumulations in enterocytes of larval carp fed a diet devoid of PC(Reference Fontagné, Geurden, Escaffre and Bergot25). Importantly, these droplets, regarded as a form of temporary TAG storage when the entry of fatty acids in the cell exceeds the output capacity(Reference Sire, Lutton and Vernier34, Reference Olsen, Myklebust, Kaino and Ringo26), were not seen in carp larvae fed dietary PC(Reference Fontagné, Geurden, Escaffre and Bergot25). Further, larvae fed the PC-supplemented diets showed improved growth and survival compared with larvae fed the PC-free diet(Reference Geurden, Charlon, Marion and Bergot23, Reference Geurden, Marion, Charlon, Coutteau and Bergot24). The present study confirms the growth-promoting effect of PC, although to a lesser extent than in first-feeding carp larvae.
The intracellular mechanisms by which luminal PL stimulate intestinal lipid export are not fully understood. In vitro studies with Caco-2 cells demonstrated an enhancing effect of PC on chylomicron synthesis and assembly by triggering the synthesis of specific apolipoproteins, such as apo B48(Reference Mathur, Born, Murthy and Field10). Alternatively, chylomicron formation can also be enhanced by an increased availability of polar lipid surface material. As such, the hydrolysed, absorbed and re-acylated sn-1 lysoPC may be directly used for intestinal chylomicron formation. In carp, however, some controversy exists regarding the intestinal lipoproteins involved in the absorption process. Iijima et al. (Reference Iijima, Aida, Mankura and Kayama35) suggested an important role for HDL since the majority of the radioactivity of orally administered fatty acids appeared first in HDL instead of chylomicrons or VLDL. This is in disagreement with earlier electron microscopic observations in carp, which revealed the presence of VLDL-like particles in the absorbing enterocytes(Reference Noaillac-Depeyre and Gas40) or with studies in other teleosts showing large chylomicrons and VLDL-like lipoproteins during lipid absorption(Reference Sire, Lutton and Vernier34, Reference Babin and Vernier41). In the present study, it is believed that the carp secreted VLDL or chylomicra rather than HDL during the absorption process since the TAG:PL ratio (>4·5) of the lipids that had entered circulation at the time of the absorption peak (peak minus baseline values) is too elevated for HDL, whose TAG:PL ratio, found to be 0·25 in carp(Reference Fainaru, Schafer, Gavish, Harel and Schwarz42), does not seem to exceed 0·6 in most other teleosts(Reference Skinner and Rogie43–Reference Ando and Mori45). The present study also shows that the TAG:PL core-to-surface ratio of the newly secreted lipoproteins was about 70 % higher in SBO- (7·8) than in SPC- (4·6) fed fish. This finding agrees with earlier reports in human subjects that lecithin ingestion promotes the production of VLDL-sized ‘small chylomicrons’ with a lower TAG:PL ratio than that of large chylomicrons produced after ingestion of a TAG oil(Reference Beil and Grundy8). Hence, the larger number of smaller chylomicrons in the SPC-fed fish, probably due to the recycling of absorbed PC, may have promoted the export of TAG from the enterocytes into circulation. It would, however, be of interest to confirm here that the (higher) postprandial PL output in SPC-fed fish is indeed of dietary and not of biliary origin given observations in mammals that the biliary lipid output is stimulated by soyabean PL(Reference Rioux, Perea, Yousef, Levy, Malli, Carrillo and Tuchweber12, Reference Polichetti, Diaconescu, Malli, Portugal, Pauli, Tuchweber, Yousef and Chanussot13) and that bile PC might be preferred over dietary PC as chylomicron PC precursor(Reference Mansbach46). Both topics received little attention in teleosts and require further work, especially in view of the reported absence of PL in the bile of carp(Reference Goto, Holzinger, Hagey, Cerre, Ton-Nu, Schteingart, Steinbach, Shneider and Hofmann47).
In addition to its role during intestinal absorption, dietary lecithin in higher vertebrates has also been documented to affect further (intravascular and hepatic) lipoprotein and lipid metabolism, resulting in modified plasma lipid profiles(Reference Wong, Nicolosi and Low11–Reference Wilson, Meservey and Nicolosi14). To our knowledge, there exist no data in fish on the effect of PL on circulating plasma lipids. In the present study, the basal plasma PL level in the 48 h unfed fish from the SPC-treatment remained almost 20 % higher than when fed the PC-free diet. This persistently higher amount of circulating PL can probably be explained by the redistribution of the PL-components of the surfaces of the (higher number of smaller) intestinal lipoproteins to the HDL fraction in the SPC-fed fish. HDL generally constitute the most abundant lipoprotein class in teleosts(Reference Babin and Vernier41) and in carp, which lack plasma albumin, HDL has been reported to assure the transport of non-esterified plasma fatty acids(Reference De Smet, Blust and Moens48) in addition to their well-documented role in cholesterol transport(Reference Iijima, Aida, Mankura and Kayama35, Reference Fainaru, Schafer, Gavish, Harel and Schwarz42). The present study, however, does not confirm the cholesterol-lowering effect of soyabean lecithin observed in mammals(Reference Wong, Nicolosi and Low11, Reference Polichetti, Diaconescu, Malli, Portugal, Pauli, Tuchweber, Yousef and Chanussot13, Reference Wilson, Meservey and Nicolosi14) since the cholesterol (baseline) levels were similar in fish from both treatments. One explanation of the hypocholesterolaemic effect of lecithin in higher vertebrates is the enhanced secretion of bile lipids, particularly of bile cholesterol and bile salts, provided in increased amounts by the metabolism of plasma HDL-cholesterol whose transport to the liver is enhanced by dietary PC(Reference Polichetti, Diaconescu, Malli, Portugal, Pauli, Tuchweber, Yousef and Chanussot13). Further studies are needed to verify whether or why dietary PL does not seem to lower circulating cholesterol in carp, which like most other teleosts is reputed to be hypercholesterolaemic by nature(Reference Babin and Vernier41).
In summary, the increased lipid digestibility and the similarity in postprandial plasma TAG output in carp when replacing a part of the dietary SBO by SPC support the idea that PC stimulates the absorption of dietary lipid in fish, without further affecting the baseline level of circulating plasma cholesterol.
Acknowledgements
We thank Peyo Aguirre for his support with the digestibility trial and Marie-Jo Borthaire and Laurence Larroquet for their assistance with the biochemical analyses. No conflicts of interest are involved in this study. The study has been conducted with institutional INRA funds.





