INTRODUCTION
Hepatitis B virus (HBV) infection is one of the leading causes of illness and death in China. According to a nationwide cross-sectional seroepidemiological study in 1992, about 60% of the population in China has a history of HBV infection, and 9·8% of persons are chronically infected with HBV. A recent national serosurvey conducted in 2006 found that the weighted prevalence of HBsAg in Chinese aged 1–59 years was 7·2% and was much lower in those aged <15 years [Reference Xia, Liu and Cao1, Reference Liang2]. Every year, an estimated 263 000 persons in China die from HBV-related liver cancer or cirrhosis, accounting for 37–50% of HBV-related deaths worldwide [Reference Goldstein3]. This disease results in a tremendous economic and healthcare burden not only on the HBV patients but also on society.
Hepatitis B vaccination is the most effective measure to prevent HBV infection and its consequences [Reference Poland and Jacobson4, Reference Namgyal5]. HBV vaccines have long-term and stable efficacy [Reference Viviani6–Reference Whittle8]. Widespread immunization programmes against HBV have been implemented in more than 100 nations and have dramatically reduced the occurrence of chronic HBV infection and hepatocellular carcinoma [Reference Chang9–Reference Viviani12].
A HBV vaccine has been available in China since 1982. It was first recommended for routine vaccination of newborns in China in 1992, with the first dose to be administered within 24 h of birth and subsequent doses at 1 and 6 months [13]. However, a pilot study of the universal HBV vaccination programme was initiated in 1986 in Long An county, Guangxi province, and then spread to several other areas of China. It consisted of routine immunization services to all newborns in those areas in order to investigate the efficacy of vaccination [Reference Xu14, Reference Li15].
In 1986 Long An county, inhabited mostly by Zhuang minorities, was a highly endemic area of hepatitis B and hepatocellular carcinoma in south China [Reference Li15]. In highly HBV-endemic areas, most infections result from perinatal transmission or infection in early childhood, which was true for Long An county [Reference Beasley16, Reference Mast17]. After the vaccination programme, the risk of acquiring HBV infection was reduced markedly [Reference Liao18].
Most studies on HBV vaccination efficacy in Long An have only investigated the HBV prevalence and protective anti-HBs levels in the vaccinated population [Reference Li15, Reference Liao18]. The aim of our survey was to observe the epidemiological changes in HBV and to analyse the current status and trends of the key characteristics of HBV infection in the entire population of Long An county, where a HBV vaccination programme has been established for over 20 years. The study included both vaccinated and unvaccinated subjects. A cross-sectional survey of HBV infection was performed in Long An county in 2005. Several HBV seroepidemiological markers were measured and compared to baseline data collected in 1985. Significant epidemiological changes in HBV prevalence were observed, and some important aspects regarding vaccination are discussed in this report.
MATERIALS AND METHODS
Background of Long An county, Guangxi, China
Long An county had a population of 370 000 and a birth rate of about 10/1000 in 2005. As a mountainous area, Long An has had a stable population composition over the past two decades. In 1985, its HBV infection rate was 75%, and HBsAg prevalence was 16·5%. Chronic hepatitis B, liver cirrhosis and hepatocellular carcinoma were the main causes of death in Long An [Reference Ding19, Reference Li20].
The universal HBV vaccination programme was first launched in Long An in 1986. During the initial 2 years of the programme, all infants and children aged <10 years were included. From 1988, all newborns were scheduled for immunization. As the pilot study for a universal HBV vaccination programme in China, several follow-up surveys among the vaccinated individuals were conducted in 1992, 1996, 2000, and 2005 to determine the magnitude and duration of the protective antibody response induced by infant vaccination [Reference Li15, Reference Gong21].
Vaccination
The plasma-derived HBV vaccine was used for the first 10 years. It was then replaced by a recombinant HBV vaccine produced from yeast beginning in 1996. The immunization schedule was three doses of HBV vaccine: the first as soon as possible after birth and subsequent doses at ages 1 and 6 months. The plasma-derived hepatitis B vaccine used from 1986 to 1996 was produced by the National Institute of Biological Products, China (5 μg/dose). The recombinant vaccine was provided by Beijing Tiantan Biological Products, China (5 μg/dose).
Participants
A house-to-house sampling investigation of HBV infection was conducted. Five villages in Long An county were included. The age of participants ranged from 3 months to 94 years. A total of 4686 subjects were included in our study by cluster sampling, representing about 30% of the entire population of the five villages in Long An county. The median age of the subjects was 34 years. There were 2290 (48·8%) males and 2396 (51·2%) females. The difference in gender proportions was not significant. Because the programme was started in 1986 (20 years prior to the current study), we divided all participants into two age groups: (1) <20 years, and (2) ⩾20 years.
Questionnaire and blood collection
Each questionnaire sought demographic data, including birthday, sex, history of HBV vaccination, and other basic information from the participants. Well-trained public health workers visited and interviewed the participants at home. Blood samples were collected by vacuum puncture. Serum was prepared and stored at −20°C prior to testing. The vaccination history card for each subject was recorded in detail and kept at the Centre of Disease Control and Prevention of Long An county.
Serological assessment
Solid-phase radioimmunoassay (SPRIA) was used to detect HBsAg, anti-HBs and anti-HBc in all blood samples collected. The protective level of being anti-HBs- and HBsAg-positive was defined as an S/N ratio of ⩾10·0, and being categorized as anti-HBc-positive required a S/CO ratio of ⩾2·1. SPRIA kits were provided by the National Institute of Biological Products, China.
Statistical analysis
All data were entered and validated using Access 2000 (Microsoft Corp., USA) and analysed with SPSS version 14.0 (SPSS Inc., USA). The difference in the prevalence of HBV markers in different age groups and sex groups were examined by χ2 test. P⩽0·05 was considered statistically significant.
Ethical issues
The survey protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Chinese CDC Ethics Committee. All study work was performed in accordance with national ethics regulations. Study participants were informed of the study purpose and of their right to keep information confidential.
RESULTS
Subjects and HBV infection
In 2005, a total of 4686 subjects took part in our study, representing about 30% of the entire population of the five villages in Long An county, Guangxi, China. All subjects were local residents; 99·5% were Zhuang minorities. Vaccination records were examined, and blood samples were taken and tested for HBsAg, anti-HBc and anti-HBs. As shown in Table 1, of those aged <20 years, 1514/1763 (85·9%) subjects studied were vaccinated for HBV, whereas only 111/2923 (3·8%) subjects aged ⩾20 years had received HBV vaccination. These results represent a great improvement of HBV vaccine coverage. Most rewardingly, as shown in Table 1, the prevalence of HBsAg positivity and anti-HBc antibodies in the <20 years group were 2·4% and 9·4%, respectively, compared to 10·5% and 70·8%, respectively, in the ⩾20 years group.
Table 1. HBV infection prevalence and vaccination history of Long An subjects in 2005

CI, Confidence interval.
The test values of the HBV infection markers are
* χ2=105·8168 and † χ2=1660·1292. Both * and † indicate P values <0·05.
It was previously reported that the average HBsAg prevalence of Long An county in 1985 was 16·5% [Reference Ding19, Reference Li20], but as shown in Table 1, the HBV prevalence had decreased to only 7·5% by 2005.
Age distribution of HBV infection markers in the entire population, 1985 vs. 2005
Using the data collected in 1985 as the baseline [Reference Li20], the changes in HBV infection markers in various age groups were examined.
As shown in Figure 1, in the 1985 data, levels of the naturally acquired protection antibody anti-HBs were positively correlated with age until a peak at age 20 years. In contrast, an 80% positive rate was achieved within the first year of birth for those vaccinated shortly after birth, the rate then decreased slowly. The lower anti-HBs-positive rate confirms the effectiveness of vaccination. At ages 9–19 years, the subjects under the universal vaccination programme had lower anti-HBs-positive rates than their counterparts prior to 1986. The anti-HBs-positive rates obtained in 2005 for the ⩾20 years age group were almost identical to the data collected in 1985 for the same age group because neither group received universal vaccination.
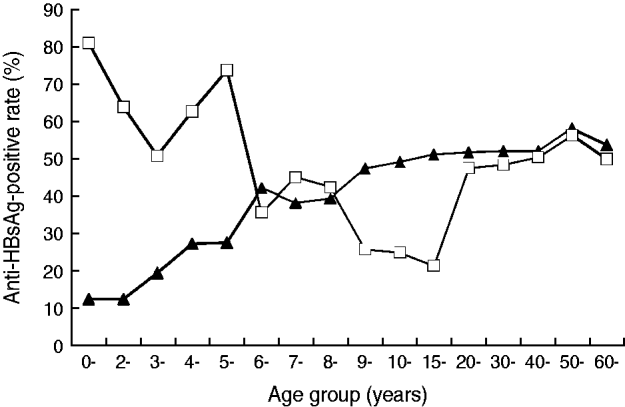
Fig. 1. The age distribution of anti-HBsAg prevalence of Long An county in 1985 (–▴–) and 2005 (–□–).
For those aged <20 years in 2005, despite the fact that 14% of them still had not received vaccination, the HBsAg prevalence for different age groups was ⩽5·0%. In marked contrast, for the same age groups in 1985, the prevalence was about 9% for the ⩾1 year age group, increased to about 15% for the ⩾2 years age group and stayed at the same high level until and after age 40 years (Fig. 2). High HBV prevalence was also observed in 2005 in subjects aged ⩾20 years. A similar trend in the anti-HBc-positive rate was also observed, as shown in Figure 3.

Fig. 2. The age distribution of HBsAg prevalence of Long An county in 1985 (–▴–) and 2005 (–□–).
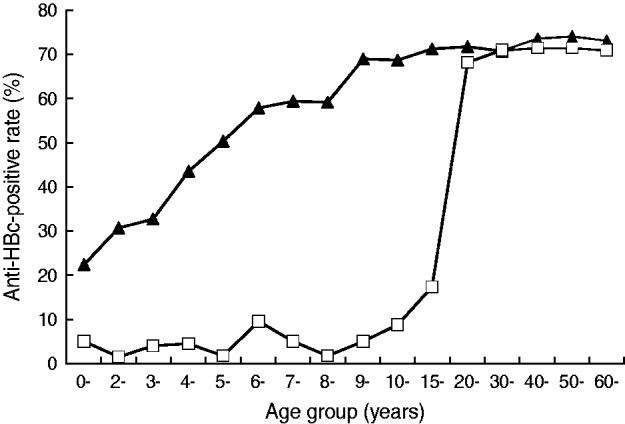
Fig. 3. The age distribution of the anti-HBc-positive rate of Long An county in 1985 (–▴–) and 2005 (–□–).
Clearly, the favourable change in HBV infection rate was mainly caused by the marked decrease of infection in children who were under the universal vaccination programme between 1985 and 2005.
The children who were born after the implementation of the universal HBV vaccination programme were further divided into four age groups (0–4, 5–9, 10–14, 15–19 years). More encouraging results were revealed, as shown in Table 2. The HBsAg-positive rate fell from 5·5% in the 15–19 years group to 1·5% in the 10–14 years group, to 0·91% in the 5–9 years group, and to 0·68% in the 0–4 years group. A trend of further decreasing HBsAg prevalence was observed in children under the universal vaccination programme. With the continuation of this universal HBV vaccination programme, we expect that Long An county could become a low-HBV prevalence area in the near future.
Table 2. HBsAg prevalence in 5-year age groups from 0 to 20 years, 2005
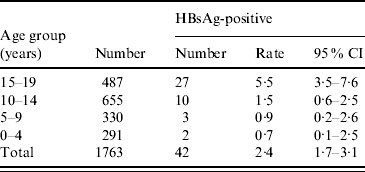
CI, Confidence interval.
Sex distribution of HBV infection
The participants in the 2005 study were 2290 males and 2396 females. The average HBsAg prevalence was 8·6% [95% confidence interval (CI) 7·5–9·8] for males and 6·5% (95% CI 5·5–7·5%) for females, which were significantly different (χ2=6·4700, P<0·05). However, when each gender was divided into two groups, those born before and after the implementation of the HBV universal vaccination programme, the difference in HBsAg-positive rates between males and females in the latter group was abolished by the universal vaccination programme (Fig. 4). For the people not covered by the universal vaccination programme, the positive rate was 13·3% for males and 8·5% for females.
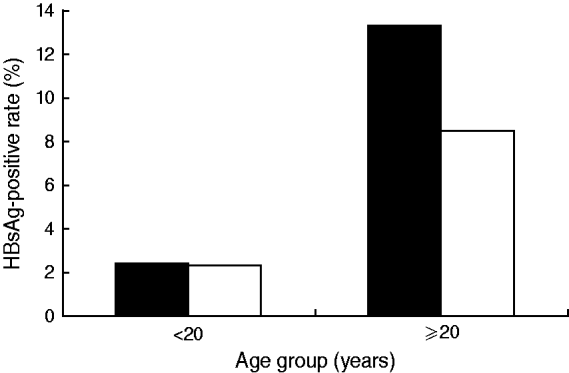
Fig. 4. Age and gender distribution of HBsAg prevalence in Long An, 2005. ▪, Male; □, female.
Timing of the first dose of HBV vaccine played a critical role in preventing future HBV infections
We compared the data from 679 subjects who received the first immunization within 24 h of birth with another group of 503 subjects who received their first dose of vaccine 1–7 days later. All of the 1182 subjects received all three doses of HBV vaccine. As shown in Table 3, the prevalence of HBsAg and anti-HBc(+) were 0·9% and 5·7%, respectively, in the group who were immunized within 24 h of birth. Those rates were much higher in the group who received the first dose of vaccine later: 2·8% and 8·7%, respectively. The differences are statistically significant (χ2=6·2900, χ2=3·993, respectively; both P values <0·05). Clearly, the administration of the first dose of HBV vaccine within 24 h of birth is critical and has significant impact on children's wellbeing.
Table 3. Timing of first dose of HBV vaccine and HBV infection
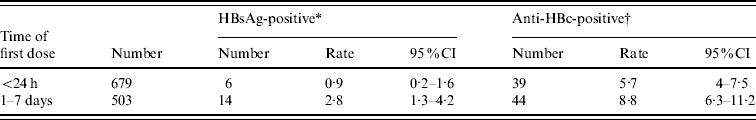
CI, Confidence interval.
The test values of the HBV infection markers are
* χ2=6·2900 and † χ2=3·993. Both * and † indicate P values <0·05.
DISCUSSION
Prior to the initiation of the universal HBV vaccination programme in 1986, Long An county was a high-prevalence area for hepatitis B and hepatocellular carcinoma in China. Over the last two decades, as one of the pilot areas of the universal HBV vaccination programme in China, mass vaccination for newborn and young children in Long An has been performed systematically and successfully. The long-term efficacy of the vaccine on the immunized population has been reported previously [Reference Li20–Reference Li22]. It was projected that with the increase in the proportion of HBV-vaccinated children, the infectivity of HBV carriers would decrease, and the total population force of HBV infection would also decrease as the prevalence of chronic HBV falls in the future [Reference Jack23]. In this study, we investigated the impact of such universal vaccination not only on immunized but on non-immunized people in Long An. Our study shows that after 20 years, the universal HBV vaccination programme has been a great success, reducing the overall HBsAg prevalence in Long An county from 16·5% in 1985 to 7·5% in 2005.
Of the 86% of children aged <20 years who received vaccination, the prevalence of HBsAg decreased to 2·4%. Together with the low anti-HBc-positive rate observed in this group, the results strongly suggest that a protective barrier against HBV infection was built up in the community. The steady decrease of 2005 HBV prevalence from 5·5% in the 14–19 years age group to 0·68% in the 0–4 years age group is extremely encouraging. We expect that with the continuation of this programme, Long An county will become a low-HBV prevalence area in the near future. However, despite the HBV vaccination programme, a 2·4% HBsAg prevalence remains in Long An. The reasons are many. According to family information and vaccination history, 27 of the 42 (2·4%) HBsAg-positive people became carriers by the age of 5 years (Table 2); 12 of the HBsAg-positive people were born to HBsAg-positive mothers, 15 of them missed the HBV vaccination, and 14 of them had not received their first dose by that time. Possible vaccination failure and S gene mutation may also play important roles. Whatever the reason, persistent HBsAg presence is an important issue that deserves more attention in further research.
In most studies conducted before the universal HBV vaccination programme, the prevalence of HBsAg was much higher in males than in females, especially in high-HBV prevalence areas [Reference Szmuness24, Reference Blumberg25]. It was suggested that these sex-based differences may be related to epidemiological, social behaviour and biological factors [Reference Wang26]. In our study, we found this difference had vanished in the <20 years age group in 2005. This phenomenon implies that long-term HBV vaccination played an important role in altering the sex distribution of HBV.
Perinatal transmission is a major factor in HBV prevalence [Reference Creati27, Reference Marion28]. Therefore, timely (within 24 h) administration of the birth dose of HBV vaccine is a particularly critical measure. In remote areas in Long An county, many children are born at home. It is not easy to achieve timely administration of HBV vaccine by routine methods of immunization. Our data confirm the importance of the timely administration of the birth dose of HBV vaccine, as it significantly reduced the risk of HBV infection. Furthermore, we found, regrettably, that almost half of vaccinated subjects had not received the vaccine within 24 h after birth, suggesting that timely administration of the birth dose still needs to be improved to control HBV prevalence effectively.
In summary, in this survey, we found significant epidemiological changes in Long An county, which included changes in HBV prevalence of the entire population, its age distribution and its sex distribution. These findings show that the implementation of the universal HBV vaccination programme in Long An county in 1986 was very successful, and the children who received the vaccine have greatly benefited from this programme. These results could also provide valuable information for future studies. However, Long An county is still a HBV-endemic area, especially in the population aged 20–50 years. Thorough hepatitis B vaccination programmes still need to be implemented. Furthermore, the strategies of antiviral therapy also need to be managed for those chronically infected, which may improve population health benefits significantly.
ACKNOWLEDGEMENTS
We thank the staff of the Centers of Disease Control and Prevention of Guangxi Province and Long An county for their great support and collaboration. We also thank the village healthcare workers for their invaluable help and assistance in performing this study.
DECLARATION OF INTEREST
None.









