Introduction
There is convincing evidence that a lifestyle characterised by a high-quality diet rich in fruit and vegetables, adequate physical activity and non-smoking contributes to healthy ageing, and that many chronic diseases occurring in older adults can be related to dietary factors(Reference Haveman-Nies, de Groot and van Staveren1,Reference Everitt, Hilmer and Brand-Miller2 ). It has been shown that high intakes of fruit, vegetables, fish and wholegrains are associated with longevity and better cardiometabolic and cognitive health, whereas dietary patterns rich in red meat and sugar-rich foods are associated with an increased risk of developing cancer and cardiometabolic diseases and increased mortality(Reference van de Rest, Berendsen and Haveman-Nies3,Reference Lu, Yu and Han4) . With ageing, dietary behaviours may be challenged by a variety of factors, including polypharmacy, polymorbidity, cognitive decline, dementia, impairment of taste and smell perception, physical inactivity and loss of social relations, which eventually can lead to inadequate nutrition(Reference Moreira, Krausch-Hofmann and Matthys5). In addition, common oral health problems in older adults including tooth loss, oral pain and wearing removable (and ill-fitting) dentures may deteriorate the ability to chew, leading to food modification and avoidance of notably crunchy and stringy foods, which can result in inadequate nutritional intake(Reference Savoca, Arcury and Leng6–Reference Quandt, Chen and Bell9).
Saliva plays an important role in the maintenance of dental and oral mucosal health, and it also exerts multiple functions in relation to the digestive processes of taste, initial breakdown of nutrients, chewing, bolus formation and swallowing(Reference Pedersen, Sørensen and Proctor10,Reference Dawes, Pedersen and Villa11) . Consequently, hyposalivation (objectively measured the reduction in salivary gland output) may cause changes in dietary behaviours due to impaired taste perception, chewing and swallowing difficulties, and discomfort when eating(Reference Pedersen, Sørensen and Proctor10). Hyposalivation is often associated with xerostomia (subjective feeling of dry mouth), itching and burning sensation of the oral mucosa, increased risk of developing dental caries, dental erosion, tooth loss and oral candidiasis(Reference Pedersen, Sørensen and Proctor10,Reference Dawes, Pedersen and Villa11) . Xerostomia and hyposalivation are common conditions in older adults, and especially in women. The prevalence of xerostomia ranges from 0⋅9 to 46 % in older adults above the age of 65 years(Reference Orellana, Lagravère and Boychuk12). The most common cause of xerostomia and hyposalivation in older adults is the intake of certain prescribed medications, including polypharmacy, and not ageing per se (Reference Smidt, Torpet and Nauntofte13,Reference Smidt, Torpet and Nauntofte14) . Older adults may modify food intake or avoid certain foods in response to xerostomia, but in spite of such potential changes in dietary behaviours, they seem to maintain an adequate dietary quality(Reference Quandt, Savoca and Leng15). Despite the common clinical observation that xerostomia and salivary gland hypofunction negatively influence food choice, evidence supporting an association between xerostomia and salivary gland hypofunction and food and nutrient intake, including intake of fruit and vegetables, in older people, is limited(Reference Quandt, Savoca and Leng15–Reference Lee, Park and Park18).
The objective of the present study was, therefore, to determine the nutrient and food intake and to test the hypothesis that xerostomia and low unstimulated and chewing-stimulated whole saliva flow rates are associated with lower daily intakes of fruit and vegetables in a sample of community-dwelling, independent Danish people aged 65–95 years. Our hypothesis was based on the assumption that intake of acidic foods including fruits may be associated with oral discomfort in terms of burning and itching, and intake of vegetables may be avoided due to taste alterations, chewing and swallowing difficulties related to salivary gland hypofunction.
Methods
Study design and study participants
This cross-sectional study included 621 community-dwelling Caucasian men and women, living in inner Copenhagen or its suburbs and aged 65–95 years, participating in a survey and health examination on oral health and lifestyle factors including dietary intake during 2004–6.
Participants were recruited among survivors of the third follow-up of the Copenhagen City Heart Study (CCHS) examination in 2001–3. The original aim of the CCHS was to focus on risk factors for coronary heart disease and stroke(Reference Schnohr, Jensen and Scharling19). Details regarding the CCHS population including invitation and examination procedures have previously been described(Reference Appleyard20,Reference Schnohr, Jensen and Lange21) . In brief, a subpopulation including 1918 men and women aged 65 years and above in 2004–6, were invited, and among these, 783 subjects agreed to participate. Inclusion criteria comprised the ability to communicate verbally and comprehend the study information and to physically attend the dental clinic at the University of Copenhagen for interview and clinical oral examination. Among both genders, non-participants were of older age than participants (P < 0⋅001), and the most common reasons for not participating were ‘old age’ and ‘poor health/not feeling well’. Out of the 783 subjects, the 621 with complete data on dietary intake, oral and general health, systemic diseases, medication intake, alcohol consumption, smoking, and clinical data on dentition, dentures and unstimulated and chewing-stimulated whole saliva flow rates were included in the present study.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the ethics committee of the capital region of Denmark (KF 01-144/01). Written informed consent was obtained from all subjects/patients. The present study was performed following the STROBE recommendations.
Interview and medical history
All participants underwent an interview with standardised questions regarding current and previous diseases, daily intake of prescribed medication, smoking and alcohol habits and xerostomia as described previously(Reference Smidt, Torpet and Nauntofte13,Reference Smidt, Torpet and Nauntofte14) . The presence of diseases and the daily intake of prescribed medication were registered and grouped into variables, describing the total numbers of diseases and prescribed medicines per day (0, 1, 2–3, 4–5 and 6+). Data on smoking habits were used to categorise participants as never smokers, former smokers and current smokers. The daily intake of alcohol (g/d) was calculated. An alcoholic beverage contains about 12 g of alcohol. A high-risk consumption was defined as a weekly intake of more than 252 g alcohol (>21 beverages) for men and 168 g (>14 beverages) for women according to the recommendations from the Danish Health Authority(22). Participants were asked if they had a daily sensation of xerostomia, if it had been persistent (i.e., present for more than 3 months), and if it affected oropharyngeal functions (speech, taste, chewing and swallowing (coded into yes or no)), including the following validated questions: ‘Does your mouth feel dry when eating a meal?’, ‘Do you sip liquids to aid in swallowing dry food?’, ‘Do you have any difficulty swallowing?’, ‘Have you had a daily sensation of dry mouth for more than 3 months?’ and ‘Does the amount of saliva in your mouth seem to be too little, too much, or you do not notice it?’(Reference Fox, Busch and Baum23). They were also asked if they had experienced taste alterations in terms of distorted or impaired taste (coded yes or no). It has previously been reported that the oldest participants of both genders had the highest frequency of low income (for women 76 % and men 59 %)(Reference Heegaard, Holm-Pedersen and Bardow24). In addition, the proportions of participants with low education (less than 1 year of education after secondary school) were the highest among the oldest women (above the age of 72 years), being 28 % for women and 15 % for men, respectively(Reference Heegaard, Holm-Pedersen and Bardow24).
Dietary assessment
The habitual intake of foods and nutrients was assessed by means of a diet history interview, where a trained interviewer (TS) questioned the participants about their habitual dietary intake in the past month. The interview was performed by pre-coded questions about meal patterns and contents, while the quantitative information on food intake was collected using photo-series, cups and measures. Calculations of the total energy (MJ), nutrient intakes (g/d) of protein, fat, carbohydrate, fibre, starch, total sugars, added sugars and sucrose, intakes of fruit and vegetables (g/d), the percentage of energy intake (E%) for protein, fat, carbohydrates and added sugar as well as vitamins were performed by means of the Dankost 3000 computer software (http://dankost.dk). This software was based on the Danish food composition database (version 7.01), developed by the National Food Institute at the Technical University of Denmark(Reference Saxholt, Christensen and Hartkopp25). The Nordic Nutrition Recommendations, which the Danish food-based dietary guidelines are based upon, were used as a reference for an adequate dietary intake(26). Accordingly, for people above the age of 65 years with a moderate physical activity level, the total daily energy intake should be on average 8⋅9 MJ (8⋅1 MJ for women and 9⋅7 MJ for men). The recommended intake for protein (energy percentage) is 15–20 % (optimal 18 %), for total fat 25–40 % and for carbohydrate 45–60 % of the total energy intake. In addition, the amount of added sugar should be less than 10 %, the amount of dietary fibres at least 25 g/d for women and 35 g/d for men, and the total intake of fruit and vegetables should be no less than 550 g/d (calculation based on a daily energy intake of 9 MJ)(26).
Measurements of whole saliva flow rates and clinical oral examination
Measurements of unstimulated and paraffin-chewing-stimulated whole saliva flow rates were performed as previously described in details(Reference Smidt, Torpet and Nauntofte14). In brief, the participants were asked to refrain from drinking, eating, chewing gum/pastilles, brushing teeth and smoking for 1 h prior to collection of saliva. The unstimulated whole saliva was sampled over a 10-min period, and the stimulated whole saliva over 5 min, using chewing on 1 g of paraffin. A normal unstimulated whole saliva flow rate ranges between 0⋅3 and 0⋅4 ml/min and a normal chewing-stimulated whole saliva flow rate ranges between 1⋅0 and 2⋅0 ml/min. The diagnosis hyposalivation (abnormal low salivary secretion) is made when the unstimulated whole saliva flow rate is ≤0⋅1 ml/min and the chewing-stimulated whole saliva flow rate is ≤0⋅7 ml/min(Reference Pedersen, Bardow and Jensen27). Finally, a clinical oral examination was performed to register the number of teeth and the presence of complete or partial removable dentures.
Statistical analysis
SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and SPSS 20 (SPSS Inc., Chicago, IL, USA) were used for statistical analyses. Mean values (sd) for continuous clinical characteristics and the corresponding proportions/frequencies for categorical data were computed and presented in tables. The distributions were compared by using one-way ANOVA for continuous variables and χ 2 test for categorical variables. Multiple linear regression analyses were performed to identify associations between intakes of fruit and vegetables (dependent variable) and xerostomia (yes/no) and unstimulated and chewing-stimulated whole saliva flow rates (ml/min). A multiple linear regression analysis was not performed with regard to taste alterations (yes/no) as they were highly associated with xerostomia and hyposalivation (P < 0⋅001). Intakes of fruit and vegetables and whole saliva flow rates were transformed by natural logarithm in order to fulfil the assumptions of linearity and normality of residuals. Adjusted regressions were also performed, adjusting for gender, age, smoking status (non-smoker, former smoker and current smoker), and alcohol intake (g/d), wearing denture/being edentulous in either the maxilla or mandible or both, numbers of systemic diseases (grouped into 0, 1, 2–3, 4–5 and 6+) and medicines taken on a daily basis (grouped into 0, 1, 2–3, 4–5 and 6+). In addition, differences in the intake of fruit and vegetables between participants being smokers and wearing a complete denture or being edentulous in the maxilla or mandible or both v. participants being non-/former smokers and/or not wearing a complete denture or being edentulous in the maxilla or mandible or both, and xerostomia (yes/no) were tested by the Mann–Whitney U test and tertiles of unstimulated (UWS) and stimulated (SWS) flow rates by one-way ANOVA. P-values less than 0⋅05 were considered statistically significant.
Results
Characteristics of the study participants
Table 1 shows the characteristics of all study participants as well as by gender. Women comprised about 60 % of the study sample. The average age of the participants was 75 years, and 86 % of them had one or more systemic diseases/medical conditions, predominantly cardiovascular and musculoskeletal diseases. Eighty-one percent had a daily intake of one or more prescribed medicines, mainly cardiovascular, musculoskeletal and gastrointestinal agents. The number of systemic diseases and the intake of prescribed medication were the highest among the oldest participants (P = 0⋅002) and among women (P < 0⋅001 and P = 0⋅002, respectively; Table 1).
Table 1. Characteristics of all study participants and by gender

Data presented as the number of participants (n) and proportions (%) and as means (and standard deviation, sd).
a Statistical significant differences between genders. High-risk consumption and hyposalivation are defined in the text.
The proportion of current smokers was 22⋅5 %. Significantly more men than women were both current and former smokers. Approximately 50 % had a daily consumption of alcoholic beverages, whereas 11 % were abstainers (Table 1). Beer and red wine were the most common types of alcoholic beverages consumed on a daily basis, either alone or in combination with other alcoholic beverages. More men (69 %) than women (41⋅5 %) had a daily consumption of one or more alcoholic beverages (P < 0⋅001). Furthermore, about 30 % of the participants (24⋅3 % women and 38⋅9 % men) had a high-risk alcohol consumption according to the guidelines of the Danish Health Authority(22). The mean intake of alcohol was 22⋅6 (±27⋅2) g/d for all participants, and higher for men than women (P > 0⋅001).
A total of 338 (53⋅9 %) of the participants were dentate with an average of 19⋅7 (±8⋅1) teeth and did not wear dentures. Seventy-seven (12⋅3 %) participants were fully edentulous and wore complete maxillary (upper) and mandibular (lower) removable dentures (Table 1). Approximately 31 % were partially edentulous; wearing both a removable partial maxillary and mandibular denture, or a partial maxillary denture in combination with either a dentate mandible or a complete mandibular denture. The remaining participants wore a complete maxillary or mandible denture in various combinations (i.e., either alone or with a partial upper, or lower denture or with a dentate mandible) (Table 1). Women tended to have more remaining teeth and wear partial removable dentures more often than men (P = 0⋅05). The odds of being a current smoker and wearing dentures/being partially or fully edentulous in either the maxilla or mandible or both (53/87 cases) were twice the odds of being non-smoker/former smoker and wearing dentures/being partially or fully edentulous (107/373 cases) (OR 2⋅1; 95 % CI 1⋅40, 3⋅14; P < 0⋅001).
Whole saliva flow rates, xerostomia and hyposalivation
The study participants had a mean unstimulated whole saliva flow rate of 0⋅20 (±0⋅19) ml/min (median 0⋅15 ml/min), which is below the normal reference range and average for unstimulated whole saliva flow rates, and thus to be considered subnormal salivary secretion. The mean chewing-stimulated whole saliva flow rate of 1⋅92 (±0⋅98) ml/min (median 1⋅75 ml/min) was within the normal range. Women had lower unstimulated and chewing-stimulated whole saliva flow rates than men (P < 0⋅001; Table 1). Approximately 35 % met the criteria of hyposalivation, and about 12 % suffered from persistent and daily xerostomia reportedly affecting their ability to taste and swallow foods. Xerostomia, hyposalivation and taste alterations were more prevalent in women than in men (P < 0⋅001; Table 1).
Dietary intake
Table 2 shows the daily intakes of energy and macronutrients, and the differences with regard to gender. The total mean energy intake was 8⋅4 (±2⋅7) MJ (median 7⋅8 MJ) for the entire study sample, which was slightly below the recommendation of 8⋅9 MJ for people above the age of 65 years with a moderate physical activity level. The male participants had an average energy intake of 8⋅6 MJ, which was below the recommendation of 9⋅7 MJ for men, whereas the female participants had an average energy intake slightly above (8⋅3 MJ) the recommendations. The total energy intake was the highest in the group aged 85–95 years (8⋅7 MJ) but did not differ significantly from that of the groups aged 65–74 and 75–84 years (Table 3).
Table 2. The daily total energy intake (megajoule, MJ), the intake of protein, fat and carbohydrate in grams (g, mean (sd)) and of the total energy (E%) and intake of selected micronutrients in the study population
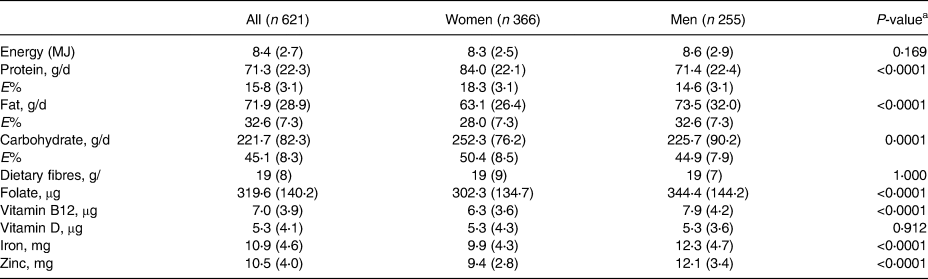
a Statistical significant differences between genders.
Table 3. Differences in intakes of fruit and vegetables (g/d) with regard to gender, age, smoking status and dental status

a Gender differences: Women had a higher intake of fruit and men had a higher intake of vegetables.
b Age group differences: Participants aged 85–95 years had lower intakes of fruits and vegetables.
c Smoking status differences: Current smokers had lower intakes of fruit and vegetables.
d Dental status differences: Fully edentulous/full denture wearers had lower intakes of fruit and vegetables than dentate non-denture wearers/partial denture wearers.
e Unstimulated (UWS) and stimulated (SWS) whole saliva flow rate (mean (sd) differences: Participants with low UWS and SWS did not differ from those with normal and high UWS and SWS with regard to intakes of fruits and vegetables.
f Participants with xerostomia had lower intakes of fruits and vegetables.
For the entire study sample, the daily consumption and the energy distribution (as E%) from total carbohydrates and total fat were within recommendations. The average protein E% was below recommendations (an optimal E% is 18 %), and the lowest intake of protein intake (69⋅5 g/d) and protein E% (13⋅8 %) were observed in the group aged 85–95 years. The average intake of dietary fibres of 18⋅8 g was below the recommended daily intake, which is 25 g for women and 35 g for men, respectively. The intake of total carbohydrates was the highest in women (Table 2) and in participants aged 85–95 years (Table 3), and thus explains the apparent higher total energy intake in these subgroups.
The intake of micronutrients (folate, vitamin B12, iron, zinc) was within the normal range, and generally higher for men (Table 2). The intake of vitamin D (mean 5⋅3 μg daily) was the same for both men and women and below recommendations.
The mean intakes of fruit and vegetables were 234⋅7 (±201⋅2) g/d (median 194 g/d) and 317⋅3 (±157⋅4) g/d (median 288 g/day), respectively (Table 3). The average total intake of both fruit and vegetables was 551 (± 271⋅1) g/d (median 511 MJ), and thus barely in accordance with the recommendations for fruit and vegetable intake for people above the age of 65 years with a daily energy intake of 9 MJ. The total consumption of fruit and vegetables did not differ significantly between men and women, but participants aged 85–95 years had a lower intake of both fruit and vegetables than those aged 65–74 and 75–84 years (P = 0⋅001; Table 3).
Current smokers and/or participants wearing a complete denture or being edentulous in the maxilla or mandible or both also had a significantly lower intake of both fruit and vegetables than non-smokers and former smokers and/or participants not wearing a complete denture or being edentulous in the maxilla or mandible or both (Table 3). Tertiles of unstimulated and chewing-stimulated whole saliva flow rates (low/normal and high) revealed no significant differences in the intakes of fruits and vegetables, whereas participants with xerostomia had lower intake than those without complaints of xerostomia (Table 3).
Unadjusted linear regression analyses showed that chewing-stimulated but not unstimulated whole saliva flow rates were directly associated with the daily intake of fruit and vegetables (P = 0⋅04), whereas xerostomia was inversely associated with the daily intake of fruit and vegetables (P = 0⋅05). After adjusting for covariates including gender, age, wearing dentures/being edentulous in the maxilla or mandible or both, smoking (current v. non-smoker/former smoker), alcohol consumption, the number of diseases and the number of medicines taken on a daily basis, the multiple linear regression analyses revealed that participants being of older age, (β −0⋅009, se 0⋅003, P = 0⋅005), current smokers (β −0⋅212, se 0⋅060, P = 0⋅0005), and wearing complete dentures/being partially or fully edentulous (β −0⋅141, se 0⋅048, P = 0⋅003) had a lower intake of fruit and vegetables, whereas the associations with chewing-stimulated whole saliva flow rates and xerostomia were no longer statistically significant (Table 4). The estimate, β, states the association (higher or lower fruit and vegetable intake) by the variables by an integer or category. In model 1, age explained 25⋅5 %, current smoking 24⋅8 % and wearing dentures 29⋅4 % of the variance in the intake of fruit and vegetables. In model 2, age explained 32⋅2 %, current smoking 23⋅6 % and wearing dentures 28⋅6 % of the variance in the intake of fruit and vegetables. In model 3, age explained 20⋅2 %, current smoking 18⋅9 % and wearing dentures 29⋅4 % of the variance in the outcome variable (fruit and vegetable intake) (Table 4).
Table 4. Multiple linear regression analyses on the association between intake in g/d of fruit and vegetables, whole saliva flow rates (ml/min) and xerostomia (yes/no), adjusted for gender, age, smoking status, and alcohol intake, wearing denture/being partially or fully edentulous, numbers of systemic diseases present and medicines on a daily basis (0, 1, 2–3, 4–5 and 6+)

β, regression coefficients (se, standard error of β). UWS and SWS, ln_unstimulated and chewing-stimulated whole saliva flow rates, respectively. The types of diseases and medication are detailed in the text. In models 1, 2 and 3, R 2 was 0⋅070, 0⋅073, and 0⋅072, respectively. The intake of fruit and vegetables was inversely associated with age, smoking and wearing dentures. For each 10 years difference in age, there was a 9 % lower daily intake of fruit and vegetables. Being a smoker and wearing dentures was associated with a daily 21 and 14⋅2 % lower intake of fruits and vegetables.
*P < 0.05.
Discussion
In the present study, including 621 community-dwelling Danish people aged 65–95 years, a lower daily intake of fruit and vegetables was associated with being among the oldest old, being a current smoker and wearing full dentures or being edentulous in one or both jaws, but not with xerostomia or low whole saliva flow rates.
In accordance with previous studies, we found that female gender and age were associated with a higher prevalence of systemic diseases, a higher intake of medication, lower unstimulated and chewing-stimulated whole saliva flow rates, as well as higher prevalence of xerostomia and hyposalivation(Reference Thorselius, Emilson and Österberg28–Reference Fure33). It has previously been shown that salivary gland hypofunction is not associated with age and gender per se, but rather is the consequence of an increase in the number of diseases and medication with increasing age, a phenomenon being most prevalent in women(Reference Smidt, Torpet and Nauntofte13,Reference Smidt, Torpet and Nauntofte14,Reference Närhi, Meurman and Ainamo30,Reference Thomson, Chalmers and Spencer32–Reference Shern, Fox and Li35) . However, a more recent meta-analysis reported lower unstimulated and stimulated submandibular/sublingual and unstimulated whole saliva flow rates in older adults, irrespective of medication intake(Reference Affoo, Foley and Garrick36). The findings of the present study indicate that the salivary gland hypofunction primarily is medication-induced as the unstimulated whole saliva flow rates were below the normal range, and the chewing-stimulated flow rates within the normal range. Most medications exert an inhibitory effect on the unstimulated salivary secretion and affect stimulated secretion to a lesser extent(Reference Smidt, Torpet and Nauntofte13). Persistent hyposalivation may result in dental decay and subsequent tooth loss. As the present study was cross-sectional, we have no data on the onset and duration of salivary gland hypofunction and the subsequent impact on oral health. Overall, we found no significant differences between gender with regard to tooth loss/remaining teeth and wearing removable dentures, but the number of dentate female participants tended to be higher. Of note, a study including almost the same sample of older adults as the present study, showed that men had more decayed coronal and root surfaces, while women had more restorations on the coronal surfaces(Reference Saxholt, Christensen and Hartkopp25), indicating that women pay more attention to oral hygiene procedures and regular dental visits, thereby preventing or delaying dental decay and tooth loss despite having lower salivary secretion than men.
With regard to lifestyle factors, we found that the proportions of smokers and consumers of alcohol on a daily basis were higher in men than women, corroborating previous findings(Reference de Groot, Verheijden and de Henauw37). It has been reported that smokers have a poorer oral health, including more prevalent tooth loss and severe periodontitis than non-smokers(Reference Adler, Modin and Friskopp38–Reference Morse, Avlund and Christensen42). In the present study, current smokers were also more likely to wear full dentures/being edentate in one or both jaws, and they also had the lowest intake of fruit and vegetables compared with former smokers and non-smokers. This indicates that a subgroup of older people with concurrent smoking and compromised oral health may have a less healthy food intake, at least when considering the intake of fruit and vegetables as a proxy for healthy dietary habits. Previous studies have also shown that tooth loss is associated with a lower intake of fruit and vegetables(Reference Krall, Dietrich and Nunn41), even in a group of male health professionals(Reference Johansson, Tidehag and Lundberg43). In addition, fruit intake has been reported to be lower in smokers than in former and non-smokers, whereas no association was found between smoking habits and intake of vegetables(Reference Joshipura, Willett and Douglass44). Additional previous findings suggest that smokers, in general, have a poorer diet than non-smokers(Reference Groth and Fagt45,Reference Laaksonen, Prattala and Karisto46) .
Oral health, smoking and alcohol consumption habits and dietary habits are closely related to socioeconomic and educational factors. A study including a similar sample of older adults as the present study revealed that the oldest old women and men (above 79 years of age) had the lowest income, and women above the age of 72 years had the lowest educational level, and generally lower income than men(Reference Heegaard, Holm-Pedersen and Bardow24). For both genders, the mean number of teeth as well as the number of smokers and alcohol consumers were the lowest among the oldest old(Reference Heegaard, Holm-Pedersen and Bardow24). We found no gender differences with regard to dental status and intake of fruit and vegetables, but a significantly higher number of smokers and alcohol consumers among the male participants. Consequently, dental health in old age seems influenced by a large number of health-related habits and the accumulative effect of these and other determinants across the lifespan, and not merely by educational and socioeconomic factors.
We found that the men had an average energy intake below and the women slightly above the recommendations. The total energy intake was the highest in the group aged 85–95 years, although it did not differ significantly from that of the other age groups. The higher total energy could be ascribed to a higher intake of total carbohydrates among women and participants aged 85–95 years.
The daily consumption and energy distribution (as E%) were in general within recommendations, except from protein and dietary fibres, which were below recommendations and lowest among participants aged 85–95 years.
The average total intake of fruit and vegetables (551 ± 271⋅1 g/d) was barely within the current recommendations for people above the age of 65 years with a daily energy intake of 9 MJ, but higher than that reported in a national investigation of dietary habits of the Danes from 2011 to 2013, where the age group 18–75 years had an average total daily intake of 389 g fruit and vegetables(Reference Pedersen, Christensen and Matthiessen47). Moreover, we found that women had a higher daily intake of fruit, but a lower intake of vegetables, than men. The total intake of fruit and vegetables did not differ between gender. Fruit (comprising citric acids) can act as the gustatory stimulus of salivary secretion, and the higher intake of fruit among female participants may reflect a way to alleviate symptoms of oral dryness. On the other hand, citric acids may also cause burning and itching sensation in the oral mucosa, especially in the case of hyposalivation. The unadjusted linear regression analysis revealed that chewing-stimulated, but not unstimulated whole saliva flow rates, were directly associated with the daily intake of fruit and vegetables, whereas xerostomia was inversely associated with the daily intake of fruit and vegetables. However, after adjusting for covariates including gender, age, wearing dentures/being edentulous in the maxilla or mandible or both, smoking (current v. non-smoker/former smoker), alcohol consumption, the number of diseases and the number of medicines taken on a daily basis, older age, current smoking, and wearing complete dentures/being partially or fully edentulous were associated with the low intake of fruit and vegetables, whereas the associations with salivary flow rates and xerostomia no longer were statistically significant.
In participants aged 75–84 years and particularly those aged 85–95 years, the intakes of fruit and vegetables were below recommendations. In Denmark, the Ministry of Environment and Food recommends a daily intake of ≥600 g of fruit and vegetables for adults (and 550 g for people aged above 65 years). It is well known that older people generally eat less than younger people primarily as a result of reduced energy requirements due to decreased levels of physical activity and basal metabolism due to age-related loss of muscle mass(Reference Poehlman and Horton48–Reference Tzankoff and Norris50). The regulation of appetite may also be altered in older people leading to ‘anorexia of ageing’(Reference Young51–Reference Morley53).
In addition, being edentulous and wearing dentures appear to be stronger determinants of a lower intake of fruit and vegetables than xerostomia and salivary gland hypofunction. Thus, participants wearing denture, particularly full dentures, had a fruit and vegetable intake below recommendations. Ill-fitting dentures, chewing difficulty and oral pain in relation to wearing dentures are probable explanations. However, limitations of the present study are that the function and symptoms related to wearing dentures were not addressed. It has previously been shown that wearing dentures are associated with a number of oral mucosal problems(Reference Lynge Pedersen, Nauntofte and Smidt54).
It may be questioned if the actual fruit and vegetable intake was overestimated, as it is well known that people tend to overestimate their self-reported intake of healthy foods(Reference Macdiarmid and Blundell55). However, previous studies have found that older people are less likely than younger (65 years v. <50 years) to underreport their food intake. Also, it is possible that the health profile of the present sample of older adults may be better than that of the general older population. Indeed, the subjects who participated were all able to attend/travel to the dental clinic, and they presumably present with better health and behaviours than the non-responders(Reference Heegaard, Holm-Pedersen and Bardow24), which can also be reflected in a high intake of fruits and vegetables. Moreover, the participants included in the present study have for decades taken part in the CCHS and are presumably more aware of a healthy diet including a high intake of fruit and vegetables, which may impact the external validity of the study.
In conclusion, we found no associations between whole saliva flow rates, hyposalivation and xerostomia and the daily intake of fruit and vegetables in this sample of older Danish people. However, smoking, being of older age and wearing denture or being edentate in one or both jaws were associated with a lower intake of fruit and vegetables, and intakes below recommendations. Age, current smoking and wearing full denture explained about 25, 25 and 29 % of the variance in the fruit and vegetable intakes. Our findings underline the importance of maintaining a functional natural dentition and providing dietary guidance in older adults to prevent dietary changes, but also paying attention to changes in dietary intake and nutritional status in older adults in cases of salivary gland function impairment and compromised oral health, as also suggested by previous research(Reference Samnieng, Ueno and Shinada16,Reference Iwasaki, Yoshihara and Ito17,Reference Kossioni56,Reference Dusek, Simmons and Buschang57) .
Acknowledgements
A special thank is addressed to the participants in the study. DDS Tina Schow and DDS, PhD Dorte Smidt are acknowledged for their assistance regarding diet history interviews, saliva sampling and clinical examinations. We also thank MSc in Statistics Claus Jensen for his biostatistical assistance.
The study was supported by the Danish Dental Association Research Foundation (FUT/Calcin) and the Faculty of Health and Medical Sciences, University of Copenhagen.
A. M. L. P., A. W. D. and B. L. H. formulated research question; A. L. M. P. and A. W. D. designed the study; A. L. M. P. and A. W. D. carried out the study; A. M. L. P. analysed the data; A. M. L. P., A. W. D. and B. L. H. interpreted the findings and wrote the article.
The authors declare that they have no conflicts of interest.





