Depression is attributed to various maladaptive affective and cognitive processes, including atypical cognitive control processes (Rizk et al., Reference Rizk, Rubin-Falcone, Keilp, Miller, Sublette, Burke and , … Mann2017). Specifically, impaired inhibition of negative thoughts, also known as rumination, is considered a major symptom in depression (Beck, Reference Beck1976; Nolen-Hoeksema & Morrow, Reference Nolen-Hoeksema and Morrow1993). However, the precise neurocognitive processes that lead to such atypical inhibition processes are currently debated. This is also the case for the neurocognitive processes related to subclinical levels of depression, which are an important precursor of depression (Li et al., Reference Li, Wei, Sun, Chen, Zhang and Qiu2015). Here, we apply a state-of-the-art computational approach—network control theory (NCT)—to quantitatively examine how different control strategies in specific brain regions relate to subclinical levels of depression. Such an approach can further elucidate the nature of this atypical inhibition and possibly serve as predictors in the occurrence of Major Depression Disorders (MDD).
In the past few years, a large-scale, whole-brain systems approach has been applied to study psychopathology (Menon, Reference Menon2011). Such an approach is moving away from examining clinical symptoms and their relation to specific brain regions (such as the amygdala in relation to depression) and is focusing on how such clinical symptoms relate to the interaction between brain regions and networks. Network approaches typically examine the interaction, and dysfunction, of three large-scale networks: The Executive Control Network (ECN), the Default Mode Network (DMN), and the Salience Network (SN). The ECN is a set of prefrontal and posterior parietal regions that are engaged during cognitive tasks that require externally directed attention, such as working memory, relational integration, response inhibition, and task-set switching (Zabelina & Andrews-Hanna, Reference Zabelina and Andrews-Hanna2016). The DMN is a set of midline and inferior parietal regions that activate in the absence of most external task demands, and is often associated with mind-wandering and other modes of spontaneous thought (Andrews-Hanna, Smallwood, & Spreng, Reference Andrews-Hanna, Smallwood and Spreng2014). The SN is a set of cingulate and fronto-insular regions that are involved in detecting, integrating, and filtering relevant interoceptive, autonomic, and emotional information (Seeley et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna and , … Greicius2007; Uddin, Reference Uddin2015).
In line with a network system approach, effort has been made to identify the role of the ECN, DMN, and SN in patients with MDD. Specifically, Cole, Repovš, and Anticevic (Reference Cole, Repovš and Anticevic2014) proposed a theory that highlighted the significance of ECN global dysconnectivity in mental disorders such as MDD (Cole, Repovš, & Anticevic, Reference Cole, Repovš and Anticevic2014). Recently, Schultz et al. (Reference Schultz, Ito, Solomyak, Chen, Mill, Anticevic and Cole2018) provided empirical evidence supporting this theory. The authors found a negative relationship between depression symptoms and a measure of global connectivity in the ECN (Schultz et al., Reference Schultz, Ito, Solomyak, Chen, Mill, Anticevic and Cole2018). Furthermore, several studies have found atypical activation in the DMN, related to rumination and processing of negative stimuli in patients with MDD (Cooney, Joormann, Eugène, Dennis, & Gotlib, Reference Cooney, Joormann, Eugène, Dennis and Gotlib2010; Hamilton, Chen, & Gotlib, Reference Hamilton, Chen and Gotlib2013; Hamilton, Chen, Thomason, Schwartz, & Gotlib, Reference Hamilton, Chen, Thomason, Schwartz and Gotlib2011; Sheline et al., Reference Sheline, Barch, Price, Rundle, Vaishnavi, Snyder and , … Raichle2009). For example, Hamilton, Furman et al. (Reference Hamilton, Furman, Chang, Thomason, Dennis and Gotlib2011) found atypical ECN and DMN activation related to maladaptive rumination processes in patients with MDD (Hamilton, Furman et al., Reference Hamilton, Furman, Chang, Thomason, Dennis and Gotlib2011). Finally, increased attention is given to the role of the SN in MDD (Hamilton, Chen, & Gotlib, Reference Hamilton, Chen and Gotlib2013; Hamilton et al., Reference Hamilton, Glover, Bagarinao, Chang, Mackey, Sacchet and Gotlib2016). This is based on the role of the SN in orienting and responding to stimuli, and its role in switching between the ECN and DMN (Sridharan, Levitin, & Menon, Reference Sridharan, Levitin and Menon2008). Such research focuses on the role of the amygdala and the anterior insula in MDD. The amygdala is involved in mnemonic and affective processing, particularly negatively valanced emotions such as fear and anxiety (Calhoon & Tye, Reference Calhoon and Tye2015). The anterior insula has widespread anatomical connections to cortical and limbic regions and is implicated in the coordination between the ECN and DMN (Iwabuchi et al., Reference Iwabuchi, Peng, Fang, Jiang, Liddle, Liddle and Palaniyappan2014; Menon & Uddin, Reference Menon and Uddin2010). Importantly, atypical functional activity and connectivity of the anterior insula have been found in several studies in patients with MDD (Iwabuchi et al., Reference Iwabuchi, Peng, Fang, Jiang, Liddle, Liddle and Palaniyappan2014). Specifically, the right anterior insula has been implicated in greater levels of maladaptive rumination (Hamilton, Furman et al., Reference Hamilton, Furman, Chang, Thomason, Dennis and Gotlib2011), and has been argued to represent a vulnerability marker for depression (Liu et al., Reference Liu, Xu, Xu, Wang, Zhao, Lv and , … Du2010).
As such, neuroimaging studies using network methods are elucidating important interactions across different neural systems related to MDD. Functional Magnetic Resonance Imaging (fMRI) is well suited for examining state-level variability across participants, given that rest- and task-based functional activity related patterns fluctuate in ways that predict cognitive measures. However, anatomical brain network analysis may better capture trait level variability across participants, by measuring stable individual differences in their neuroanatomy that might constrain neural and cognitive states. This is due to the unique information embedded in the brain’s anatomical network organization, that has been demonstrated to organize much of observable functional activity such as that observed in fMRI (Hermundstad et al., Reference Hermundstad, Bassett, Brown, Aminoff, Clewett, Freeman and , … Miller2013; Hermundstad et al., Reference Hermundstad, Brown, Bassett, Aminoff, Frithsen, Johnson and , … Carlson2014; Medaglia et al., Reference Medaglia, Huang, Karuza, Kelkar, Thompson-Schill, Ribeiro and Bassett2018b). Such information can be measured via diffusion tractography, measuring white matter tract connectivity in typical and clinical populations (Sotiropoulos & Zalesky, Reference Sotiropoulos and Zalesky2017).
Indeed, an association between white matter abnormalities and MDD has been established (Sexton, Mackay, & Ebmeier, Reference Sexton, Mackay and Ebmeier2009; White, Nelson, & Lim, Reference White, Nelson and Lim2008). Several studies using diffusion tensor imaging (DTI) have examined whole brain white matter connectivity related to MDD (De Witte & Mueller, Reference De Witte and Mueller2017; Gong & He, Reference Gong and He2015; Griffa, Baumann, Thiran, & Hagmann, Reference Griffa, Baumann, Thiran and Hagmann2013; Murphy & Frodl, Reference Murphy and Frodl2011; Rizk et al., Reference Rizk, Rubin-Falcone, Keilp, Miller, Sublette, Burke and , … Mann2017). However, these studies have resulted in conflicting findings, with some reporting MDD-related differences in white matter connectivity (Bai et al., Reference Bai, Shu, Yuan, Shi, Yu, Wu and , … Zhang2012; Korgaonkar, Fornito, Williams, & Grieve, Reference Korgaonkar, Fornito, Williams and Grieve2014) and others showing no differences (Choi et al., Reference Choi, Holtzheimer, Franco, Kelley, Dunlop, Hu and Mayberg2014; Qin et al., Reference Qin, Wei, Liu, Yan, Luo, Yao and Lu2014). Furthermore, most studies of MDD have focused on the amygdala and its connectivity to other brain regions (De Witte & Mueller, Reference De Witte and Mueller2017). A recent DTI study examined the relationship between cognitive control processes and white matter integrity in unmedicated young and midlife patients with MDD (Rizk et al., Reference Rizk, Rubin-Falcone, Keilp, Miller, Sublette, Burke and , … Mann2017). The authors found that unlike control participants, patients with MDD failed to exhibit an association between Stroop interference and white matter integrity in the anterior cingulate cortex (Rizk et al., Reference Rizk, Rubin-Falcone, Keilp, Miller, Sublette, Burke and , … Mann2017). However, the authors argue for the importance of further studies elucidating the relation between white matter integrity and cognitive control mechanisms in patients with MDD.
Thus, individual differences in subclinical levels of depression may be related to variance in whole brain white matter connectivity, which may impact efficient cognitive control processes. However, the current research on the influence of depression on white matter integrity is contradictory and debated (Rizk et al., Reference Rizk, Rubin-Falcone, Keilp, Miller, Sublette, Burke and , … Mann2017). This may be due to small sample sizes usually collected in such studies or the focus on white matter integrity, as opposed to white matter connectivity, as related to depression. In the current study, we apply computational NCT in relation to individual differences in subclinical depression. This allows us to computationally examine how whole brain structural connectivity theoretically “controls” dynamic brain processes in relation to individual differences in subclinical depression.
From an engineering perspective, network control is a process in which a system is deliberately shifted or guided along a particular trajectory to support specific goals (Tang & Bassett, Reference Tang and Bassett2018). This guidance is usually theoretically examined by simulating injection of signals into the system via deliberate perturbations. Recently, NCT has been applied to study the dynamics of large-scale neural systems (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015; Medaglia et al., Reference Medaglia, Gu, Pasqualetti, Ashare, Lerman, Kable and Bassett2016; Yan et al., Reference Yan, Vértes, Towlson, Chew, Walker, Schafer and Barabási2017). For example, Yan et al. (Reference Yan, Vértes, Towlson, Chew, Walker, Schafer and Barabási2017) applied NCT to investigate the significance of controllability of specific neurons in Caenorhabditis Elegans on its locomotion behavior. Importantly, these predictions were empirically examined and verified by ablating specific neurons identified as significant controllers (Yan et al., Reference Yan, Vértes, Towlson, Chew, Walker, Schafer and Barabási2017), thus demonstrating the feasibility of this computational theoretical approach in examining control strategies and dynamics in such neural systems.
Investigating the controllability of neural dynamics is computationally challenging, requiring to model non-linear neural dynamics and the neural structural connectivity that gives rise to such dynamics (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015). Thus, a common practice in the general application of NCT is based on linear models of dynamic processes (Liu, Slotine, & Barabási, Reference Liu, Slotine and Barabási2011). Accordingly, the application of control theory in neuroscience is built upon anatomical connectivity networks combined with a simplified, linear model of such neural dynamics (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015). This assumption of linear dynamics is commonly accepted and is based upon prior models linking anatomical brain networks to resting state functional dynamics (Abdelnour, Voss, & Raj, Reference Abdelnour, Voss and Raj2014; Bettinardi et al., Reference Bettinardi, Deco, Karlaftis, Van Hartevelt, Fernandes, Kourtzi and , … Zamora-López2017; Cole, Ito, Bassett, & Schultz, Reference Cole, Ito, Bassett and Schultz2016; Galán, Reference Galán2008; Honey et al., Reference Honey, Sporns, Cammoun, Gigandet, Thiran, Meuli and Hagmann2009; Honey, Thivierge, & Sporns, Reference Honey, Thivierge and Sporns2010; Muldoon et al., Reference Muldoon, Pasqualetti, Gu, Cieslak, Grafton, Vettel and Bassett2016). Importantly, Muldoon et al. (Reference Muldoon, Pasqualetti, Gu, Cieslak, Grafton, Vettel and Bassett2016) demonstrated how a non-linear computational model of neural dynamics validates controllability measures computed based on the simplified linear model. Thus, while the forefront of computational neuroscience aims to develop methods to map the relation between structural and functional signals (e.g., Medaglia et al., Reference Medaglia, Huang, Karuza, Kelkar, Thompson-Schill, Ribeiro and Bassett2018b), controllability measures built on a simplified linear model have proven their fruitfulness.
Recent applications of NCT to neural systems have proposed a set of three controllability metrics that quantify the contributions made by individual brain regions in “driving” the brain network from one state into another (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015). Here, “state” refers to the magnitude of neurophysiological activity across brain regions at a single time point. Average controllability quantifies the theoretical extent to which a specific brain region can easily “drive” the brain into easy to reach states with little energy and has been observed in DMN regions (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015; Pasqualetti, Zampieri, & Bullo, Reference Pasqualetti, Zampieri and Bullo2014). Modal controllability quantifies the theoretical extent to which a specific brain region can easily “drive” the brain into states that require a substantial amount of energy, or are difficult to reach states, and has been observed in fronto-parietal regions (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015). Boundary controllability quantifies the theoretical extent to which a specific brain region lies at the “boundary” between network sub-communities, contributing to the integration between them. Brain regions with high boundary controllability have been associated with attention systems (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015). Together, these three control roles define different continua in brain networks: Brain regions may vary in their tendency to drive the brain to or away from specific types of states or into integrated or segregated states. However, these measures are mathematical abstractions and the significance of these measures in studying behavior is still open. A growing number of studies is, however, currently establishing a link between NCT and cognition (Kenett et al., Reference Kenett, Medaglia, Beaty, Chen, Betzel, Thompson-Schill and Qiu2018; Medaglia et al., Reference Medaglia, Gu, Pasqualetti, Ashare, Lerman, Kable and Bassett2016; Medaglia et al., 2018a; Tang et al., Reference Tang, Giusti, Baum, Gu, Pollock, Kahn and , … Bassett2017).
A few recent studies have demonstrated the feasibility of applying NCT to study cognition. Medaglia et al. (Reference Medaglia, Gu, Pasqualetti, Ashare, Lerman, Kable and Bassett2016) related modal and boundary controllability to performance on a variety of tasks that demand executive control (such as the Stroop task), and it is the first to ground cognitive control in network controllability measures. Tang et al. (Reference Tang, Giusti, Baum, Gu, Pollock, Kahn and , … Bassett2017) investigated whole brain network controllability measures related to typical neurocognitive development. The authors found that the relative strength of average controllability of subcortical brain regions predicted improved cognitive performance as related to development. Kenett et al. (Reference Kenett, Medaglia, Beaty, Chen, Betzel, Thompson-Schill and Qiu2018) applied NCT to examine differences in controllability measures across the whole brain related to intelligence and creativity. The authors found a positive relation with average controllability and intelligence, and a positive relation with modal controllability and creativity across different brain regions (Kenett et al., Reference Kenett, Medaglia, Beaty, Chen, Betzel, Thompson-Schill and Qiu2018). The authors also find opposite relations between boundary controllability, intelligence, and creativity. Thus, different controllability measures across different brain regions can be related to cognitive processes in typical populations. Recently, Jeganathan et al. (Reference Jeganathan, Perry, Bassett, Roberts, Mitchell and Breakspear2018) applied NCT analysis on a sample of participants with bipolar depression and participants that are in high risk of bipolar depression. The authors found decreased average controllability in both of these groups compared with controls in several brain regions including the right inferior frontal gyrus, insula, and the pre-central gyrus (Jeganathan et al., Reference Jeganathan, Perry, Bassett, Roberts, Mitchell and Breakspear2018).
Thus, the application of NCT in neurocognitive research advances our understanding of regions’ theoretical roles in driving activity across the brain as related to cognitive processes in typical and clinical populations. In the current study, we apply NCT to white matter anatomical connectivity networks in a large sample of participants (N=349) who were assessed for subclinical depressive symptoms. For each participant, we extracted anatomical connectivity matrices based on diffusion tractography, and computed average, modal, and boundary controllability for regions across the whole brain. We then examined and compared the relation of each of the controllability measures to the depression measure. This allows us to quantitatively examine theories on the roles of the ECN, DMN, and SN regions in driving brain network dynamics as related to subclinical depression. While we are theoretically motivated to focus on these network systems, we conduct a whole-brain analysis to examine differences in controllability across all possible brain regions. This is motivated by the possibility that different brain regions actually regulate such dynamics, whereas functional imaging studies are the consequences of underlying dynamic-driving roles across the brain. In line with studies that have revealed atypical hyper activity and connectivity within the ECN and DMN, we expected to find higher average controllability in brain regions related to these systems. In line with studies implicating the right anterior insula in atypical switching between ECN and DMN in MDD, we expected to find a significant relation between boundary controllability and subclinical levels of depression in this region.
1. Methods
1.1. Participants
The sample was collected as part of a large research project (http://fcon_1000.projects.nitrc.org /indi/retro/southwestuni_qiu_index.html) exploring the associations among individual differences in brain structure and function, cognitive function, and mental health (Liu et al., Reference Liu, Wei, Chen, Yang, Meng, Wu and , … Qiu2017). Participants were recruited from Southwest University by means of the campus network, advertisements on bulletin boards and leaflets, or through face-to-face communications on campus. Before enrolling in the study, each participant was screened with a set of exclusion procedures involving self-reported questionnaires as well as structured and semi-structured interviews. All participants were required to be right-handed, and none had a history of psychiatric disorder, cognitive disability, substance abuse, or MRI contraindications.
In the current study, we only included participants that completed the Beck Depression Inventory (BDI, see below), which consisted of 351 participants. In addition, we excluded participants who had BDI scores with values higher than the cut-off score for severe clinical depression (BDI score of 30; Beck, Steer, & Carbin, Reference Beck, Steer and Carbin1988). Thus, the final sample included 349 participants (156 male, 191 female; average age of 20 years, SD=1.27) with an average BDI score of 7 (SD=5.5, skewness=.92). This research project was approved by the Southwest University Brain Imaging Center Institutional Review Board, and written informed consent was obtained from each participant. Participants received payment depending on time and tasks completed.
1.2. Materials
1.2.1. Behavioral measures
Depression Assessment—Depression was assessed using the BDI (Beck, Ward, Mendelson, Mock, & Erbaugh, Reference Beck, Ward, Mendelson, Mock and Erbaugh1961). The BDI is a 21-item self-report questionnaire measuring the severity of depressive symptoms within the past week. Participants who score higher in the BDI exhibit more depressive symptoms. The BDI is a reliable and widely used measure that assesses the severity of depressive symptoms from non-clinical to clinical samples (Beck, Steer, & Carbin, Reference Beck, Steer and Carbin1988).
1.2.2. MRI data acquisition
Imaging data were collected using a 12-channel head coil on a Siemens 3T Trio scanner (Siemens Medical Systems, Erlangen, Germany) at the Brain Imaging Center, Southwest University. High-resolution, three-dimensional T1-weighted structural images were obtained using a Magnetization Prepared Rapid Acquisition Gradient-echo sequence (TR/TE=1,900 ms/2.52 ms, FA=9°, resolution matrix=256×256; slices=176; thickness=1.0 mm; voxel size=1×1×1 mm3). Diffusion tensor images were obtained using a diffusion-weighted, single shot, spin echo, EPI sequence (TR/TE=11,000/98 ms, matrix=128×128, field of view=256×256 mm, voxel size=2×2×2 mm3, 60 axial slices, 2 mm slice thickness, b value 1=0 s/mm2, b value 2=1,000 s/mm2) in 30 directions and repeated acquisition of data three times to increase the signal-to-noise.
DTI data were reconstructed in DSI Studio (www.dsi-studio.labsolver.org) using q-space diffeomorphic reconstruction (QSDR; Yeh, Wedeen, & Tseng, Reference Yeh, Wedeen and Tseng2011). QSDR first reconstructs diffusion-weighted images in native space and computes the quantitative anisotropy (QA) in each voxel. These QA values are used to warp the brain to a template QA volume in Montreal Neurological Institute (MNI) space using the statistical parametric mapping nonlinear registration algorithm. Once in MNI space, spin density functions were again reconstructed with a mean diffusion distance of 1.25 mm using three fiber orientations per voxel. Fiber tracking was performed in DSI Studio with an angular cut-off of 35, step size of 1.0 mm, minimum length of 10 mm, spin density function smoothing of 0, maximum length of 400 mm, and a QA threshold determined by its signal in the colony-stimulating factor. Deterministic fiber tracking using a modified FACT algorithm was performed until 1,000,000 streamlines were reconstructed for each individual. These parameters were chosen based on previous neurocognitive studies applying NCT (Betzel, Gu, Medaglia, Pasqualetti, & Bassett, Reference Betzel, Gu, Medaglia, Pasqualetti and Bassett2016; Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015; Kenett et al., Reference Kenett, Medaglia, Beaty, Chen, Betzel, Thompson-Schill and Qiu2018; Medaglia et al., 2018a; Tang et al., Reference Tang, Giusti, Baum, Gu, Pollock, Kahn and , … Bassett2017).
Anatomical scans were segmented using FreeSurfer (Fischl, Reference Fischl2012) and parcellated using the connectome mapping toolkit (Cammoun et al., Reference Cammoun, Gigandet, Meskaldji, Thiran, Sporns, Do and , … Hagmann2012). Based on previous research (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015; Hermundstad et al., Reference Hermundstad, Bassett, Brown, Aminoff, Clewett, Freeman and , … Miller2013; Medaglia et al., Reference Medaglia, Gu, Pasqualetti, Ashare, Lerman, Kable and Bassett2016), a parcellation scheme including 234 brain regions (Cammoun et al., Reference Cammoun, Gigandet, Meskaldji, Thiran, Sporns, Do and , … Hagmann2012) was registered to the B0 volume from each participant’s DTI data. The B0 to MNI voxel mapping produced via QSDR was used to map region labels from native space to MNI coordinates. To extend region labels through the grey-white matter interface, the atlas was dilated by 4 mm (Cieslak & Grafton, Reference Cieslak and Grafton2014). Dilation was accomplished by filling non-labeled voxels with the statistical mode of their neighbors’ labels. In the event of a tie, one of the modes was arbitrarily selected. Each streamline was labeled according to its terminal region pair. Finally, we conducted automatic quality control analysis to assess the quality of the DTI data (Roalf et al., Reference Roalf, Quarmley, Elliott, Satterthwaite, Vandekar, Ruparel and , … Gur2016). For each participant’s DTI data, we computed their temporal signal-to-noise ratio (tSNR). tSNR was computed by averaging each brain voxel’s mean and standard deviation, after brain masking and motion correction. This analysis did not reveal any participants with an outlier tSNR values. As such, no participant was excluded based on this analysis.
From these data, we constructed structural connectivity networks that map streamline connections between 234 cortical and sub-cortical regions. In these anatomical connectivity matrices, brain regions are defined as nodes, and a link between two nodes represents the number of streamlines connecting them, normalized for their density (Sotiropoulos & Zalesky, Reference Sotiropoulos and Zalesky2017).
1.2.3. Network controllability analysis
To assess the ability of a certain brain region to influence other regions in different ways, we adopt the control theoretic notion of controllability. Controllability of a dynamical system refers to the possibility of driving the state of a dynamical system to a specific target state by means of an external control input (Tang & Bassett, Reference Tang and Bassett2018). Classic results in control theory ensure that controllability of the network is equivalent to the controllability Gramian matrix, which determines whether a linear system is controllable (Summers, Cortesi, & Lygeros, Reference Summers, Cortesi and Lygeros2016). A rigorous mathematical formulation of network controllability in brain networks can be found in Gu et al. (Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015). From the Gramian matrix, different controllability measures can be computed for each node (brain region) in the network. Here, based on previous research of network controllability in brain networks, we compute for each participant and each brain region their average controllability, modal controllability, and boundary controllability (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015; Medaglia et al., Reference Medaglia, Gu, Pasqualetti, Ashare, Lerman, Kable and Bassett2016; Pasqualetti, Zampieri, & Bullo, Reference Pasqualetti, Zampieri and Bullo2014).
Average controllability identifies brain regions that, on average, can drive the system into different states with little effort (input energy). A state can be defined as the vector of neurophysiological activity magnitudes across brain regions at a single time point. Thus, regions with high average controllability can move the brain to many easily reachable states (Figure 1d). Thus, these regions may be important in allowing the brain to move smoothly between many cognitive functions that require little cognitive effort. Previous work has identified brain regions that demonstrate high average controllability, such as the precuneus, posterior cingulate, superior frontal, paracentral, precentral, and subcortical structures (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015).
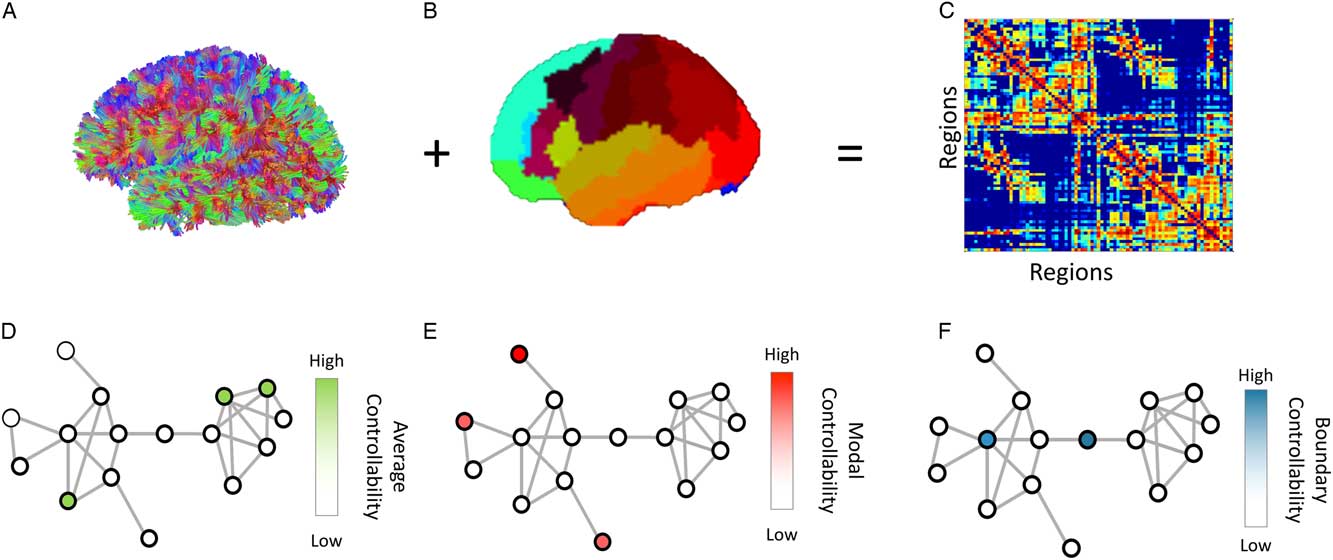
Figure 1 Overview of Methods: (A) We performed diffusion tractography for each participant, and (B) applied a probabilistic whole-brain parcellation. (C) anatomical connectivity matrices are constructed that represents the number of streamlines between pairs of regions, normalized by density. Finally, we define a simplified model of brain dynamics and simulate network control to quantify (D) average, (E) modal and (F) boundary controllability for each node (brain region) in the network for each participant. Figure adapted from Kenett et al. (2018).
Modal controllability identifies brain regions that can drive the brain into different states that require high effort to achieve (those which require substantial input energy). Thus, regions with high modal controllability can move the brain to many difficult to reach states (Figure 1e). From a cognitive perspective, these regions may be important in switching the brain between functions that require significant cognitive effort. Previous work has identified brain regions that demonstrate high modal controllability, such as the postcentral, supramarginal, inferior parietal, pars orbitalis, medial orbitofrontal, and rostral middle frontal cortices (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015).
Boundary controllability identifies brain regions that can drive the brain into states where different cognitive systems are either coupled or decoupled (Figure 1f). >From a cognitive perspective, these regions may be important in gating, synchronizing, or otherwise manipulating information across different cognitive processes. Previous work has identified brain regions that demonstrate high boundary controllability, such as the rostral middle frontal, lateral orbitofrontal, frontal pole, medial orbitofrontal, superior frontal, and anterior cingulate cortices (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015).
Boundary controllability identifies network nodes that lie at the boundaries between network communities, as defined across all possible levels of hierarchical modularity in a network (Tang & Bassett, Reference Tang and Bassett2018). As such, an initial identification of brain modules (or communities) is required. While data-driven approaches have been developed to achieve such an identification, identifying brain modular organization remains an open challenge (see Medaglia et al., Reference Medaglia, Gu, Pasqualetti, Ashare, Lerman, Kable and Bassett2016). Here we chose to side step this issue and use a modular assignment that was computed via a data-driven approach that analyzed a large independent sample of resting state functional data using the same parcellation atlas. This approach, based on the method developed by Mišić et al. (Reference Mišić, Betzel, Nematzadeh, Goñi, Griffa, Hagmann and , … Sporns2015), uses a consensus analysis to identify a partition that maximizes the modular partition of a large sample of independent datasets (Mišić et al., Reference Mišić, Betzel, Nematzadeh, Goñi, Griffa, Hagmann and , … Sporns2015). This partition identified 12 systems which are in line with neural systems identified in previous research (Dosenbach et al., Reference Dosenbach, Nardos, Cohen, Fair, Power, Church and , … Lessov-Schlaggar2010). Using this a priori independent modularity partition controls for the stochastic nature of the boundary controllability method and is justified by the identified relation between anatomical connectivity and resting state functional data (Honey et al., Reference Honey, Sporns, Cammoun, Gigandet, Thiran, Meuli and Hagmann2009).
1.2.4. Analysis overview
Our analysis process is as follows (Figure 1): We defined anatomical brain networks by subdividing the entire brain into 234 anatomically distinct brain regions (network nodes) in a commonly used anatomical atlas (Cammoun et al., Reference Cammoun, Gigandet, Meskaldji, Thiran, Sporns, Do and , … Hagmann2012; Daducci et al., Reference Daducci, Gerhard, Griffa, Lemkaddem, Cammoun, Gigandet and , … Thiran2012; Hagmann et al., Reference Hagmann, Cammoun, Gigandet, Meuli, Honey, Wedeen and Sporns2008). Following prior work (Bassett, Brown, Deshpande, Carlson, & Grafton, Reference Bassett, Brown, Deshpande, Carlson and Grafton2011; Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015; Hermundstad et al., Reference Hermundstad, Bassett, Brown, Aminoff, Clewett, Freeman and , … Miller2013; Hermundstad et al., Reference Hermundstad, Brown, Bassett, Aminoff, Frithsen, Johnson and , … Carlson2014), we connected nodes (brain regions) by the number of white matter streamlines identified by a commonly used deterministic tractography algorithm (Cieslak & Grafton, Reference Cieslak and Grafton2014). This procedure results in sparse, weighted, undirected structural brain networks for each participant. To control for volume confounds between pairs of brain regions i and j, streamline counts were normalized by dividing by the sum of streamlines brain region i has, which resulted in a measure of streamline density (Medaglia et al., Reference Medaglia, Gu, Pasqualetti, Ashare, Lerman, Kable and Bassett2016). Next, a simplified model of brain dynamics was applied to simulate network control and quantify average, modal, and boundary controllability for each brain region for each participant, as described above (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015; Tang & Bassett, Reference Tang and Bassett2017). Intuitively, a node’s average and modal controllability values are negatively related (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015; Wu-Yan et al., Reference Wu-Yan, Betzel, Tang, Gu, Pasqualetti and Bassett2018). This intuition was verified in a previous study analyzing the same dataset (Kenett et al., Reference Kenett, Medaglia, Beaty, Chen, Betzel, Thompson-Schill and Qiu2018).
We then conducted a whole-brain correlation analysis between BDI and each of the network controllability measures for all brain regions for all participants. As the BDI measure is skewed (skewness=.92), we conducted a Spearman rank correlation analysis for average, modal, and boundary controllability, controlling for multiple comparisons by calculating the false discovery rate (Benjamini & Hochberg, Reference Benjamini and Hochberg1995; Benjamini & Yekutieli, Reference Benjamini and Yekutieli2001) with a false positive rate of 0.05. The brain networks were then visualized via the BrainNet Viewer (http://www.nitrc.org/projects/bnv/; Xia, Wang, & He, Reference Xia, Wang and He2013). Anatomical labels were determined using the Brainnetome Atlas (http://atlas.brainnetome.org), which uses state-of-the-art multimodal neuroimaging techniques to provide a current fine-grained, cross-validated atlas and contains information on both anatomical and functional connections (Fan et al., Reference Fan, Li, Zhuo, Zhang, Wang, Chen and , … Jiang2016).
2. Results
We applied the network controllability analysis, as described above. To verify that the white matter connectivity matrices and controllability measures we compute are consistent with previous studies we conduct two initial inspections. First, we examine the consistency of our white matter connectivity matrices. To do so, we compare our white matter connectivity matrices to an external, published, data set of DTI data from 270 participants (Jung, Mead, Carrasco, & Flores, Reference Jung, Mead, Carrasco and Flores2013). To compare the consistency of the white matter connectivity matrices in our sample, we conducted the following analysis: First, for each participant in each sample we computed Pearson’s correlation across all pairs of vectors of their white matter connectivity matrices. Next, we computed the mean and standard deviation of the correlation matrix for each participant. Finally, we calculated the average over the average correlation distribution and the average over the standard deviation distribution for all participants in each sample. Since comparing across different DTI data sets is a challenge (Lebel, Treit, & Beaulieu, Reference Lebel, Treit and Beaulieu2017), we only demonstrate that we achieve comparable results for the white matter consistency correlation distribution of our current data set (mean=0.03, SD=0.16) and this second sample (mean=0.03, SD=0.16).
Second, we examined whether our controllability measures computed over the sample map on to previously reported brain regions. To do so, we follow the approach conducted by Gu et al. (Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015): We average the controllability scores for each brain region over all participants to derive a mean average, modal, and boundary score for each of our 234 brain regions. Next, for each controllability measure independently we examine the 30 brain regions with the highest scores for that controllability measure. In accordance with Gu et al. (Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015), the top-30 average controllability brain regions included a majority of DMN regions such as the inferior parietal lobe and the medial frontal gyrus; the top-30 modal controllability brain regions included a majority of ECN regions such as the cingulo-operculum regions and also the insula; and the top-30 boundary controllability brain regions included a majority of attention regions including superior frontal areas and the frontal poles. Thus, our controllability analysis is consistent with previous reports on the dispersion of network controllability measures across the brain (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015).
Next, we correlated the different network controllability measures across all brain regions with the BDI measure. This analysis revealed several brain regions that survived false discovery rate correction (Table 1; Figure 2), including a significant negative correlation with boundary controllability and BDI in the right anterior insula (adjusted p<.001). Furthermore, five regions exhibited a significant positive correlation with average controllability and a significant negative correlation with modal controllability: An area in the right inferior parietal lobe (IPL: average: adjusted p<.001; modal: adjusted p<.001), bilateral Lingual gyrus (right: average: adjusted p<.001; modal: adjusted p<.001; left: average: adjusted p<.05; modal: adjusted p<.05), and an area in the right lateral-occipital cortex (average: adjusted p<.05; modal: adjusted p<.05). A region within the left post-central gyrus also showed a negative correlation with average controllability and a positive correlation with modal controllability (average: adjusted p<.05; modal: adjusted p<.05).
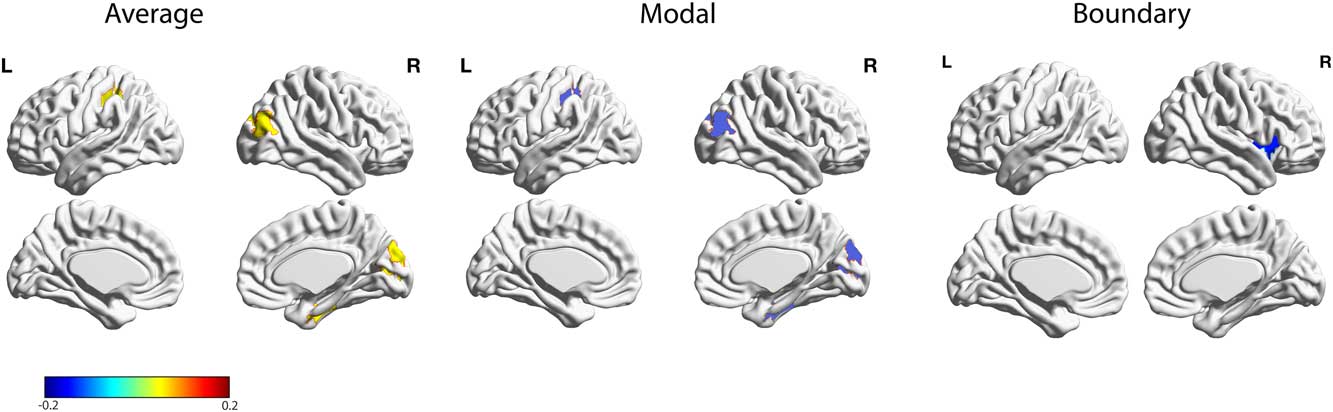
Figure 2 Relations between BDI and individual differences in average, modal, and boundary controllability anatomical brain networks. Maps highlight brain regions with significant correlation values that survived FDR correction. Warmer/colder colors indicate a positive/negative correlation between controllability and behavior.
Table 1 Whole-brain correlation analysis between Beck Depression Inventory and network controllability measures (average, modal, and boundary) for the entire sample

Notes: Only correlations with brain regions that survived false discovery rate (FDR) are presented. All correlation values reported survived FDR correction; x, y, z coordinates represent the peak maximal voxel in Montreal Neurological Institute space. Anatomical labels were determined using the Brainnetome Atlas (BA) (http://atlas.brainnetome.org).
*p<.05; ***p<.001.
3. Discussion
In the current study, we applied a novel computational approach—NCT —to quantify the relation between the role of different brain regions in theoretically “controlling” whole brain neural dynamics related to subclinical depressive symptoms. We argue that NCT can advance our understanding of evoked control processes related to subclinical levels of depression, as measured by the BDI. Our approach is motivated by previous initial work that has implicated the importance of average controllability in typical development and intelligence (Kenett et al., Reference Kenett, Medaglia, Beaty, Chen, Betzel, Thompson-Schill and Qiu2018; Tang et al., Reference Tang, Giusti, Baum, Gu, Pollock, Kahn and , … Bassett2017) and modal and boundary controllability in cognitive control tasks and creativity (Kenett et al., Reference Kenett, Medaglia, Beaty, Chen, Betzel, Thompson-Schill and Qiu2018; Medaglia et al., Reference Medaglia, Gu, Pasqualetti, Ashare, Lerman, Kable and Bassett2016; Medaglia et al., 2018a). We conducted a NCT analysis on a large sample of participants (N=349) who underwent diffusion tract imaging, alongside behavioral measurement of depressive symptoms. Our findings extend past work linking brain structure to subclinical depression by uncovering controllability effects within specific brain regions associated with depressive symptomology.
One main finding of the present study is a significant negative correlation between boundary controllability and BDI in the right anterior insula. The insula is part of the SN, which is implicated in coordinating behavioral responses through the detection and orientation toward internal and external stimuli (Menon, Reference Menon2011; Seeley et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna and , … Greicius2007; Uddin, Reference Uddin2015). The right anterior insula is considered a key node in the SN, involved in mediating dynamics between the ECN and DMN (Uddin, Reference Uddin2015). Several studies have implicated this region in MDD (Diener et al., Reference Diener, Kuehner, Brusniak, Ubl, Wessa and Flor2012; Hamilton, Furman et al., Reference Hamilton, Furman, Chang, Thomason, Dennis and Gotlib2011; Iwabuchi et al., Reference Iwabuchi, Peng, Fang, Jiang, Liddle, Liddle and Palaniyappan2014; Manoliu et al., Reference Manoliu, Meng, Brandl, Doll, Tahmasian, Scherr and , … Sorg2014; Strigo, Matthews, & Simmons, Reference Strigo, Matthews and Simmons2010; Wiebking et al., Reference Wiebking, de Greck, Duncan, Tempelmann, Bajbouj and Northoff2015). Manoliu et al. (Reference Manoliu, Meng, Brandl, Doll, Tahmasian, Scherr and , … Sorg2014) conducted a resting state functional connectivity analysis to investigate the relationship between the anterior insula dysfunction, altered brain network interaction, and severity of depression in MDD. The authors found that decreased functional connectivity of the right anterior insula within the SN was significantly correlated with the severity of MDD symptoms; such symptoms were also related to atypical functional connectivity between sub-systems of the ECN and DMN (Manoliu et al., Reference Manoliu, Meng, Brandl, Doll, Tahmasian, Scherr and , … Sorg2014).
Manoliu et al. interpret their findings as supporting the hypothesis that dysfunction of the right anterior insula may be associated with abnormal interactions between ECN and DMN in patients with MDD. This abnormal interaction is a result of impaired right anterior insula-mediated control of network interaction (Menon, Reference Menon2011). Previous studies have implicated the pivotal role of the right anterior insula in modulating interactions between the ECN and DMN (Sridharan, Levitin, & Menon, Reference Sridharan, Levitin and Menon2008), and clinical neuroscience has reported atypical activity and connectivity within these networks in patients with MDD (Hamilton, Furman et al., Reference Hamilton, Furman, Chang, Thomason, Dennis and Gotlib2011). As such, the significant negative correlation between boundary controllability of the right anterior insula related to BDI reported in the present study strengthens and extends these previous findings. Importantly, NCT allows us to quantitatively characterize the role of the right anterior insula in mediating interactions between the ECN and DMN. Such a negative relation may be related to a diminished ability to integrate between ECN and DMN, which inhibits suppression of maladaptive and repetitive thought, linked to hyper activity in the DMN (Hamilton, Furman et al., Reference Hamilton, Furman, Chang, Thomason, Dennis and Gotlib2011).
We also found a significant positive relation between BDI and average controllability in several right hemisphere brain regions, including the IPL, lingual gyrus, fusiform, and cuneus. While still debated, a few studies have found atypical activation in the right IPL related to depression (Hao et al., Reference Hao, Yang, Wang, Zhang, Xie, Luo and , … Qiu2015; Li, et al., Reference Li, Wei, Sun, Chen, Zhang and Qiu2015; Sheline et al., Reference Sheline, Barch, Price, Rundle, Vaishnavi, Snyder and , … Raichle2009). Based on the role of the right IPL in attentional processing of emotional stimuli (Canli et al., Reference Canli, Sivers, Thomason, Whitfield-Gabrieli, Gabrieli and Gotlib2004), Hao et al. (Reference Hao, Yang, Wang, Zhang, Xie, Luo and , … Qiu2015) found that depressed patients exhibited higher IPL activation when processing sad emotional stimuli. Li et al. (Reference Li, Wei, Sun, Chen, Zhang and Qiu2015) found decreased grey matter volume in a sample of women with subclinical depression. The authors interpreted this finding as indicating that reduced IPL volume may induce inefficient attentional control on negative emotion processing (Li et al., Reference Li, Wei, Sun, Chen, Zhang and Qiu2015). While the bilateral lingual gyrus and the lateral occipital gyrus areas are less commonly related to depression, a few studies have revealed abnormal activation in these areas (Jung et al., Reference Jung, Kang, Won, Nam, Lee, Tae and Ham2014; Keedwell et al., Reference Keedwell, Drapier, Surguladze, Giampietro, Brammer and Phillips2009; Veer et al., Reference Veer, Beckmann, Van Tol, Ferrarini, Milles, Veltman and , … Rombouts2010). Keedwell et al. (Reference Keedwell, Drapier, Surguladze, Giampietro, Brammer and Phillips2009) demonstrated an emotional processing bias towards negative information in the lingual gyrus and the primary visual cortex, and Veer et al. (Reference Veer, Beckmann, Van Tol, Ferrarini, Milles, Veltman and , … Rombouts2010) found decreased functional connectivity of the bilateral lingual gyrus in MDD. In the context of the present study, we suspect that subclinical depressive symptoms may be related to deficits in controlling visual-affective information flow, as related to higher average controllability in these areas.
Finally, we found a weak significant positive correlation between modal controllability and depression in the post-central gyrus. Although the postcentral gyrus has been linked to modal controllability in non-clinical samples (Gu et al., Reference Gu, Pasqualetti, Cieslak, Telesford, Yu, Kahn and Bassett2015), to our knowledge, this is the first study linking structural and functional deficits in this region to depressive symptomology . Future work should replicate and further examine how controllability within the postcentral gyrus relates to subclinical and clinical depressive symptoms.
The current study adds to a growing amount of studies that have demonstrated the strength of applying NCT to study neural dynamics related to different cognitive phenomena (Kenett et al., Reference Kenett, Medaglia, Beaty, Chen, Betzel, Thompson-Schill and Qiu2018; Medaglia et al., Reference Medaglia, Gu, Pasqualetti, Ashare, Lerman, Kable and Bassett2016; Medaglia et al., 2018a; Tang et al., Reference Tang, Giusti, Baum, Gu, Pollock, Kahn and , … Bassett2017). In relation to clinical population, to the best of our knowledge, only one study similar to ours exist (Jeganathan et al., Reference Jeganathan, Perry, Bassett, Roberts, Mitchell and Breakspear2018). Jeganathan et al. (Reference Jeganathan, Perry, Bassett, Roberts, Mitchell and Breakspear2018) investigated average controllability and network characteristics of white matter connectivity networks in participants with bipolar depression, participants with high risk of bipolar depression, and controls. The authors found average controllability deficits (lower compared with controls) in a left lateralized network of brain regions that included the inferior frontal gyrus, insula, and the post-central gyrus in participants with bipolar depression. The authors also found average controllability deficits in a right lateralized network of brain regions that included the prefrontal cortex and striatal regions in participants with high risk of bipolar depression. The authors interpret their findings regarding the altered average controllability in participants with bipolar depression as related to altered connectivity between these regions, mediating dysfunctional cognition with emotional homeostasis (Jeganathan et al., Reference Jeganathan, Perry, Bassett, Roberts, Mitchell and Breakspear2018). It is important to note that while our study is related to the work of Jeganathan et al. (Reference Jeganathan, Perry, Bassett, Roberts, Mitchell and Breakspear2018), we examined a sample of participants with subclinical levels of depression and examined a broader range of controllability measures. Regardless, the work of Jeganathan et al. (Reference Jeganathan, Perry, Bassett, Roberts, Mitchell and Breakspear2018) and the current study demonstrate how such a quantitative approach can be applied to study psychopathology such as MDD and bipolar depression. Further research is needed to quantify the spectrum of varying degrees of depression and the neural dynamics leading to these varying states.
The focus of the current study is on atypical inhibition and rumination as signals of maladaptive cognitive control related to depression and how NCT can be applied to quantify such signals. However, it is important to note that the BDI measures both a psychological–cognitive factor and an affective–somatic factor (Steer, Ball, Ranieri, & Beck, Reference Steer, Ball, Ranieri and Beck1999; Storch, Robert, & Roth, Reference Storch, Robert and Roth2004). While rumination is attributed to hyper-connectivity in the DMN (Hamilton, Furman et al., Reference Hamilton, Furman, Chang, Thomason, Dennis and Gotlib2011), Burrows, Timpano, and Uddin (Reference Burrows, Timpano and Uddin2017) have recently proposed that rumination is related to dysfunction in the SN, specifically the insula (Burrows, Timpano, & Uddin, Reference Burrows, Timpano and Uddin2017). This hypothesis is supported by atypical connectivity patterns between the SN and DMN related to rumination (Kaiser et al., Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer, Minkel and , … Pizzagalli2016), and also to recent findings that altered insula timing in coupling between ECN and DMN is altered in patients with MDD (Hamilton, Furman et al., Reference Hamilton, Furman, Chang, Thomason, Dennis and Gotlib2011). Burrows, Timpano, and Uddin (Reference Burrows, Timpano and Uddin2017) interpret this atypical timing of the anterior insula as potentially indicating processing of heightened salience of negative information in participants with MDD. Our main finding of a negative correlation between boundary controllability in the anterior insula and BDI supports the hypothesis on the role of the SN in rumination and offers a potential bridge across the two factors measured by the BDI.
A few limitations in our study exist. First, the controllability metrics are calculated over the entire space of all possible states. In reality, however, neural systems occupy a restricted space of biologically viable configurations, generally avoiding pathological states (e.g., seizures) and states that require too much energy to reach. While methods for controlling transitions between specific configurations in a restricted state space are beginning to be explored (Betzel et al., Reference Betzel, Gu, Medaglia, Pasqualetti and Bassett2016; Gu et al., Reference Gu, Betzel, Mattar, Cieslak, Delio, Grafton and , … Bassett2017), those methods are not yet fully developed and require the researcher to specify states of interest. For this reason, the focus of the present analysis was on the behavior relevance of average, modal, and boundary controllability, which do not require such researcher input.
A second limitation is in the method we used to measure structural connectivity, which was based on DTI data. DTI may under-sample some white matter fibers, particularly those linking hemispheres or those that cross paths with other fibers (Wedeen et al., Reference Wedeen, Wang, Schmahmann, Benner, Tseng, Dai and , … de Crespigny2008). This can also partially account for the weak correlations, albeit significant, found in our data. Future efforts should apply diffusion spectrum imaging to improve estimates of structural network architecture.
Third, our boundary controllability analysis was based on an independent a priori brain modularity partition, which was in turn based on a modularity analysis of resting-state functional imaging data (Mišić et al., Reference Mišić, Betzel, Nematzadeh, Goñi, Griffa, Hagmann and , … Sporns2015). This partition was chosen as an objective initial template for the analysis and also based on the relation between structural connectivity networks and resting-state data (Cole et al., Reference Cole, Ito, Bassett and Schultz2016; Hermundstad et al., Reference Hermundstad, Brown, Bassett, Aminoff, Frithsen, Johnson and , … Carlson2014). However, the partition we chose may partially bias our results. Future research is needed to establish independent a priori partitions based on structural networks, which will increase the reliability and validity of the boundary controllability analysis.
Finally, recently the ability of specific nodes in a network (such as anatomical brain networks) to drive the system into a specific state has been questioned (Menara, Gu, Bassett, & Pasqualetti, Reference Menara, Gu, Bassett and Pasqualetti2017; Tu et al., 2018). In the current study, we were interested in investigating the theoretical notion of NCT and individual differences in depressive symptoms, without committing to linking between cognitive control processes and network controllability.
In conclusion, while rumination and atypical inhibition are considered indicators of atypical cognitive control process in depression, they are far from understood. We propose a method to computationally quantify the role of different brain regions in theoretically “controlling” the brain as related to subclinical levels of depression symptoms. Our results provide unique and novel evidence on how individual differences in controllability measures across the brain correlate with behavioral measures of depression symptoms. Thus, our results demonstrate the feasibility of applying NCT to advance our understanding of different drivers of neural dynamics relate to subclinical levels of depression.
Acknowledgments
JDM acknowledges support from the Office of the Director at the National Institutes of Health (1-DP5-OD-021352-01).
Conflict of Interest
The authors declare no conflicts of interest.





