Introduction
The value of citizen science projects has often been ascribed to their effectiveness as tools to promote community engagement and public educational outcomes (Dickinson et al., Reference Dickinson, Zuckerberg and Bonter2010; Bonney et al., Reference Bonney, Shirk, Phillips, Wiggins, Ballard, Miller-Rushing and Parrish2014). Their value as scientific research has been questioned, however, with particular concerns about data quality (Crall et al., Reference Crall, Newman, Stohlgren, Holfelder, Graham and Waller2011; Ward, Reference Ward2014) and the credibility of the results (Conrad & Hilchey, Reference Conrad and Hilchey2011). Many citizen science projects have run for decades and it is now recognized that the generation of long-term data sets, at large spatial scales, is reliant on well-designed projects involving volunteers (Devictor et al., Reference Devictor, Whittaker and Beltrame2010; Hochachka et al., Reference Hochachka, Fink, Hutchinson, Sheldon, Wong and Kelling2012; Tulloch et al., Reference Tulloch, Possingham, Joseph, Szabo and Martin2013a; Bonney et al., Reference Bonney, Shirk, Phillips, Wiggins, Ballard, Miller-Rushing and Parrish2014). Cooper et al. (Reference Cooper, Shirk and Zuckerberg2014) reviewed the contribution of data generated by citizen scientists to studies of climate change impacts on birds, and found that most studies used such data extensively, although the contributions of volunteers were not always stated explicitly.
Citizen science monitoring programmes are making increasingly important contributions to wildlife conservation. Many such projects are at spatial and temporal scales unachievable by individual or teams of researchers (Dickinson et al., Reference Dickinson, Zuckerberg and Bonter2010; Bonney et al., Reference Bonney, Shirk, Phillips, Wiggins, Ballard, Miller-Rushing and Parrish2014; Cooper et al., Reference Cooper, Shirk and Zuckerberg2014) and are particularly valuable in estimating population trends and the impacts of management, thus facilitating effective conservation decisions for declining species (van Swaay et al., Reference van Swaay, Nowicki, Settele and van Strien2008; Tulloch et al., Reference Tulloch, Possingham, Joseph, Szabo and Martin2013a). The quality and potential biases of citizen science data are of concern, and appropriate experimental design (Conrad & Hilchey, Reference Conrad and Hilchey2011), sampling protocols (Crall et al., Reference Crall, Newman, Stohlgren, Holfelder, Graham and Waller2011) and data analysis (Roos et al., Reference Roos, Johnston and Noble2012; Dennis et al., Reference Dennis, Freeman, Brereton and Roy2013) are needed to ensure that the maximum scientific value is extracted. Issues of particular concern include varying abilities of volunteers in identifying target species (Ward, Reference Ward2014), variability in sampling effort between sampling sites (Dennis et al., Reference Dennis, Freeman, Brereton and Roy2013), including lack of repeated sampling at some sites resulting in patchy data sets (Bonney et al., Reference Bonney, Shirk, Phillips, Wiggins, Ballard, Miller-Rushing and Parrish2014), and spatial variability in sampling, often as a consequence of the self-selection of participants and the sites surveyed (Cooper et al., Reference Cooper, Shirk and Zuckerberg2014). There are clearly benefits to be had from citizen science surveys but survey designers must take care to account for methodological shortcomings that may contribute additional sources of variation to counts of birds and other wildlife (Dickinson et al., Reference Dickinson, Zuckerberg and Bonter2010).
In response to concerns about a possible decline of the Endangered Carnaby's black-cockatoo Calyptorhynchus latirostris an extensive citizen science survey, the Great Cocky Count, has been undertaken annually since 2010. Each survey involves several hundred volunteers, and in 2014 > 290 known and putative roost sites were surveyed (Finn et al., Reference Finn, Barrett, Groom, Blythman and Williams2014). As the only source of data on the abundance of Carnaby's black-cockatoo, these surveys provide valuable information on the population trend. We report the results from the first 5 years (2010–2014) of this community-based monitoring programme, and discuss the implications for citizen science monitoring of other threatened species.
Carnaby's black-cockatoo is a long-lived, charismatic species endemic to south-western Australia. Individual flocks once exceeded 5,000 birds (Perry, Reference Perry1948; Johnstone et al., Reference Johnstone, Johnstone and Kirkby2009) but flocks of > 500 are now a rarity (Finn et al., Reference Finn, Barrett, Groom, Blythman and Williams2014). As a result of land clearing for urbanization and agriculture the species has undergone substantial range contraction (Saunders, Reference Saunders1990), with an estimated population decline of > 50% during the past 50 years (Saunders & Ingram, Reference Saunders, Ingram, Saunders, Arnold, Burbidge and Hopkins1998; Garnett & Crowley, Reference Garnett and Crowley2000; Cale, Reference Cale2003). Fragmentation of the remaining habitat has resulted in two geographically distinct subpopulations, with limited gene flow between them (White, Reference White2011). The northern subpopulation is the larger of the two (DEC, 2012) and migrates annually between the breeding areas and the west coast; the southern subpopulation moves between the breeding areas and the south coast region (Saunders, Reference Saunders1980; Saunders et al., Reference Saunders, Mawson and Dawson2011; White et al., Reference White, Bunce, Mawson, Dawson, Saunders and Allentoft2014). After migrating to coastal regions the cockatoos mass into flocks and roost communally at sites throughout the region (Shah, Reference Shah2006; Berry, Reference Berry2008; Berry & Owen, Reference Berry and Owen2009). Estimates of the total population size of the species vary widely (11,000–60,000; DEC, 2012) and there is no quantitative information on the size of either subpopulation. The lack of an accurate population estimate or any analysis of accumulated monitoring data has impeded understanding of the population dynamics and assessment of the long-term viability of populations of Carnaby's black-cockatoo (DEC, 2012).
To obtain reliable estimates of population size of a mobile species such as Carnaby's black-cockatoo is challenging, and the best method is to count individuals arriving at nocturnal roosts (Shah, Reference Shah2006; Berry, Reference Berry2008; Berry & Owen, Reference Berry and Owen2009). On the west coast the northern part of the Swan Coastal Plain bioregion (Thackway & Cresswell, Reference Thackway and Cresswell1995) supports the largest subpopulation during the non-breeding period (Johnstone et al., Reference Johnstone, Johnstone and Kirkby2009). This region includes the city of Perth, the state capital of Western Australia, which is expanding rapidly (WAPC, 2010). The cockatoo's predominant food sources in the region, native Banksia spp. woodlands and plantations of exotic Pinus pinaster, are subject to ongoing removal for urbanization and the management of groundwater recharge (EPA, 2007), and significant further clearing is planned for housing and extraction of raw materials (WAPC, 2010). Widespread clearing of Banksia spp. woodland has accelerated since 1950, and 61% of the original extent has been cleared (WAPC, 2010). Extensive pine plantations established since the 1920s have progressively replaced Banksia woodlands as the major food source for the cockatoos in the region, inadvertently offsetting the loss of native habitat (Perry, Reference Perry1948; Saunders, Reference Saunders1974; Butcher, Reference Butcher2007; Stock et al., Reference Stock, Finn, Parker and Dods2013). However, all the remaining pine plantations in the northern part of the region are scheduled for harvesting before 2031 (Valentine & Stock, Reference Valentine and Stock2008). This ongoing clearance of vegetation is likely to have a substantial negative impact on Carnaby's black-cockatoo and will further reduce the region's carrying capacity (Stock et al., Reference Stock, Finn, Parker and Dods2013).
We report the results of the five annual monitoring surveys carried out during 2010–2014, with the aims of (1) determining any trends in roost utilization and flock size, (2) assessing the effectiveness of the citizen science monitoring programme, and whether any changes are needed, and (3) identifying any implications for citizen science monitoring of comparable species.
Study area
The study was conducted on the Swan Coastal Plain, which extends along the south-west coast of Australia. Perth (population 1.9 million), the fourth largest city in Australia, is located in the centre of the region (Fig. 1). The climate is Mediterranean, with cool wet winters and a prolonged summer drought. Most of the native vegetation in the region, predominantly Banksia woodlands and coastal heathland, has been cleared for urbanization, industry and agriculture.

Fig. 1 The study area in south-western Australia, showing the locations of sites surveyed annually for Carnaby's black-cockatoo Calyptorhynchus latirostris during 2010–2014.
Methods
Roost counts
Sampling sites were at known or presumed nocturnal roosting locations for Carnaby's black-cockatoo, determined from a 2006 survey and from reports from scientists and members of the public. A database of these sites is maintained by the count organizers, and newly reported sites are added each year. Individual or groups of volunteers were assigned to these sites to conduct annual, synchronized surveys (roost counts) on a set date in April (the Great Cocky Count) during 2010–2014. We examine only the results for the northern Swan Coastal Plain region, as count data from other areas are sparse. On the coastal plain flocks consist entirely of Carnaby's black-cockatoo, whereas in forested areas to the east of the study area they may also include the superficially similar Baudin's black-cockatoo Calyptorhynchus baudinii. Although the two taxa cannot be reliably distinguished genetically (White, Reference White2011; White et al., Reference White, Bunce, Mawson, Dawson, Saunders and Allentoft2014), they are morphologically distinct, have different diets, and occupy different habitats (Saunders, Reference Saunders1982; Johnstone & Kirkby, Reference Johnstone and Kirkby2008). As this study was restricted to the northern Swan Coastal Plain, roosting birds consisted entirely of Carnaby's black-cockatoo (Johnstone & Kirkby, Reference Johnstone and Kirkby2008). Surveys were conducted at previously surveyed sites (resurveys) or at new sites. Count organizers prioritized resurveying of sites where birds had been recorded roosting in previous years, but volunteer turnover and other factors (e.g. destruction of roost sites) meant that it was not possible to survey all previously surveyed sites during each annual survey. Thus, the frequency of resurveys was calculated as the number of resurveys completed divided by the total number of possible resurveys.
In the days preceding each survey observers were encouraged to follow the birds to locate the roosting area precisely. On the day of the survey, birds were tallied during a fixed time interval, from 30 minutes before until 30 minutes after sunset, as they arrived at and occupied the roost trees. Any birds that arrived but subsequently departed to roost elsewhere were excluded. Complete details of the methodology are included in the annual reports (Burnham et al., Reference Burnham, Barrett, Blythman and Scott2010; Kabat et al., Reference Kabat, Scott, Kabat and Barrett2012a,Reference Kabat, Barrett and Kabatb; Finn et al., Reference Finn, Barrett, Groom, Blythman and Williams2014). Important considerations for data analysis and interpretation of the results are that (1) the annual surveys included both known and putative roost locations, (2) new sites were added to the survey each year because count organizers actively sought to identify new roost sites (e.g. through field surveys by scientists or community reports) and because volunteers were able to nominate sites that they subsequently surveyed themselves, and (3) the sampled sites and volunteer observers, as well as the assignment of volunteers to sites, all differed from year to year.
Data analysis
As most of the birds were counted at relatively few sites (see Results), the total number recorded at each site was used to rank sites in terms of relative importance. Many roost counts yielded tallies of zero (see Results), which may be attributable to either normal variation in the number of birds roosting at a particular site (which varies from day to day as well as at broader time scales), or a design problem (e.g. the site is never used as a roosting area as it is unsuitable). Counts of the latter type are problematic because although they are irrelevant to the study they can influence estimates of mean roost size and hence any trend (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009). They also cause so-called zero-inflation or excess zeros: extra records of zeros, exceeding the number expected as part of normal variation in counts. These problems are common in citizen science surveys (Kéry & Schmid, Reference Kéry and Schmid2004; Schmeller et al., Reference Schmeller, Henle, Loyau, Besnard and Henry2012) and appropriate statistical methods have been developed to deal with them (Cunningham & Lindenmayer, Reference Cunningham and Lindenmayer2005). A suitable approach, and the method employed here, is to use a zero-inflated negative binomial model that allows for the excess counts of zero and incorporates over-dispersion to account for any unknown sources of variation (Link & Sauer, Reference Link and Sauer1997; Sauer et al., Reference Sauer, Link and Royle2004a; Roos et al., Reference Roos, Johnston and Noble2012; Tonachella et al., Reference Tonachella, Nastasi, Kaufman, Maldini and Rankin2012).
This method models the data in two stages: firstly, the probability of a site being occupied each year is determined, and then for the occupied sites the mean count for each year is estimated using a log-linear model. The annual trend in both occupancy and mean roost size is also estimated. The statistical model for the occupied sites assumes that the count data follow a negative binomial distribution, the mean of which is determined by two explanatory variables: the annual trend in mean roost size, and a site effect that allows for correlation in the repeated surveys at each site. As the surveys sampled a significant fraction of the subpopulation of Carnaby's black-cockatoos in the region and were restricted to a few sites, the site effect was treated as fixed rather than random. The use of the negative binomial distribution (rather than the Poisson or lognormal distribution) allows for potential excess variation as a result of the many unmodelled sources of variation (overdispersion) in the roost counts (Richards, Reference Richards2008). Although additional explanatory factors could be incorporated in this model, particularly relating to site characteristics, no additional information about roost site characteristics is available yet. The zero-inflated negative binomial model is comparable to those used for similar surveys, such as bird counts in North America (the Christmas Bird Count, International Shorebird Survey and Breeding Bird Survey; Sauer et al., Reference Sauer, Link and Royle2004a,Reference Sauer, Niven and Linkb; Butcher et al., Reference Butcher, Niven and Sauer2005) and Australia (Barrett et al., Reference Barrett, Silcocks, Cunningham, Oliver, Weston and Baker2007), annual counts of whales (the Great Whale Count; Tonachella et al., Reference Tonachella, Nastasi, Kaufman, Maldini and Rankin2012), the UK Butterfly Monitoring Scheme (Dennis et al., Reference Dennis, Freeman, Brereton and Roy2013), and surveys of hedgehogs (Hogwatch; Roos et al., Reference Roos, Johnston and Noble2012). The details, merits and limitations of the methodology have been discussed elsewhere (Howe et al., Reference Howe, Geissler and Harrington1989; Dobbie & Welsh, Reference Dobbie and Welsh2001; Sauer et al., Reference Sauer, Link and Royle2004a,Reference Sauer, Niven and Linkb; Cunningham & Lindenmayer, Reference Cunningham and Lindenmayer2005; Fletcher et al., Reference Fletcher, Mackenzie and Villouta2005; Humbert et al., Reference Humbert, Mills, Horne and Dennis2009; Roos et al., Reference Roos, Johnston and Noble2012). This method is superior to simple linear regression models of counts or log-transformed counts because variation in the counts is modelled more realistically (Cunningham & Lindenmayer, Reference Cunningham and Lindenmayer2005; Richards, Reference Richards2008). The generalized linear models procedure (GENMOD) of SAS/STAT v. 9.3 (SAS Institute Inc., Cary, USA) was used for model estimation.
Results
During the 5-year period 2010–2014 a total of 738 roost counts were conducted at 265 actual or purported nocturnal roost sites (Table 1). Retention of volunteers varied from year to year, with approximately one third to half of participants having undertaken surveys previously. The number of sites surveyed increased steadily each year, to a peak of 186 in 2014, with a mean of 150 (range 124–186). The number of actual roost sites (i.e. surveyed sites with a positive count) was only a small fraction (20–30%) of the number of sites surveyed each year. The discovery rate of actual roost sites has declined steadily, with the majority (65%) identified and surveyed in the first year of the study and only five new roosts (6%) discovered in each of the fourth and fifth years.
Table 1 Descriptive statistics for the five annual surveys of nocturnal roost sites of Carnaby's black-cockatoo Calyptorhynchus latirostris during 2010–2014, with the date of survey, number of volunteers, volunteer fidelity, number of site surveys, number and percentage of occupied roosts, and number and percentage of newly discovered roosts.

1 The percentage of volunteers who had participated in a previous survey
2 Includes some sites outside the study area
At the majority of the sites (n = 176, 66%) no birds were recorded during any of the annual surveys. Birds were recorded at only 89 sites during the 5-year period, and 42 of these sites account for almost all (95%) of the records of Carnaby's black-cockatoo in the region (Supplementary Table S1). The relative importance of the roost sites in terms of the total number of records declined steadily: three roost sites accounted for 28% of all records and the 10 largest sites accounted for > 54% of records, the next 10 for a further 24%, and the next 10 for a further 10%.
The largest roosts were most likely to be resurveyed in the years after initial discovery, and the resurvey rate declined with diminishing roost size (Table 2). The 10 largest roost sites were resurveyed on 33 of 36 possible occasions after discovery but the resurvey rate declined steadily at progressively smaller and less important roost sites. The proportion of repeat surveys that recorded birds at the same roost site in subsequent years indicates the fidelity of Carnaby's black-cockatoo to roost sites. Fidelity was greatest for the 10 largest roost sites, with 91% of repeat surveys recording birds present, whereas for the 47 smallest sites, which accounted for only 5% of all records, fidelity was only 39% (Table 2). The 20 largest, most consistently occupied roosts (62–91% re-occupancy rate) were resurveyed most frequently (89–92% resurvey rate), whereas the next 20 largest sites, with moderate numbers of birds but lower repeat occupancy rates (38–58%), were resurveyed less frequently (69–74%), and sites with no birds had the lowest resurvey rate (50%).
Table 2 The number of annual surveys of nocturnal roost sites of Carnaby's black-cockatoo during 2010–2014, with the percentage of birds recorded at each site group, the number of repeat surveys, the number of repeat surveys with birds present, and roost site fidelity.
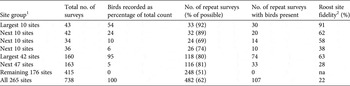
1 Sites ranked by decreasing total counts for the period 2010–2014
2 The percentage of repeat surveys of roost sites in which birds were present
For the 89 actual roost sites, 153 of the 323 surveys (47%) yielded counts of zero (Supplementary Tables S1 & S2), supporting our use of a zero-inflated model in the analysis of trends. Ignoring the remaining 139 sites, which were never occupied and do not affect the estimated occupancy rates, site occupancy declined over time at a significant rate (year trend parameter = −0.18, Wald χ2 = 4.76, P = 0.03, equivalent to an annual decline in occupancy rate of 4.2–4.5%; Fig. 2). The trend in mean roost count was a decline at an annual rate of 6.8% (year trend parameter = −0.07, Wald χ2 = 1.48, P = 0.22; Fig. 3). During 2010–2014 the estimated decline in the abundance of Carnaby's black-cockatoo, incorporating the declines in both roost site occupancy and mean roost size, was 45%, or 13.8% per year. In contrast, the total counts for the region showed no trend during the period (Fig. 3).

Fig. 2 Probability of occupancy of nocturnal roost sites by Carnaby's black-cockatoo in the Swan Coastal Plain region of Western Australia (Fig. 1) from annual surveys during 2010–2014, estimated using a logistic regression model, with 95% confidence limits. The dashed line indicates the estimated trend.

Fig. 3 (a) Mean roost size (logarithmic scale), with 95% confidence limits, and (b) total counts of Carnaby's black-cockatoo at nocturnal roost sites in the Swan Coastal Plain region from annual surveys during 2010–2014. The mean roost size and trend (dashed line) were estimated using a log-linear zero-inflated regression model.
Discussion
The survey methodology used in the Great Cocky Count presents three issues that should be considered in interpreting any estimate of a population trend for Carnaby's black-cockatoo, and may be used to inform other citizen science surveys. Firstly, not all of the known roost sites were sampled in each year of the study, and newly identified sites were added each year. As a consequence, a slightly different set of sites was surveyed each year. Secondly, in citizen science surveys where observers choose their sampling sites, or where the survey coordinators prioritize previously occupied sites, there is likely to be a bias towards allocating new observers to survey sites where cockatoos are known to roost, rather than to sites where they are absent. Although volunteers received no tangible reward for locating or surveying roost sites, it is to be expected that they would seek to conduct counts at occupied roosts, as for interested observers this is a reward in itself. In contrast, it may be expected that sites where no birds are present are less likely to be resurveyed. Similarly, the emphasis on obtaining a minimum population count of Carnaby's black-cockatoos in the study area (based on the repeated, annual survey of a large number of known roosts in the region) rather than a population estimate (based on sampling of a representative sample of sites across the region) encouraged the coordinators of the Great Cocky Count to prioritize the allocation of observers to known roosts. This bias was reflected in the results: the largest, most consistently occupied roost sites were resurveyed most frequently, whereas sites with no birds had the lowest resurvey rate.
A bias towards surveying known roosts is problematic if, for example, flocks abandon known roost sites and relocate to new or previously unoccupied sites during the next survey year. If these new or previously unoccupied sites are less likely to be surveyed, the shift in occupancy may go undetected, potentially leading to an overestimate of the rate of decline. This may not be a problem for the Great Cocky Count, where the number of observers greatly exceeds the number of occupied sites; however, it would be prudent to rationalize the surveys by re-allocating observers from patchily surveyed, consistently unoccupied sites to strengthen the resurvey rates at other sites, in accordance with the suggestion of Tulloch et al. (Reference Tulloch, Mustin, Possingham, Szabo and Wilson2013b) to deploy observers to fill gaps in survey coverage. Examination of the spatial relationship between sites and the movement of marked birds between them would assist in determining the extent to which flocks shift to other roosts from year to year. Care should also be taken to articulate the issues that may arise when a citizen science survey does not involve sampling in the strict sense, but instead approximates a census both in its design (e.g. surveying a large proportion of occupied roosts in the region) and objectives (e.g. obtaining a minimum population count). Finally, variation between observers in their ability to count cockatoos accurately, annual variation in the observers participating in the surveys, and local and annual weather conditions pertaining during the surveys all potentially affect the detectability of birds and may therefore influence the accuracy of counts (Kavanagh & Recher, Reference Kavanagh and Recher1983; Cunningham et al., Reference Cunningham, Lindenmayer, Nix and Lindenmayer1999; Lindenmayer et al., Reference Lindenmayer, Wood and MacGregor2009). These sources of variation can present problems for analysis that are common to other wildlife counts that employ citizen scientists (Butcher et al., Reference Butcher, Niven and Sauer2005; Roos et al., Reference Roos, Johnston and Noble2012; Tonachella et al., Reference Tonachella, Nastasi, Kaufman, Maldini and Rankin2012), although the benefit of obtaining a large, comprehensive data set tends to compensate for this (Dickinson et al., Reference Dickinson, Zuckerberg and Bonter2010). Recent improvements in statistical methods have provided practical ways of overcoming some of the problems with point counts (Farnsworth et al., Reference Farnsworth, Nichols, Sauer, Fancy, Pollock, Shriner and Simons2005), and the model used here is less susceptible to such problems. The way forward for the Great Cocky Count is to continue the repeated sampling of a (largely) fixed set of monitoring sites using the standard survey protocol, but aim to incorporate additional explanatory factors (including site and observer characteristics) into the statistical model to improve the precision of trend estimates, the accuracy of counts, and understanding of roost use.
There are three main implications of our findings for citizen science programmes. Firstly, consistent resurveys of sites should be a goal, particularly for highly mobile species such as Carnaby's black-cockatoo. To achieve this, observers should be redeployed from empty sites to ensure sampling is consistent at the most important sites. Secondly, appropriate analysis is needed, accounting for both variation in resurvey rates and the potential excess of empty sites (‘zeroes’). The model used here, which accounts for the zero-inflated data of the Great Cocky Count, is one such method. Thirdly, incorporation of additional explanatory factors, such as observer effects, should be considered at the design stage. If it had been known that occupancy rates and flock sizes were to be effectively analysed separately, appropriate covariates (e.g. roost tree characteristics, availability of drinking water, distance from roads, or other site factors) could have been recorded and would have assisted in determining the reasons why some roosts are inconsistently occupied. These findings reflect those of other studies of citizen science programmes (Devictor et al., Reference Devictor, Whittaker and Beltrame2010; Dennis et al., Reference Dennis, Freeman, Brereton and Roy2013) but emphasize the need to account for additional sources of variation to improve the accuracy of the estimated trend (Hochachka et al., Reference Hochachka, Fink, Hutchinson, Sheldon, Wong and Kelling2012).
Programmes such as the Great Cocky Count provide opportunities for community members to contribute their observations to broader programmes investigating the ecology and population trends of prominent threatened species (Dickinson et al., Reference Dickinson, Zuckerberg and Bonter2010). Our experience with the Great Cocky Count is instructive in this regard. The growth of the Great Cocky Count has increased the replication and geographical spread of observations to provide a more complete picture of how a threatened species is responding to a landscape undergoing rapid urban development. Relating population change in a species to environmental factors is a crucial step in making monitoring data from citizen science projects relevant to management (van Swaay et al., Reference van Swaay, Nowicki, Settele and van Strien2008; Dennis et al., Reference Dennis, Freeman, Brereton and Roy2013; Tulloch et al., Reference Tulloch, Possingham, Joseph, Szabo and Martin2013a). There is clearly scope to expand the role of the Great Cocky Count survey in identifying and assessing relationships between roost counts and environmental variables, and as an ongoing data source that can be used to test these models. The need for site-specific covariates for factors that influence counting efficiency is well established for count-based surveys (Sauer et al., Reference Sauer, Link and Royle2004a; Farnsworth et al., Reference Farnsworth, Nichols, Sauer, Fancy, Pollock, Shriner and Simons2005) but is yet to be incorporated in the analysis of citizen science-based projects (Dickinson et al., Reference Dickinson, Zuckerberg and Bonter2010). A statistical framework that incorporates these covariates could provide a more realistic and accurate model of population trends in the region. Similarly, an analysis that incorporates appropriate treatment of the spatial arrangement of the sampling sites would be of value. The model used here can be easily extended to incorporate observer effects, habitat data or any other relevant explanatory information for individual sampling sites or at broader scales; this should be investigated to determine if a more accurate knowledge of observer variability and roost site utilization can be achieved. Maintaining a dialogue among biologists, statisticians, managers and count organizers to apply methods that accommodate the limitations of the survey and maximize the information obtained from the Great Cocky Count is important for future conservation and population monitoring of Carnaby's black-cockatoo. Our findings show that, given sufficiently large numbers of surveys and appropriate statistical analysis, even small changes in abundance (such as the annual 4% decline in roost occupancy rates) can be detected. The Great Cocky Count has two features that make it good science: the sampling protocol and effort at each site is fixed, and the resurvey rate is high (> 90%) for the most important sites. The fidelity of the cockatoos to a relatively limited number of roost sites facilitates forward planning of surveys. Any single annual survey may be subject to potentially large variation as a result of weather and seasonal variation in abundance, but this has not been a problem for other once-per-year sampling studies (O'Brien et al., Reference O'Brien, Thorne, Rosenzweig and Shapiro2011). The annual Great Cocky Count is supplemented with additional roost counts around the main survey date, and future analyses incorporating these data may improve estimates (e.g. Dennis et al., Reference Dennis, Freeman, Brereton and Roy2013).
The Great Cocky Count provides new data on the location and dynamics of nocturnal roosts and population trends for Carnaby's black-cockatoo on the Swan Coastal Plain. The majority of roosting birds were recorded at relatively few sites and the largest roosts were occupied more consistently across the 5 years of the survey. The characteristics of these preferred roosting sites are unknown, but factors such as isolation from disturbance and reliable access to water may be important (Saunders, Reference Saunders1980; Berry, Reference Berry2008; Berry & Owen, Reference Berry and Owen2009). Further study is needed to assess these and any other factors that may affect use of roost sites: identifying these sources of variation will improve understanding of roost site dynamics, improve future monitoring and provide guidance on whether the suitability of sites for roosting can be improved. The large nocturnal roost sites identified should be protected from disturbance; in the past, clearing of trees has caused birds to abandon roost sites and the ultimate fate of such birds is unknown (Burnham et al., Reference Burnham, Barrett, Blythman and Scott2010).
The Great Cocky Counts provide the first systematic basis for estimating trends in the northern subpopulation of Carnaby's black-cockatoo, and several important findings have emerged. Firstly, the number of cockatoos on the Swan Coastal Plain is decreasing. In the first coordinated survey of Carnaby's black-cockatoo on the Swan Coastal Plain, undertaken in 2006 using similar techniques to the Great Cocky Count, 26 observers recorded 4,510 individuals at only 13 roost sites (Shah, Reference Shah2006). In 2014 > 300 observers surveyed 180 roost sites and counted 6,671 individuals at 38 occupied roosts. Despite the > 10-fold increase in numbers of survey sites and volunteers, the total count of Carnaby's black-cockatoo in the region has remained largely unchanged. Secondly, since 2010 there has been a decline in the number of new roost sites discovered each year and this indicates that the Great Cocky Count is now sampling a large proportion of the birds that spend the non-breeding period on the Swan Coastal Plain. Thirdly, analysis of the Great Cocky Counts using an appropriate statistical model indicates there is a significant and ongoing decline in the roost occupancy rate of Carnaby's black-cockatoo on the Swan Coastal Plain, with an estimated annual decline of 14% in the number of individuals roosting in the region. This decline of a threatened species is of concern.
There are two potential explanations for the observed trend: (1) the decline at known sites may be attributable to the loss of birds from the study area, or (2) birds may have relocated from known to new roost sites, and the trend is the result of birds being displaced from existing to new sites each year. For the former, the trend analysis reported here is appropriate and provides an estimate of the losses from the region, although the true fate of such birds (mortality or emigration) is unknown. For the latter, the total counts provide a better estimate of abundance and population trend, provided that the birds at newly discovered roost sites have relocated from previously occupied roosts. A combination of both these mechanisms may be the ultimate reason for the observed decline in mean roost counts and occupancy rate. However, there are no completed studies that provide support for either scenario and it would be prudent to take a precautionary approach until better information becomes available. One thing is certain: despite increased knowledge of roost sites and improved survey focus there has been no increase in the number of occupied roosts.
Several putative causes of decline in Carnaby's black-cockatoo have been identified. The species’ two major food sources, Banksia spp. woodlands and pine plantations, have been substantially reduced in extent and are increasingly fragmented. Banksia woodlands in the region have been subject to ongoing clearing for urban development and consequently are being assessed for federal listing as a threatened ecological community. Of the 24,000 ha of pine plantations present in the region in 2004, 9,000 ha (40%) has been cleared (Gnangara Coordinating Committee, 2009; Stock et al., Reference Stock, Finn, Parker and Dods2013). In addition to these major food sources the cockatoos are also known to utilize non-native food sources such as garden plants. Urban consolidation (i.e. the process of increasing housing density in an existing urban area) is also progressively reducing these food resources. Population expansion among other parrots (galahs Eolophus roseicapilla, corellas Cacatua spp. and forest red-tailed black-cockatoos Calyptorhynchus banksii naso) and corvids on the coastal plain has increased competition for roost sites and food sources, and results in antagonistic interactions, sometimes causing death and injury, particularly for young birds (H. Finn, pers. obs.; C. Groom, pers. comm.). There has also been increased reporting of bird deaths from vehicle strike, and mass deaths during extreme weather events, which may be associated with climate change in the region (Saunders et al., Reference Saunders, Mawson and Dawson2011). The relationship between the abundance of Carnaby's black-cockatoo and these factors requires urgent attention.
In conclusion, data from the Great Cocky Counts provide a valuable insight into roost site use and population trends in Carnaby's black-cockatoo. The results from five annual surveys indicate a substantial and ongoing decline of the population at roost sites in the northern Swan Coastal Plain region. Although the total annual counts have not shown a pronounced decline, this is probably because newly identified roost sites have been added each year, which may mask an underlying decline. If the rate of decline at roost sites is representative of the population in this region, such a rapid decline is clearly unsustainable for this long-lived, slow-breeding species. Our analyses highlight the need to employ analytical methods that account for annual variation in survey effort and appropriate treatment of zero counts. Continued surveys of the large, consistently occupied roosts are particularly important to facilitate monitoring of population trends in the region. There is a need to prioritize these roost sites to ensure that the largest, most important sites are surveyed each year, while also accounting for possible shifts to new or previously unoccupied sites.
Acknowledgements
We thank the hundreds of volunteer citizen scientists who contributed the data for this study; the staff of BirdLife Australia and the Department of Parks and Wildlife involved in coordinating Great Cocky Counts during 2010– 2014, particularly Quinton Burnham, Mark Blythman, Raana Scott, Alexander Kabat and Tamara Kabat; Christine Groom for insights into the challenges of citizen science, and the solutions; Michael Craig, Sarah McEvoy, Carly Bishop, Brett Glossop and an anonymous reviewer for their comments; and Blair Pellegrino for preparing Fig. 1.
Biographical sketches
Matthew Williams, Colin Yates and Geoff Barrett are ecologists focusing on the conservation of threatened species and ecosystems in Western Australia. Matthew Williams is a biometrician and has a research interest in the ecology and conservation of Lepidoptera. Colin Yates is interested in the evolution and ecology of south Western Australia's biodiversity and its vulnerability to global environmental change. Geoff Barrett is the regional ecologist for the Swan Region, where the present study was conducted, and has a particular interest in ornithology. Will Stock's research has focused on the ecology and conservation of Mediterranean-type ecosystems. Hugh Finn coordinated the 2014 Great Cocky Count, and his research interest is the ecology of threatened species.






