Introduction
The Alzheimer Society of Canada reported an estimated 597,300 individuals living with dementia in Canada in 2020, and this number is estimated to reach one million individuals in 2030. 1 Unfortunately, many people living with dementia do not receive a timely diagnosis, and reports show high levels of undetected dementia globally. Reference Burke and Goldfarb2,Reference Lang, Clifford and Wei3 Today, dementia costs the Canadian economy and healthcare system an estimated $10.4 billion annually, and these costs are expected to continue to grow with the aging population. Reference Manuel, Garner and Finès4
Alzheimer’s disease (AD) is a neurodegenerative disorder that accounts for 60-70% of patients with dementia and is characterized by a decline in memory and cognition. Reference Garcia-Alloza, Subramanian and Thyssen5–Reference McKhann, Knopman and Chertkow7 In Canada, there are currently no approved therapeutics which stop or delay the progression of this debilitating disease. Recently, there has been progress in monoclonal antibodies (mAbs) that target amyloid-β (Aβ), which have shown promising results in the earlier stages of the disease. Reference van Dyck, Swanson and Aisen8,Reference Sims, Zimmer and Evans9 However, the arrival of a disease-modifying therapy (DMT) for AD will most likely overwhelm the Canadian healthcare system, as the influx of patients seeking a diagnosis and potential treatment would put a strain on already-limited healthcare capacity. Recent analyses show that Canada would have the longest and most persistent wait times for a DMT for AD among the G7 countries. Reference Mattke and Wang10 There is a clear need to assess and address barriers in the Canadian healthcare system that are delaying early diagnosis and treatment of AD, prior to the potential arrival of DMTs.
A group of Canadian stakeholders with expertise in dementia and other neurological illness convened in the Fall of 2022 to discuss ways in which models of care for AD would need to evolve ahead of the introduction of DMTs. There were three regional forums which took place in Calgary, Toronto, and Quebec City, comprised of a multidisciplinary group referred to here as the “Canadian Dementia Expert Group.” The objectives were to prioritize key barriers in the AD patient pathway, understand how other existing healthcare models could be applied to address such barriers, and design solutions to evolve the care pathway for new innovations, including potential DMTs and novel biomarkers.
An important aspect of introducing potential Alzheimer’s DMTs is the healthcare system cost associated with these treatments. This was explored by the Institute for Clinical and Economic Review’s California Technology Assessment Forum, utilizing drug pricing in the United States. Reference Wright, Lin and Whittington11 At this time, Canada-specific drug pricing information is not yet available. One analysis showed potential cost recovery to the Canadian healthcare system through the use of DMTs, via reduced long-term care usage. Reference Jun, Shi and Mattke12 Ultimately, a true pharmaco-economic analysis, relevant to Canada, will be essential. However, this was beyond the capability of our Canadian Dementia Expert Group.
Here, we aim to review the recommendations from the Canadian Dementia Expert Group, focusing on the rationale behind early detection and diagnosis of AD, the barriers in the Canadian healthcare system which prevent access to timely biomarker testing, and recommendations to facilitate safe and appropriate access to Alzheimer’s DMTs, if they are approved for use by Health Canada.
The AD Continuum & Pathophysiology
AD is defined biologically as the presence of two major hallmarks: extracellular amyloid-β (Aβ) plaque deposition and intracellular neurofibrillary tangles (NFT). Reference Jack, Bennett and Blennow13,Reference Pinheiro and Faustino14 Inflammation, oxidative stress, disruption of intracellular nutrient transport, synaptic loss, and neuronal degeneration accompany accumulation of Aβ and NFT. These neurobiological changes lead to the onset of clinical symptoms, which can include changes in short-term memory, language, general cognition, mood, and behavior. Reference Pinheiro and Faustino14,Reference Ghahremani, Wang and Chen15 While the precise sequence of events remains unknown, it is believed that extracellular Aβ plaque deposition occurs prior to intracellular NFT formation. Reference Ghahremani, Wang and Chen15 Importantly, data suggest that Aβ deposition alone is not sufficient to cause cognitive deterioration directly, though its abundance is directly correlated with the extent of cognitive decline. Reference Jack, Bennett and Blennow13,Reference Resnick, Sojkova and Zhou16
The onset of AD neuropathological changes precedes the emergence of clinical symptoms by an estimated average of 10–20 years. Reference Villemagne, Burnham and Bourgeat17–Reference Braak, Thal, Ghebremedhin and Del Tredici22 Historically, the diagnosis of AD has focused on clinical symptoms. In the mild cognitive impairment (MCI) stage, individuals may experience memory loss and show abnormality on cognitive tests, while daily independence remains generally intact. Reference Aisen, Cummings and Jack23,Reference Jack, Albert and Knopman24 In the dementia stage, memory and other cognitive symptoms become more severe, with individuals requiring assistance performing daily activities. Reference Aisen, Cummings and Jack23,Reference Jack, Albert and Knopman24 The AD dementia stage can further be broken down into mild, moderate, and severe stages depending on the severity of interference with daily activities. Reference McKhann, Knopman and Chertkow7,Reference Jack, Albert and Knopman24
With the emergence of biomarkers that characterize the pathophysiological changes in AD, the National Institute on Aging and the Alzheimer’s Association (NIA-AA) research framework provides a biological definition of AD. Reference Jack, Bennett and Blennow13 The framework includes three general groups of biomarkers: (1) biomarkers of Aβ plaques (labeled “A”) such as cortical amyloid PET ligand binding or low CSF Aβ42; (2) biomarkers of fibrillar tau (labeled “T”) such as cortical tau PET ligand binding or elevated CSF phosphorylated tau (p-tau); and (3) biomarkers of neurodegeneration or neuronal injury (labeled “(N)”) such as CSF total tau (t-tau), CSF neurofilament light protein (NfL), FDG PET hypometabolism, and cortical atrophy on MRI. Reference Jack, Bennett and Blennow13,Reference Zetterberg and Bendlin25 Further, the NIA-AA Clinical Staging system accounts for the AT(N) designation across the clinical cognitive continuum, from normal cognition to subjective cognitive decline, to mild cognitive impairment and syndromic dementia, incorporating potential behavioral change in advance of cognitive decline. Reference Jack, Bennett and Blennow13 Stage 1 describes individuals with biomarker evidence of Alzheimer’s disease who are asymptomatic. Reference Jack, Bennett and Blennow13 Stage 2 describes individuals who have normal performance on objective cognitive tests but have a subjective concern of cognitive decline or neurobehavioral changes. Reference Jack, Bennett and Blennow13 Stage 3 describes individuals who have objective cognitive impairment on testing not severe enough to impact general everyday functioning. Reference Jack, Bennett and Blennow13 Stages 4 through 6 describe progressive loss of function and are characterized as mild, moderate, and severe dementia. Reference Jack, Bennett and Blennow13
It has been proposed that interventions in upstream events (Aβ or tau aggregation) can mitigate downstream deleterious events (synaptic loss or neuronal death), thus slowing development of dementia. Reference Selkoe and Hardy26 While there are therapies available to treat the symptoms of AD, there are currently no Health Canada-approved medications to treat and target the underlying pathology of AD. Promising results have been reported recently using monoclonal antibodies (mAbs) which reduce Aβ levels in early AD (broadly defined as MCI or mild AD dementia, or NIA-AA Clinical Stages 3 or 4), Reference Jack, Bennett and Blennow13 leading to United States Food and Drug Administration approvals of such medications. 27,28 However, the process of introducing these new therapies into Canadian provincial healthcare systems is far from straightforward. As the COVID-19 pandemic has shown, even the most sophisticated healthcare systems can be overwhelmed by a rapid surge in demand for services. The introduction of a potential DMT for early AD may result in a similar development, in which the current healthcare system capacity is insufficient to cope with the expected volume of patients. Reference Mattke and Wang10 Shifts and adaptations of current models of care for AD patients will be required in order for such medications to be accessible to Canadians who are assessed as eligible to receive them.
Facilitating the Future Detection and Management of Early AD
The landscape of AD disease-modifying therapies is constantly evolving. If approved, such therapies will require fundamental changes in the way healthcare is delivered for people living with AD. While there are several compounds on the horizon, our focus is on the most advanced class of medication, monoclonal antibodies (mAbs) targeting Aβ-positive MCI and mild AD dementia. These passive immunotherapies activate microglia to engulf and remove fibrillar Aβ and/or block the aggregation of smaller Aβ species from forming plaques. Reference Avgerinos, Ferrucci and Kapogiannis29 Recently, the anti-Aβ mAbs lecanemab and donanemab have demonstrated efficacy in slowing clinical decline in the MCI and mild dementia stages of biomarker-confirmed AD. Reference van Dyck, Swanson and Aisen8,Reference Sims, Zimmer and Evans9 If approved, extensive medical infrastructure would be required to safely administer antibody therapy either by intravenous infusions or as subcutaneous injections. Reference Gauthier and Rosa-Neto30,Reference Chertkow, Rockwood and Hogan31 Figure 1 conceptualizes a future pathway for early AD diagnosis, assessment for eligibility, and treatment with disease-modifying therapies, based on current clinical trial requirements.
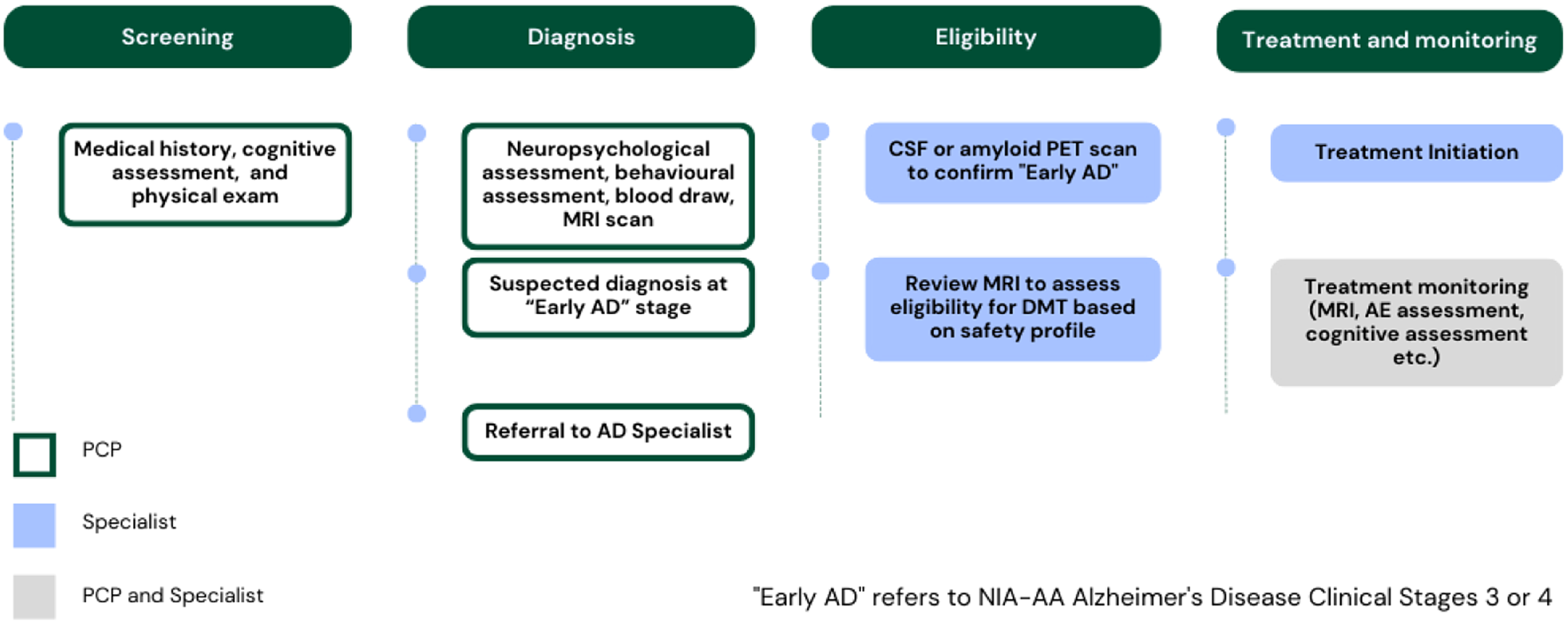
Figure 1: Early AD patient pathway to screen, diagnose, determine eligibility, and initiate DMTs, once available.
In Canada, most care for dementia is currently provided by primary care physicians, but with the introduction of DMTs, there would be a need to shift to specialist-based dementia diagnosis with biomarkers. Reference Chertkow, Rockwood and Hogan31 Anti-Aβ mAbs require the evaluation of Aβ status prior to administration, either by amyloid PET scan or by lumbar puncture to analyze CSF for Aβ, total tau, and phospho-tau. Reference Chertkow, Rockwood and Hogan31–Reference Cummings, Apostolova and Rabinovici33 While there are many advancements underway developing blood/plasma Aβ and tau biomarkers, they are not ready for clinical use. Reference Chertkow, Rockwood and Hogan31,Reference Smirnov, Ashton and Blennow34
A major potential side effect of treatment with anti-Aβ mAbs is amyloid-related imaging abnormalities (ARIA). Reference Cummings, Aisen, Apostolova, Atri, Salloway and Weiner32,Reference Cummings, Apostolova and Rabinovici33,Reference Sperling, Jack and Black35 Since anti-Aβ mAbs target removal of Aβ from both the parenchyma and cerebral vasculature, vessels with preexisting Aβ vascular pathology might become transiently more susceptible to leakage of vascular contents. Reference Sperling, Jack and Black35 This pathology results in ARIA-E (edema) if the leakage consists of proteinaceous fluid, and ARIA-H (hemorrhage) if the leakage consists of blood products. Reference Sperling, Jack and Black35 Should patients decide to undergo treatment with an anti-Aβ mAb, a baseline pre-treatment MRI would be required to ensure there is no elevated risk of ARIA due to existing multiple microbleeds prior to treatment. Reference Cummings, Aisen, Apostolova, Atri, Salloway and Weiner32,Reference Cummings, Apostolova and Rabinovici33 Once on treatment, monitoring with additional MRIs would be required, especially during the initial stages of treatment, as ARIA-E and ARIA-H typically occur early in the course of treatment. Reference Barakos, Purcell and Suhy36 Clinical trial protocols require up to four MRIs in the first year of treatment, a significant burden on healthcare resources. Reference Cummings, Aisen, Apostolova, Atri, Salloway and Weiner32,Reference Cummings, Apostolova and Rabinovici33,Reference Ostrowitzki, Lasser and Dorflinger37 Should a patient develop ARIA, mAbs therapy would be interrupted, and additional MRIs would be required. Reference Cummings, Aisen, Apostolova, Atri, Salloway and Weiner32,Reference Cummings, Apostolova and Rabinovici33 In clinical trials, treatment often resumed once ARIA resolved. Reference Cummings, Aisen, Apostolova, Atri, Salloway and Weiner32,Reference Cummings, Apostolova and Rabinovici33
Overall, substantial healthcare resources and coordination between multiple stakeholders will be required to realize future care pathways with DMTs. Several models have shown that the Canadian healthcare system is not equipped to manage the influx of patients and the requirement for diagnosis, eligibility determination for DMTs, and safety monitoring. Reference Mattke and Wang10,Reference Liu, Hlavka and Coulter38
Canadian Dementia Expert Group Recommendations
Multiple groups of experts involved in dementia and other neurological care gathered in 2022 to discuss the future needs of the healthcare system and ways to create change ahead of potential DMT approvals. Forums took place in Western Canada (Calgary, AB), Ontario (Toronto, ON), and Quebec (Quebec City, QC) with a total of 6 meeting chairs and 30 participants. 18 Canadian cities were represented across the participants, with a balance of males and females, and healthcare professionals from academic and community practice settings. The objectives of the meetings were to (1) understand and prioritize key barriers in the current patient pathway that would affect the ability to treat patients with potential DMTs; (2) understand how other existing clinic models, including those used in multiple sclerosis (MS), could be applied to address key barriers in AD; and (3) assess how to best design optimal care pathways and implement these models in the future. The expert group was composed of AD specialists (neurologists, geriatricians, psychiatrists), primary care providers (including general practitioners and nurse practitioners) with experience in AD care, AD biomarker experts, neuroradiologists, MS specialists, and dementia government policy experts.
Across the three regions in Canada, participants overwhelmingly identified similar barriers within the current AD care pathway, and there was alignment that addressing barriers within the diagnostic and treatment initiation stage of the pathway would be needed (Table 1). The groups prioritized the need to support the timely identification of early AD, which includes increasing capacity of primary care, and addressing general public stigma toward AD. All groups also felt that access to resources to confirm DMT eligibility (amyloid PET, CSF biomarkers, specialists) would only worsen if a DMT was approved. Currently, amyloid PET is limited to research settings and is very costly. CSF testing for amyloid status is available for clinical use and reimbursed by some provinces, but often through complicated mechanisms. For example, in the province of Quebec, CSF testing for Aβ is reimbursed using a process called “Authorization for Medical Biology Services Not Available in Quebec” which requires the signature of two physicians, including one that is a medical biochemist designated by the Health Ministry. 39 Historically, hospitals in Canada have sent CSF samples to laboratories outside of Canada, not only resulting in a delay in diagnosis but adversely impacting a critical window of opportunity for treatment, clinical trials, and other supportive services for patients and their families. In an effort to demonstrate the benefit of providing direct access to CSF biomarker testing to providers in Canada, St Paul’s Hospital in Vancouver, Canada launched the National Alzheimer’s Disease Biomarker Testing Program in 2021, through a special access designation. 40 While CSF testing is now available across Canada through this program, capacity investment levels and differing reimbursement pathways from province to province continue to create barriers to equitable access for all.
Table 1: Summary of key barriers to integrating future AD DMTs into care pathways
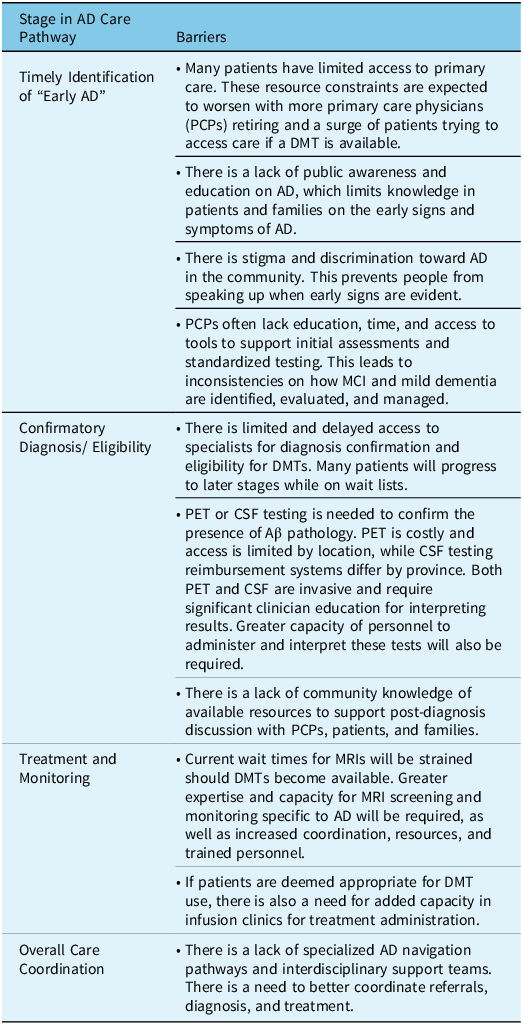
The challenges continue once patients are deemed eligible for treatment with DMTs. MRI monitoring and ongoing infusions or injections would put a strain on specialists, nurses, technologists, and radiologists. MRI wait times were seen as a barrier in all regions. As well, the infrastructure for administering intravenous or subcutaneous DMTs is non-existent within the current AD care pathway. Finally, across all of these barriers, a common challenge identified was the need to better coordinate care across multidisciplinary teams.
In order to overcome these challenges, the Canadian Dementia Expert Group prioritized six short-term recommendations (Table 2). The majority of the recommendations were consistent and applicable in all three regions. Recommendations were prioritized as highly impactful if they could be implemented in the foreseeable future, and have a triple effect on patients, caregivers, and the healthcare system.
Table 2: Summary of recommended short-term recommendations to improve AD models of care in Canada
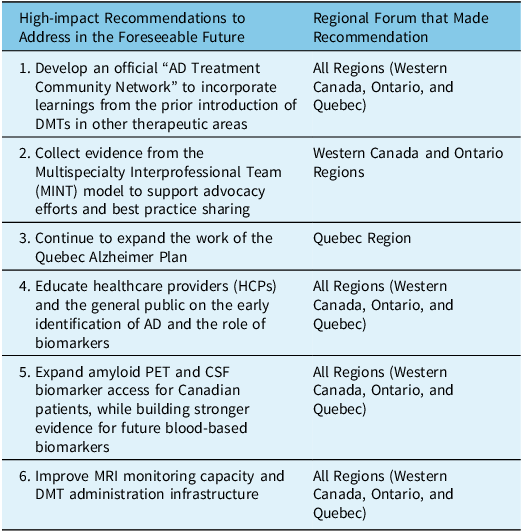
Recommendation to Develop an Official “AD Treatment Community Network” to Incorporate Learnings From the Prior Introduction of DMTs in Other Therapeutic Areas
The Canadian Dementia Expert Group included multiple sclerosis (MS) specialists and nurses who shared their experience introducing some of the first MS DMTs into their clinic models. The exchange between disease areas in these forums provided strong insights and parallels that could be leveraged to inform change in the healthcare system. Developing an “AD Treatment Community Network” would be helpful nationally to promote discussion about introducing AD DMTs if available, while incorporating learning from other fields. Conferences and other forums would be helpful in connecting clinicians and patient advocacy groups within these areas, to work together to create change in the model of care.
There are a number of insights gained by looking at the MS multidisciplinary clinic model. The first therapy proven to be effective in altering the natural history of relapsing-remitting MS was in 1993 (i.e. interferons). Reference Murray41 Today, there are over 20 DMTs for patients with MS. 42 Parallels can be drawn between MS treatment in the early 1990s and AD treatment today. However, the scale of AD in the aging population is magnitudes greater than the population affected by MS, which is a limitation of our analysis.
MS stakeholders noted that it was critical that they quickly established and funded MS multidisciplinary clinics, which partnered with radiologists, specialized nurses (to support infusions and injections), social workers, occupational therapists, drug navigators, lab medicine specialists, and other ancillary resources. Radiology partnerships enabled clinics to secure ongoing access to MRI in MS. Specialized nurses developed extensive knowledge of MS pathology, diagnosis, symptom management, and treatment, contributing to therapeutic decisions, side effect management, and therapeutic response monitoring. Nurses also help manage patient expectations and play a key role in patient education. Specialized MS clinics were able to build evidence on improved health outcomes (e.g. slowed disease progression, improved quality of life) and cost savings of the clinic model. Government and other sources of funding were then approached based on this evidence. National programs were also developed to educate the public and PCPs on understanding the initial symptoms of MS, and why early MS diagnosis is important.
The MS community has built well-established consensus guidelines on the diagnostic criteria for MS, which are continuously updated as new evidence emerges. A similar approach should be taken in AD, especially on biomarker interpretation and MRI use for safety monitoring. Such guidelines should be disseminated nationally, as education of specialists will be important to reduce potential skepticism surrounding early AD diagnosis and treatment.
As noted, strategies employed in creating MS clinics across Canada may need to be scaled up significantly in AD, as the prevalence of AD is about six times that of MS. 1,Reference Gilmour, Ramage-Morin and Wong43 It is also recommended that the AD community seek open dialogue with clinicians in other therapeutic areas that implement DMTs, such as cancer care, wherein patients often require regular MRI and PET scans to assess their initial eligibility for treatment and to monitor disease response. Developing systems of care for patients with early AD modeled to include the robust elements of integrated provincial cancer care systems would enhance provincial and thus national dementia care overall.
Recommendation to Collect Evidence from the MINT Model to Support Advocacy Efforts and Best Practice Sharing
Currently, referral numbers to AD specialists are high, as some PCPs may be unfamiliar with identification and assessment of cognitive symptoms, and initiation of standard-of-care symptomatic treatments. Reference Chodosh, Peitti and Elliott44–Reference Prins, Hemke, Pols and Moll van Charante46 In Canada, where there is a shortage of AD specialists, national guidelines emphasize that dementia care should be centered in primary care. 47–Reference Ismail, Black and Camicioli51 However, studies have shown this can be challenging, with many individuals with dementia going undiagnosed in the community. Reference Lang, Clifford and Wei3 Multispecialty Interprofessional Team (MINT) memory clinics across Canada offer multidisciplinary dementia care provided by primary care teams who have received standardized nationally accredited training. There are over 100 MINT sites in Canada, and the success of this model is based on collaboration between primary care, dementia specialist care, and community agencies. Reference Lee and Hillier52 This model, or one similar, could improve patient access to diagnostic tests, specialists, and follow-up resources.
The Canadian Dementia Expert Group noted that the economic benefit of the MINT model has been independently evaluated, with published data demonstrating MINT clinics to be less expensive, while improving quality of life as compared to usual care. Reference Wong, Lee and Walker53 Several other studies have demonstrated high levels of patient and caregiver satisfaction within MINT clinics, improved care through partnerships between MINT clinics and the Alzheimer Society, high levels of healthcare provider satisfaction, and substantial capacity-building for dementia care within primary care. Reference Lee, Slonim and Hillier54–Reference Lee, Hillier and Heckman58 Although this model can create local capacity, a major limitation is that sufficient funding may not be available to allow all regions to adopt this model. The reliance on multidisciplinary care may also be challenging to implement when there is already-limited access to primary care resources across Canada. 59
In the short term, learnings and best practices from multidisciplinary models such as MINT memory clinics can be shared with primary, specialty, and community care in order to facilitate early diagnosis, which is central to appropriate DMT use.
Recommendation to Expand the Work of the Quebec Alzheimer Plan
Experts in the Quebec region recommended leveraging the already-established model of care known as the Quebec Alzheimer Plan, to continue to build capacity for AD diagnosis and treatment. Reference Arsenault-Lapierre, Godard-Sebillotte and Sourial60 PCPs in Quebec work within multidisciplinary teams called Family Medicine Groups (FMGs), with access to nurses, social workers, and occasionally occupational therapists. The interdisciplinary model has been endorsed by the government of Quebec with two protocols available for AD: (1) diagnosis; and (2) post-diagnostic care. 61 Under the Plan, the province of Quebec has designated primary care as responsible for detecting MCI and early Alzheimer’s disease, and within this model, nurses are empowered and trained to administer cognitive tests, reserving the more complex patients for tertiary care. This is very much in line with national dementia guidelines which emphasize that dementia care should be centered in primary care. 47–Reference Ismail, Black and Camicioli51 Not only has this structure been found to increase the quality of care provided to patients with dementia, but physicians have also had increased confidence in their competence to diagnose and manage dementia. Reference Vedel, Sourial, Arsenault-Lapierre, Godard-Sebillotte and Bergman62,Reference Vedel and Couturier63
While there is a strong level of support for the Quebec Alzheimer Plan already, the Quebec Dementia Expert Group recommended continued investment in the Plan’s Phase III implementation. 64,65 Appropriate levels of investment and resources should be made available to enhance support for timely diagnosis and care. A central recommendation was to continue to elevate the role of nurse practitioners in Quebec, as they can play a pivotal role in supporting earlier diagnosis. Additionally, the group recommended implementing a navigator model within FMGs (ensuring that patients and caregivers are supported throughout their journey, including moving between primary and specialty care), and assigning both an HCP and nurse champion to each team.
As part of Phase III of the Quebec Alzheimer Plan, strengthening of secondary and tertiary memory clinics will be essential, as these clinics will assist with interpretation of biomarker testing, confirmation of early AD diagnosis, and administration and monitoring of DMTs alongside PCPs and FMGs. 64,65 They will also be involved in training and education at all levels, including networking between medical specialties (imaging, laboratory, and clinical) around AD diagnosis and DMT use. The clinical and functional parameters of these memory clinics should be made official, and their status recognized.
There is a greater need for education of all stakeholders involved in the Quebec Alzheimer Plan. Education can be provided on disease awareness and diagnosis through FMGs, in partnership with academic and government bodies. There is also a need to evolve payment models, as the current system creates a disincentive to spending more time with patients during a cognitive assessment.
Recommendation to Educate HCPs and the General Public On the Early Identification of AD and Biomarkers
The Canadian Dementia Expert Group determined that a significant barrier is the lack of primary care education on using available tools to identify MCI and mild dementia. Reference Vedel, Sourial, Arsenault-Lapierre, Godard-Sebillotte and Bergman62 Recommendations were made for primary care to use scales to measure cognition, behavior, and function, administered to both the patient and care partner. Reference Tang‐Wai, Smith and Bruneau66 Early detection fundamentally requires family member and friend participation, as ignoring them is a frequent cause of delayed diagnosis. Reference Briggs and O'Neill67 Addressing this barrier through education would increase primary care confidence in identifying which patients could potentially benefit from DMTs, and optimizing outcomes for those who are not eligible.
For specialized healthcare practitioners including neurologists, geriatricians, geriatric psychiatrists, and laboratory medicine specialists, education should focus on the utility and interpretation of PET and CSF biomarkers. This knowledge will be essential in managing the eligibility requirements for DMTs in a healthcare system with strained diagnostic resources. Education will need to be provided for radiologists on how to assess MRI scans for amyloid-related imaging abnormalities (ARIA). New educational initiatives could be accredited by the College of Family Physicians of Canada and the Royal College of Physicians and Surgeons of Canada, and disseminated in partnership with the Alzheimer Society of Canada and other local agencies, which can further advocate for government investment in healthcare practitioner education.
A recommendation was made to increase public education around the differences between healthy aging and mild cognitive impairment. Through partnership with patient groups such as the Alzheimer Society, social media campaigns were recommended as a potential means to educate the public and combat stigma. Testimonials from celebrities, patients with lived experience, and caregivers could be leveraged for such campaigns. Reducing public stigma tied to cognitive impairment should lead to earlier consultations with a healthcare provider.
Potential approval of DMTs for AD will lead to more people seeking opinions on whether they are eligible for treatment, or even if they are at risk for cognitive decline. Equipping primary care teams and other physicians with the knowledge to assess individuals at risk, how to communicate that risk, and how to provide prevention and/or cognitive enhancement strategies will be important in the future model of care. International roadmaps are already available on how to improve brain health services and implement models of dementia prevention. Reference Frisoni, Altomare and Ribaldi68
Recommendation to Expand Amyloid PET and CSF Biomarker Access for Canadian Patients, While Building Stronger Evidence for Future Blood-Based Biomarkers
Access to amyloid PET and CSF biomarker analyses was highlighted as a major barrier to overcome prior to the potential arrival of AD DMTs. There is a need to optimize access to existing resources, as well as secure funding for expansion. Contact should be made with nuclear medicine sites across the country, to gauge current PET capacity. Lumbar punctures for CSF analysis are scalable, less expensive, and more accessible than PET scans. Lumbar puncture has been demonstrated to be safe and effective in patients with AD, and given the demonstration of high diagnostic accuracy of measurement of Aβ and tau proteoforms in CSF, this technology should be used more widely in memory clinics. Reference Stiffel, Bergeron and Mourabit Amari69 Increasing ease of access to CSF testing for Aβ and tau through provincial reimbursement pathways, and increasing capacity to perform lumbar punctures, will help ensure patients who are eligible for DMTs can swiftly receive them.
Blood-based biomarkers may become an effective screening tool in primary care given the less-invasive specimen collection requirements. In the future, blood-based biomarkers may even replace PET and CSF for confirmatory diagnosis of Alzheimer’s disease, which will aid in the determination of DMT eligibility. Currently, there are no Health Canada-approved blood-based biomarker products available, which in part reflects the need to collect more evidence to establish their validity in the diagnosis of AD. The Canadian Dementia Expert Group recommended a focused effort to build further evidence on blood-based biomarkers through research groups and industry partnerships in Canada. As new data become available, the NIA-AA Research Framework and Clinical Staging should be leveraged to review this information and assess how to best incorporate blood-based biomarkers into the future AD care pathway. Reference Jack, Bennett and Blennow13
A first pan-European workflow for biomarker use in “middle-old age” to diagnose AD at the MCI to mild dementia stage has been proposed. Reference Festari, Massa and Ramusino70 Frailty and comorbidities, rather than age alone, may be more important future considerations when interpreting limited existing biomarker data in older individuals.
ApoE genetic testing has been proposed as an integral aspect of risk stratification for possible amyloid-related imaging abnormalities (ARIA) associated with DMT use. Reference Cummings, Apostolova and Rabinovici33 ApoE genotyping capacity should be surveyed across the country, to assess current access. There is a need to create and distribute standardized ARIA risk charts for each possible ApoE genotype, to assist AD specialists in interpreting results to patients, as there are insufficient dedicated genetic counselors to address this discussion in all cases.
Recommendation to Improve MRI Monitoring Capacity and DMT Administration Infrastructure
The availability of MRI will be a critical rate-limiting step for diagnosis, treatment eligibility, and safety monitoring of DMT use. The Canadian Dementia Expert Group recommended convening a forum in collaboration with neuroradiologists nationwide, to determine standardized sequencing protocols for MRI in dementia care, especially relating to monitoring and management of ARIA. Clinician education on the detection and management of ARIA will also be required, to enable technologists, neuroradiologists, and clinicians to align on key requirements across centers in Canada.
The neuroimaging forum suggested above could also explore how to optimize MRI access, beyond simply purchasing more scanners. Recommendations may include creating shorter MRI time slots, providing pre-filled MRI requisition forms, and creating automatic future booking systems, to help centers forecast workload. Fostering ongoing relationships with neuroradiologists across the country will be essential to accommodate the increased patient volume if DMTs become available.
If intravenous DMTs are approved in Canada, there will be a need to expand intravenous infusion clinic capacity to allow for more widespread administration of DMTs. Specialty care and memory clinics will need to evolve to coordinate care and enable more frequent visits for drug administration, monitoring, and counseling. Multiple Sclerosis (MS) clinic models can offer guidance on how this infrastructure can be created. Community-based intravenous administration clinic capacity may also be leveraged for AD care.
Conclusion
The Canadian healthcare system is not prepared for the potential arrival of Alzheimer’s disease-modifying therapies (DMTs), as it currently cannot accommodate the need for early diagnosis, biomarker testing, and MRI monitoring that is essential for treatment with these agents. These challenges will only grow based on the increase in the number of people living with AD and existing barriers in the current care pathway. The Canadian Dementia Expert Group recommendations outlined in this review begin a discussion to support timely access to DMTs for eligible patients, if such agents are approved for use by Health Canada. These recommendations are aligned with priorities set out in both the Canadian National Dementia Strategy and the Quebec Alzheimer Plan and focus on awareness, education, and quality of care. Reference Arsenault-Lapierre, Godard-Sebillotte and Sourial60,71 Ultimately, there is great need for provincial healthcare systems to prioritize dementia care and support infrastructure development. In the anticipated era of disease-modifying therapies, all stakeholders must be ready to work collaboratively with persons living with AD and their families, to enact change in the AD model of care.
Acknowledgments
The authors thank the participants of the Canadian Dementia Expert Group for their contributions to the recommendations provided in this review. Participants included:
Western Canada Forum: Dr Alexandre Henri-Bhargava, Dr Sheny Khera, Dr Michael Yeung, Fred Horne, Dr Mari L. DeMarco, George Andrews, Margaret Prociuk, Dr Oliver David, Karyn Fischer
Ontario Forum: Dr Mark Freedman, Dr Sandra Black, Dr Linda Lee, Dr Amer M. Burhan, Dr Deborah Norrie, Dr Sharon Cohen, Jennifer Whitlock, Kyle Fitzgerald, Dr Daniel Wong, Dr Donald Lee
Quebec Forum: Dr Pierre Duquette, Julie Brunet, Dr Claude Patry, Karoline-Mona Demers, Josée Poirier, Sylvie Grenier, Dr Pedro Rosa-Neto, Dr Guy Lacombe, Dr Christian Vinette, David Levine, Claire Webster
Funding
This manuscript and the Canadian Dementia Expert Group forums were funded by F. Hoffmann-La Roche Ltd (Mississauga, ON, Canada).
Competing interests
Dr Andrew Frank has received support for this manuscript and the Canadian Dementia Expert Group forums, as noted above.
Dr Michael Borrie has received funding/grants to his institution from MITNEC-C6, CIHR Institute on Aging for the Canadian Collaboration on Neurodegeneration in Aging, NIH Alzheimer Disease Neuroimaging Initiative, Biogen, Alector, Eisai, Abbvie, and Eli Lilly. He has received consultancy fees to his institution from Biogen and Hoffmann-La Roche Ltd He has received support for this manuscript and the Canadian Dementia Expert Group forums, as noted above.
Dr Serge Gauthier is a board member of the Sharon Francis Foundation. He has served on advisory boards/consultancies for ENIGMA US, AmyriAD, and TauRx. He has received honoraria from Lundbeck Korea, Eli Lilly Canada, and Eisai Canada. He has received support for this manuscript and the Canadian Dementia Expert Group forums, as noted above.
Dr Ging-Yuek Robin Hsiung is the current president of the Consortium of Canadian Centres for Clinical Cognitive Research (C5R). He has acted as Site Investigator in clinical trials in Alzheimer’s Disease and was paid accordingly by Biogen, Cassava, and Eli Lilly. He has received consulting fees from Biogen, NovoNordisk, and Hoffmann-La Roche Ltd He has received support for this manuscript and the Canadian Dementia Expert Group forums, as noted above.
Dr Zahinoor Ismail is funded by the Alzheimer’s Drug Development Foundation, Brain Canada, CCNA, CIHR, Gordie Howe CARES, NIA, and Weston Foundation. He has served on advisory boards/consultancies for Acadia, Biogen, Lundbeck, Otsuka, Hoffmann-La Roche Ltd., and the Canadian Agencies for Drugs and Technologies in Health, and is co-chair of the Government of Canada Ministerial Advisory Board for Dementia. He has received honoraria from Lundbeck/Otsuka and his institution has received fees from Acadia, Biogen, and Hoffmann-La Roche Ltd He has received support for this manuscript and the Canadian Dementia Expert Group forums, as noted above.
Dr Louis Verret has acted as Principal Investigator in clinical trials in Alzheimer’s Disease and was accordingly paid by Hoffmann-La Roche Ltd., Biogen, IntelGenX, and NovoNordisk. He received personal fees for consulting work and advisory boards, speaker bureaus, and lectures from Hoffmann-La Roche Ltd and Biogen. He has received support for this manuscript and the Canadian Dementia Expert Group forums, as noted above.
Melanie Wilson is employed by Hoffmann-La Roche Ltd (Mississauga, ON, Canada).
Statement of authorship
AF, MB, SG, RH, ZI, LV, and MW contributed substantially to the review conception and strategy. AF, MB, SG, RH, ZI, LV, and MW were responsible for the critical review of the manuscript outline and draft reports, and all approved the final version for submission.





