INTRODUCTION
The National Immunization Programme (NIP) in The Netherlands is an effective programme with a national vaccination coverage rate of about 95% [Reference Lier1], inducing a high level of immunization protecting the population against outbreaks of infectious diseases. Through the voluntary and free-of-charge NIP, infants and young children are currently offered vaccination against diphtheria, pertussis, tetanus, poliomyelitis, Haemophilus influenzae type B, mumps, measles, rubella, pneumococcal disease, meningococcal group C disease and hepatitis B (risk groups only). Most of these infectious diseases have become very rare in The Netherlands. However, outbreaks of poliomyelitis in 1992–1993 [Reference Oostvogel2], measles in 1999–2000 [Reference Hof3], rubella in 2004–2005 [Reference Hanhé4] and mumps in 2007 [Reference Karagiannis5] have occurred in low vaccination coverage regions (LVR). In these regions, part of the population rejects participation in the NIP for religious reasons, and as a result some infectious diseases have not yet been eliminated. These regions represent an important obstacle in the otherwise successful control of vaccine-preventable infectious diseases in The Netherlands.
Rubella vaccination for girls aged 11 years was introduced in the NIP in 1974. In 1987, it was replaced by universal vaccination using a mumps-measles-rubella (MMR) combination vaccine offered at ages 14 months and 9 years. Even though rubella is a mild infection in children, vaccination is of great public health importance since infection during pregnancy can lead to miscarriage and congenital rubella syndrome (CRS) [Reference Banatvala and Brown6]. The risk of complications is highest when the woman is infected during the first half of the pregnancy [Reference Banatvala and Brown6]. Screening for absence of rubella antigens provides the possibility of offering postpartum vaccination against rubella to women who lack protection, i.e. present a negative serology test (seronegative). Postpartum vaccination would protect the foetus in a subsequent pregnancy. Vaccination during pregnancy with the live attenuated vaccine is not advisable, although no complications have been reported in inadvertently vaccinated pregnant women [Reference Banatvala and Brown6].
Immigrant women originating from a non-Western country may represent another possible risk group in the event of an epidemic in The Netherlands; in several studies the seroprevalence of rubella was found to be lower in this group compared to pregnant women in the general Dutch population [Reference Buiker and Schout8–Reference Van der Zwan14] (L. Mollema, personal communication).
All pregnant women in The Netherlands are offered antenatal screening for hepatitis B, HIV, syphilis, rhesus-D factor, irregular antibodies, haemoglobin status and determination of blood group. Screening for rubella antibodies has been recommended for immigrant women and women living in LVR [Reference Elsacker15]. However, this recommendation was not supported by an economic evaluation and rubella screening was not included in the antenatal screening programme, thus it is not standard practice in routine midwifery. In contrast, antenatal screening for rubella antibodies is routinely carried out in other European countries, including Germany [Reference Best, Enders, Banatvala and Peckham16] and France [Reference Lévy-Bruhl, Six and Parent17], whereas in Italy [Reference Ciofi degli Atti18] and the UK [Reference Best, Enders, Banatvala and Peckham16] it is recommended. A successful screening and postpartum vaccination programme may help to prevent rubella during pregnancy, reducing the risk of babies being born with CRS. The cost-effectiveness of such a programme is one component of an evidence-based decision and provides important input in public health policy.
The current study presents a cost-utility analysis of a screening and vaccination programme for rubella in pregnant women in The Netherlands, using recent data from the 2004–2005 outbreak. We analysed three different scenarios: (1) screening non-vaccinated pregnant women in LVR; (2) screening all pregnant women in LVR; and (3) screening non-vaccinated pregnant women throughout The Netherlands.
METHODS
We estimated cost-utility ratios of screening pregnant women for rubella antibodies, with subsequent postpartum vaccination of seronegative women to avoid rubella in later pregnancies. All infants born with one or more defect (defects of the CNS, hearing defect, heart defect) from women with laboratory-confirmed rubella infection during pregnancy observed during the 2004–2005 epidemic in The Netherlands were included, together with two cases of fetal death due to rubella infectionFootnote † [Reference Hanhé4].
The cost for a screening and vaccination programme was weighted against healthcare costs saved and quality-adjusted life-years (QALYs) gained by prevention of rubella infection in pregnancy. If one scenario turned out to be more costly but also more effective in terms of QALYs gained, an incremental cost-utility ratio was calculated, dividing incremental costs by incremental QALYs gained. The resulting ratio shows the cost per extra QALY gained if a more effective, but more costly scenario was chosen. Costs were discounted using a rate of 4% and life-years gained using a rate of 1·5% in accordance with the Dutch guidelines for pharmacoeconomic research [19]. The analyses were made from a healthcare-related costs perspective. Costs were expressed in euros (€), price level 2007.
Scenarios
The three scenarios consisted each of subgroups representing different groups of women with distinct characteristics regarding rubella status and willingness to accept screening and vaccination. These characteristics determine the number of screenings offered, the costs of screening and vaccination, as well as the number of preventable complications of rubella in pregnancy.
We investigated three possible scenarios for screening: (1) screening all non-vaccinated pregnant women in LVR (non-vaccinated LVR), (2) screening all pregnant women in LVR (all LVR); and (3) screening all non-vaccinated pregnant women in The Netherlands, including pregnant, first-generation, non-Western immigrant women (non-vaccinated NL). We defined LVR as municipalities where >5% of voters voted for the Orthodox Reformed party (SGP) in the 2006 elections using information retrieved from national statistics [20] (Fig. 1a). SGP is an orthodox Calvinist political party that bases its point of view on the bible and represents orthodox protestant groups including some who refrain from vaccination. Following this criteria, 60 of the ~460 Dutch municipalities met the definition of a LVR. The reason for choosing this criteria and not, for instance, municipalities with the highest proportion of unvaccinated individuals, was that epidemics of vaccine-preventable diseases in The Netherlands are often largely confined to orthodox protestant communities, which are not protected by herd immunity of the general Dutch population due to their social and geographical clustering. In general, municipalities with low vaccination coverage overlap with those with a high percentage of religious voters.

Fig. 1. (a) Geographical representation of voters on Orthodox Reformed party (SGP) in 2006; (b) geographical dispersion of rubella infection in The Netherlands, epidemic 2004–2005. There was statistically significant positive relation between the proportion of SGP votes and number of rubella cases (over-dispersed Poisson regression). Result regression, see Appendix, Table A1. [Source: Volksgezondheid Toekomst Verkenning, Nationale Atlas Volksgezondheid. Bilthoven: RIVM, Nationale Atlas Volksgezondheid (http://www.zorgatlas.nl), version 3.15, 25 September 2008.]
Table A1. Association between Orthodox Reformed party (SGP) voters and number of rubella cases in the municipalities

CI, Confidence interval.
The categories represent percentage SGP voters in municipality (Fig. 1a, b). Relative rates are estimated using Poisson regression with over dispersion.
The 2004–2005 epidemic
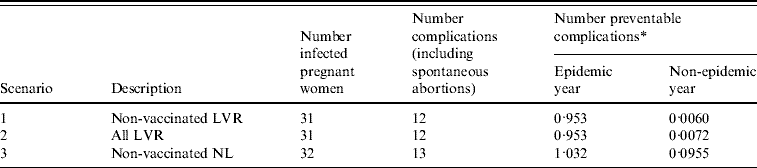
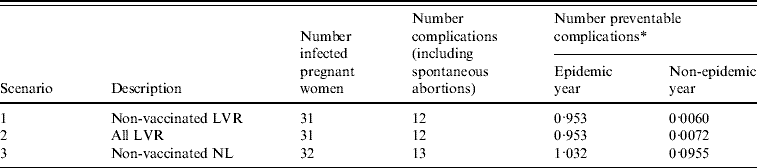
During the epidemic there were 32 pregnant women notified with rubella infection [Reference Hanhé4]. Thirty-one of these women were living in LVR, one was living outside these regions. All women belonged to a group who were not vaccinated on religious grounds (Fig. 1b). Of these 32 women, two suffered spontaneous abortions and 11 infants were born with defects associated with congenital rubella. Of 25 women with known parity, for 44% (11 women) it was not their first pregnancy.
Time perspective of the economic analysis
By means of a screening and vaccination programme rubella complications could be averted in the event of an epidemic, and to a lesser degree, in the years between epidemics. The probability of an outbreak occurring is, however, difficult to predict. The two most recent rubella outbreaks in The Netherlands occurred within an 8-year interval. We used this to form an assumption about the frequency of outbreaks and thus estimated the cost-effectiveness of a screening and vaccination programme (beginning in year 0) for a time span of 16 years during which a rubella outbreak occurred twice, during years 7 and 15, respectively.
However, not all complications occurring during an epidemic or non-epidemic year would be prevented if screening and vaccination were offered. The willingness to accept screening and vaccination, which differs per target group, together with the effect of screening and vaccine efficacy, partly explains why not all rubella complications would be prevented. Furthermore, since pre-conception advice or screening is not regularly provided, rubella complications in a first pregnancy would also not be prevented. The number of prevented complications in a non-epidemic year was adjusted for the probability that a complication would arise in the target group and the chance that a pregnant woman belongs to that specific target group.
Screening programme
The cost of a screening and vaccination programme depends on the number of eligible women, i.e. number of (non-vaccinated) pregnant women and their willingness to accept screening and/or vaccination. The percentage of non-vaccinated women in each scenario was estimated as 1 minus the average vaccination coverage for the second dose of MMR based on the 1994 birth cohort. In the municipalities included in LVR, vaccination coverage was on average 90·2%, while outside LVR it was on average 98·7%, resulting in 9·8% and 1·3% non-vaccinated women in the respective target groups [Reference Lier1]. It was further assumed that all first-generation non-Western immigrant women were not vaccinated. The number of pregnant women that would be offered screening in the different (sub)groups was estimated as the number of liveborn infants multiplied by the percentage of women for whom it was the first pregnancy, using information gathered from national statistics [21] (Table 1). In addition, we included 20% of first-generation immigrant women who were on at least their second pregnancy [21]. This was based on the assumption that the first pregnancy of 80% of these women had been handled in The Netherlands, therefore these women would have already been offered participation in the programme (Table 1).
Table 1. Number of screenings offered and accepted, and number of accepted vaccinations, per subgroup and total per scenario (willingness to accept screening is 95% in all groups)

LVR, Low vaccination coverage regions; NL, Netherlands.
* Seroprevalence 11% (95% CI 6–19) [Reference Ruijs22].
† Seroprevalence in pregnant women participating in the ‘Amsterdam Born Children and their Development’ (ABCD) study (L. Mollema, personal communication).
‡ Assuming the same seroprevalence in non-vaccinated population in whole of The Netherlands as in vaccinated LVR (97·9, 95% CI 96·9–98·8) [Reference Haas23].
§ Assumed the same for all groups except non-vaccinated women in LVR, which is based on preliminary results, later published in [Reference Elsacker15] [results in [Reference Ruijs22] were 17% (95% CI 2–48)]. The impact of the willingness to vaccinate is investigated in the sensitivity analysis.
In general, only women experiencing their first pregnancy were included, as it was assumed that a woman expecting her next child would have already been offered and accepted or rejected screening and vaccination (assuming no women changed their mind after rejecting vaccination the first time). This was done in order to avoid double-counting screening costs in a programme that continues for several years.
Costs of the programme
Programme costs comprise the cost of the screening test and honorarium, together with MMR vaccination for seronegative women that accept postpartum vaccination. Since the screening would take place alongside the antenatal healthcare visits at 12 weeks' gestation, the standard blood sample drawn during this visit would also be used for the rubella screening, therefore no extra costs for such a visit were included:

We assumed that the vaccination would take place during a visit to the general practitioner (GP) and included costs for one such visit. Costs for vaccination were:

The estimated number of screenings offered and numbers of seronegative women can be found in Table 1; unit costs are described in the Appendix.
QALYs gained
A QALY is a combination of a (health-related) quality of life (QoL) weight for a specific health state and the duration of time spent in that health state. Congenital rubella-associated defects are assumed to be permanent, i.e. the duration of time spent in that state is equivalent to the expected life years remaining after birth. QoL weights were taken from non-age-weighted disability weights using the formula:
The disability weights adopted were derived from disability-adjusted life years (DALYs) calculated for the Dutch population [Reference Melse24]. Although based on different methodologies, under the assumptions made here (non-age-weighted and lifelong inferior health states) the QALY and DALY approaches are similar [Reference Sassi25]. The QALYs lost due to congenital rubella complications were estimated as:

QALYs lost due to complications were estimated as a weighted average based on the 2004–2005 outbreak and a QoL weight of 1·0 for a healthy infant. QALYs lost due to rubella-associated fetal death were calculated as a loss of an entire life, using life expectancy at birth in 2005 (78·8 years) and a discount rate of 1·5% (resulting in 46·2 years). Potentially prevented complications in an epidemic were estimated for each subgroup as:

These calculations formed the base for the potentially gained QALYs, estimated as average QALYs lost multiplied by the number of preventable cases (used in Tables 2 and A4), as well as costs saved. (For further details see the Appendix.)
Saved costs
Healthcare costs were calculated as costs per defect summed by the number of defects. The weighted average of the costs for the different defects seen in the epidemic was used as an estimate of costs saved by averting a complication (excluding healthcare costs directly related to fetal death). We included 5 years of saved costs in the base analysis. This is a conservative assumption about cost savings. Based on data from an online database describing cost-of-illness for different diagnoses in The Netherlands, we assumed 45% of costs for congenital complications occur during the first 5 years of life [26]. (For further details of these calculations are given in the Appendix.)
Non-epidemic years
In non-epidemic years, additional costs could be saved and QALYs gained. We assumed that one case of rubella infection in a pregnant woman occurs in each non-epidemic year in The Netherlands, with a probability that 1/3 infections would have led to complications [Reference Hof27]. It was assumed that 25% of these cases occurred in the native Dutch population and 75% in the immigrant population. This assumption was based on different (informal) sources including surveillance data [Reference Hof27].
Sensitivity analyses
Sensitivity analyses (one-way) were performed for the following parameters: willingness to vaccinate, no occurrence of an epidemic within the defined 16-year period, one (instead of 1/3) rubella complications prevented in the inter-epidemic period, <44% of women expecting their second child, postpartum vaccination without antenatal screening, fetal death excluded as a complication of rubella infection, expected remaining life-years 25% lower for a child born with complications and lifelong treatment costs for children with complications.
RESULTS
The number of preventable congenital rubella complications for each scenario is presented in Table 2 together with the potential number of QALYs gained due to the screening and vaccination programme. Preventing a complication of rubella infection during pregnancy would lead to an average of 22·9 QALYs gained (see Appendix, Table A2). The costs for the screening and vaccination programme are a direct consequence of the number of screenings accepted, proportion of seronegative women and number of vaccinations accepted (Table 1). The yearly expected costs of a screening programme are €17 900, €107 800 and €266 600, for scenarios 1, 2 and 3, respectively. Scenario 1 (Non-vaccinated LVR) entails the smallest number of eligible women. Scenario 2 (All LVR) is an extension of the first scenario and scenario 3 (Non-vaccinated NL) involves screening the largest number of women.
The screening and vaccination programme during the 16-year period would be cost-effective if targeted at non-vaccinated women in LVR (€1100/QALY gained) (Table 3), if judged by an unofficial but often quoted limit of €20 000 for preventive interventions in The Netherlands. Based on the same limit, the other two scenarios would not be cost-effective.
Table A2. Number of lost quality-adjusted life-years (QALYs) per defect and average per complication, based on rubella epidemic 2004–2005

QoL, Quality of life.
Table 3. Costs and cost-effectiveness ratios per scenario [price level 2007 (€)], costs discounted 4%, life-years gained 1·5%

QALYs, Quality-adjusted life-years; LVR, Low vaccination coverage regions; NL, Netherlands.
* Equation (1)+equation (2). Number of screenings and vaccinations per target group is given in Table 1, unit costs in Table A5.
† From Table A4.
‡ Number preventable complications in epidemic [equation (5)]×average cost and average QALYs lost per complication, respectively (Tables A2, A3).
§ Net cost=screening & vaccination costs−savings.
Calculating the incremental cost-effectiveness ratio of the extra gain in QALYs by implementing scenario 3 instead of scenario 1, i.e. including all non-vaccinated pregnant women in The Netherlands instead of only non-vaccinated women in LVR would cost €97 700/QALY gained.
We further performed a number of sensitivity analyses for scenario 1, changing some of the assumptions made in the base-case analysis (Fig. 2). In two of the sensitivity analyses the favourable cost-effectiveness ratio became unfavourable: (i) if willingness to accept vaccination by native women in LVR was zero (€112 000/QALY gained) and (ii) if there was no epidemic during the 16-year period (€105 000/QALY gained). Further calculations showed that the cost-effectiveness ratio in scenario 1 was below the cost-effectiveness limit when the willingness to vaccinate was above 4%. This means that if <4% of the non-vaccinated seronegative women accepted vaccination, scenario 1 would not be cost-effective. Postpartum vaccination without screening, whereby all women would be vaccinated regardless of serological status, would also be cost-effective for non-vaccinated women in LVR (€17 000/QALY gained). If the expected remaining life expectancy was lower for an infant born with rubella complications or if the probability of preventing complications was 1 in a non-epidemic year, the cost-effectiveness would be more favourable than in the base case. If <10% of women were on at least their second pregnancy it would not be cost-effective to screen according to scenario 1 (€20 100/QALY gained). If both costs and life-years gained were discounted with the same rate (4%) the cost-effectiveness ratio would change to €2100/QALY gained.

Fig. 2. Sensitivity analysis of scenario 1 [non-vaccinated women in low vaccination coverage regions (LVR)]. CRS, congenital rubella syndrome.
For the other two scenarios changing these assumptions influenced the cost-effectiveness of a few analyses. When calculating lifelong (up to 77 years, age-adjusted) healthcare costs for treatment of congenital complications (i.e. CNS and heart defects), instead of the conservative assumption of 5 years, scenario 1 would be cost-saving and scenarios 2 and 3 would be cost-effective. Costs for hearing disabilities above the age of 5 years were excluded in the lifelong costs since available cost estimates were not related to congenital defects. Assuming that the rubella infection occurring in a pregnant woman in a non-epidemic year always led to an infant born with complications (instead of a chance of 1/3) then scenarios 2 and 3 would have a cost-effectiveness ratio between €26 900 and €28 100/QALY gained. Not discounting costs and effects led to lower cost-effectiveness ratios. As a consequence the cost-effectiveness ratio in scenario 2 fell below the threshold (€18 600/QALY gained). The undiscounted ratio would be €28 300/QALY gained for scenario 3.
DISCUSSION
Screening pregnant women for rubella antibodies in order to offer postpartum vaccination to seronegative women is cost-effective if targeted at unvaccinated women in LVR in The Netherlands. We also investigated the cost-effectiveness of a screening and vaccination programme for two other scenarios: including all pregnant women in LVR regardless of vaccination status, and a nationwide programme including all non-vaccinated pregnant women in the country. These two latter scenarios were cost-effective if cost savings due to avoided treatment costs for prevented complications were lifelong, as shown in the sensitivity analysis. In the base-case analysis we included 5 years of cost savings for prevented complications, a conservative assumption. However, 45% of costs due to congenital complications are expected to occur in the first 5 years of life [26].
The saved costs are based on public data on treatment costs of congenital defects and are not specified for congenital defects due to rubella infection, leading to a somewhat uncertain estimate of costs that could be saved. Other societal costs not included in this analysis are the cost of physical adjustments to the home or special arrangements at school, e.g. extra teachers or special education for a disabled child. Due to lack of reliable data, production losses due to long- or short-term absence from work by parents taking care of a disabled child are also not included. The inclusion of such preventable costs would lead to a more favourable cost-effectiveness ratio. No indirect cost savings, i.e. production losses, were included due to lack of reliable data.
A survey among young women in one municipality in the region where rejection of vaccination is often made because of religious reasons showed that about 17% (2/12) would be willing to receive a MMR vaccination [Reference Ruijs22]. The sensitivity analysis showed that the difference between our assumption (i.e. 20%, based on the preliminary results from Ruijs et al. [Reference Ruijs22]) and the later results did not influence the results substantially. However, the willingness to vaccinate would probably be even lower for women belonging to the orthodox protestant groups because the women who accepted the MMR vaccination did not belong to these groups [Reference Ruijs22]. The current study showed that the willingness to vaccinate would have to be at least 4% in the non-vaccinated women belonging to the orthodox protestant risk groups in LVR for scenario 1 to be cost-effective. If lower, the cost-effectiveness ratio would increase above the unofficial cost-effectiveness limit of €20 000 seemingly employed in The Netherlands.
Assumptions on the occurrence of rubella epidemics greatly influence the cost-utility estimates. If no outbreak occurred during the 16-year period, scenario 1 would no longer be cost-effective. As the epidemiology of rubella in The Netherlands has changed since the introduction of vaccination, it is difficult to make predictions about occurrence of future outbreaks. No extensive sensitivity analysis was performed on this aspect, since the disease burden is strongly correlated with the inter-epidemic interval: a longer interval would increase the incidence of complications since the seroprevalence in pregnant women would be lower. Furthermore, increasing the time period would also increase the uncertainty of the estimates of the cost-effectiveness. Regrettably, only the data from the 2004–2005 outbreak was sufficiently detailed for such an analysis.
The health-related QoL weights should ideally be preference-based, estimated by a validated QoL instrument. Due to a lack of such estimates for the specific CRS defects, disease burden estimates were used and converted into a QALY estimate. These disease weights are country specific for The Netherlands, and originate from the same source (based on a panel of experts) [Reference Melse24].
Ad-hoc screening in pregnant immigrant women and women in LVR already takes place, but the extent of this is not documented. This practice may have lessened the number of pregnant women becoming infected during the 2004–2005 epidemic. Consequently, if the recommended policy entailed screening only pregnant women in LVR, thus excluding immigrant women outside LVR, there could possibly be more infants born with rubella defects during an epidemic. We therefore recommend that this unofficial practice and the consequences of its cessation are assessed before recommending the cessation of any current screening practices.
Immigrant girls (up to age 12 years) and young women (up to age 18 years) are vaccinated against rubella when entering The Netherlands. On ethical grounds it can be argued that all immigrant women of childbearing age should be screened and vaccinated if necessary, as they are entitled to the same protection against preventable infectious disease as the native population.
If the screening and vaccination programme against rubella is implemented, we believe a good alternative would be to offer screening at the regular antenatal visit where other screening also takes place. In fact, this assumption was made in our calculations, where we added no extra costs for blood sampling. One alternative for the postpartum vaccination would be to vaccinate directly after the delivery or at the after-birth care of the mother. If that were the case, the costs for vaccination would be lower since no extra GP visit would have to be made, resulting in a more favourable cost-effectiveness ratio.
Another option would be to include screening and vaccination in a pre-conception advice as advised by the Health Council of The Netherlands [28]. One of the best advantages would be that a foetus in the first pregnancy would also be protected.
Fundamental to our results is the assumption that there is a willingness to accept vaccination in order to protect future children. As all rubella cases in pregnancy during the 2004–2005 epidemic were in unvaccinated, orthodox protestant women, the acceptance of vaccination in this group is extremely important. The acceptance among women – particularly in the orthodox protestant risk groups – would have to be further investigated before implementation.
APPENDIX
Estimation of average QALY lost
The average number of QALYs lost was estimated for an unspecified complication using disability weights estimated for the Dutch population [Reference Melse24]. Since one child can suffer from several defects due to the maternal rubella infection, the QoL weights are multiplied to arrive at an estimate for the combination of defects, the standard way of combining one or more defects into one weight [Reference Van Baal29]. Number of QALYs lost is summed for the defects present in the 2004–2005 epidemic. The weighted average of these is then used as the measure of the potential QALYs gained for one unspecified complication. (Table A2) The life expectancy at birth [78·8 years (46·2 years when discounted 1·5%)] was used to calculate the QALYs.
Estimation of average costs due to congenital defect
Unit costs per defect are based on total healthcare costs in 2003 for diagnoses of hearing disability, congenital heart defect and congenital CNS defect for children aged 0–4 years, as found in the database, describing cost of illness in The Netherlands, using the definition of costs ‘Zorgrekeningen CBS’Footnote † [26]. To arrive at a cost per patient (unit cost per defect) we divide total costs per defect by number of hospital-admitted patients aged 0–4 years with the diagnosis, respectively, from national statistics [21]. Costs for the first 5 years from birth are included under the assumption that most of the healthcare costs accrue in this period (45% of the costs of defects of the CNS and the heart appear in the first 5 years). Ninety-five percent of these costs are for hospital costs, except for hearing defects, where 65% are hospital costs. The unit costs for hearing defects are slightly overestimated because there are more children with hearing defects than is assumed based on number of hospital admissions. Possible medical costs for treatment due to spontaneous abortion are not included in the costs due to CRS defects. The average yearly costs are estimated at €32 600, which during 5 years is €156 900 (€163 000 undiscounted) (Table A3).
Table A3. Annual unit cost per defect and average cost of complications [price level 2007 (€)]

* Inflated from 2003 with consumer price index.
QALYs gained and costs saved in a non-epidemic year
The number of preventable complications in a non-epidemic year is based on the assumption that one pregnant woman is infected with rubella annually. The probability that infection leads to a complication is 1/3, and the probability that the infected woman belongs to the specific subgroup is 25% for native women and 75% for immigrant women [Reference Hof27]. These aspects lead to the different probability of complications in each subgroup (Table A4, column E).
Table A4. Number of quality-adjusted life-years (QALYs) gained and saved costs in a non-epidemic year [price level 2007 (€)]

The preventable complications are dependent on the chance that it is the second pregnancy and the chance that the women in each group accept screening and vaccination, and the vaccine effectiveness (95% in all groups). Of the 32 infected women parity is known for 25 of them: 11 or more were expecting their second or more child (44%). These probabilities are multiplied by the average QALY estimates and costs, respectively, and summed for each scenario. This results in potentially gained QALYs and saved costs per scenario (Table A4).
Unit costs, screening and vaccination programmes
Unit costs for the screening programme are costs for the test and an honorarium. The blood sample would not entail extra costs since it would be part of the standard sample taken at the antenatal healthcare visits at about 12 weeks' gestation. It is further assumed that the vaccination is given by a GP and that the sensitivity and specificity of the serological test is 100% (Table A5).
Table A5. Unit costs for screening and vaccination [price level 2007Footnote * (€)]

* Inflated with consumer price index
† Reimbursement to pharmacy per prescription, €6·10 [31]
ACKNOWLEDGEMENTS
We thank Dr J. van de Kassteele for statistical help regarding Figure 1(a, b).
DECLARATION OF INTEREST
None.