Chronic high dietary glycaemic index (GI) and glycaemic load (GL) have been shown to increase the risk of chronic diseases such as diabetes and CVD among adults(Reference Barclay, Petocz and McMillan-Price1–Reference Liu and Chou3). However, the current evidence regarding dietary GI and GL and their associations with disease risks, among children and adolescents, is limited and mixed. Data from longitudinal studies such as the DOrtmund Nutritional and Anthropometric Longitudinally Designed (DONALD) study(Reference Buyken, Cheng and Gunther4, Reference Cheng, Karaolis-Danckert and Libuda5) did not show any prospective association between dietary GI or GL and percentage of body fat or BMI. There has also been no study to investigate the effect of dietary GI or GL in childhood on long-term health risks. On the other hand, low dietary GL has been shown to improve cognitive performance(Reference Benton, Maconie and Williams6, Reference Gilsenan, de Bruin and Dye7), and some evidence from randomised controlled trials(Reference Ebbeling, Leidig and Sinclair8, Reference Fajcsak, Gabor and Kovacs9) also suggested that a low-GL diet was beneficial in the treatment of obesity in children and adolescents.
Despite the potential health benefits, data about the dietary GI and GL of a nationally representative sample of children and adolescents have not been reported in the literature, and there have only been a few investigations of GI and GL among children and adolescents in larger samples or national surveys in other countries(Reference Forbes, Storey and Fraser10–Reference Nielsen, Bjornsbo and Tetens12). Therefore, the aims of the present study are to (1) describe the dietary GI and GL of Australian children and adolescents, (2) analyse their relation to other nutrients and (3) identify the major foods contributing to their GI and GL, using data from the most recent Australian national dataset available to date, the 2007 Australian National Children's Nutrition and Physical Activity Survey (2007ANCNPAS)(13). Furthermore, we examined age group and sex differences with regard to these aims.
Materials and methods
The 2007 Australian National Children's Nutrition and Physical Activity Survey
The 2007ANCNPAS was commissioned in 2007 by the Australian Commonwealth Department of Agriculture, Fisheries and Forestry, and the Australian Food and Grocery Council(13). The methodology of the 2007ANCNPAS was previously described in detail(14). In brief, the survey measured the dietary intakes of food and beverages as well as the use of supplements using the 24 h recall method, administered twice during the survey period. These data were collected on children aged 2–16 years (n 4834) between 22 February and 30 August 2007. Dietary intake data were entered into a purpose-built database, with nutrition compositions based on the AUSNUT2007 database(15).
Data cleaning
Children who completed only one 24 h recall (n 179) were excluded from the analysis. The plausibility of the remaining food intake data was assessed using the Goldberg cut-off(Reference Goldberg, Black and Jebb16) for specific physical activity level (PAL), which was determined from information collected about physical activity with a pedometer. Where PAL data were not available (n 2438), we have utilised the lower 95 % CI of a PAL of 1·5 (i.e. 0·93) and the upper 95 % CI of a PAL of 1·7 (i.e. 2·73) as the cut-off values for under- and over-reporting. These PAL cut-off points were approximately the 25th and 75th percentile of those children who had a PAL determined from the survey. Participants with the energy intake:BMR ratio outside the 95 % CI were excluded from the analysis. We excluded 360 under-reporters and 100 over-reporters based on this method. We also excluded eleven participants who reported unusually high intakes of foods (e.g. six cups of rice in a meal). The final dataset included 4184 participants, where 51 % were male. The demographic characteristics of the participants are summarised in Table 1.
Table 1 Demographic characteristics of the subjects included in the analyses
(Number of subjects and percentages, n 4184)
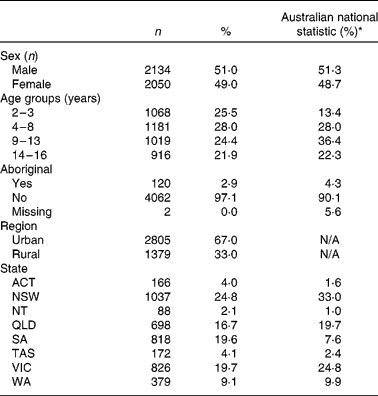
N/A, not available.
* Data from the 2006 Australian Census for children and adolescents aged 2–16 years.
Linking published glycaemic index values to 2007 Australian National Children's Nutrition and Physical Activity Survey food items
GI values were assigned to individual food items recorded in the 2007ANCNPAS dataset based on a method previously described by our group(Reference Louie, Flood and Turner17) with a small modification at step 1, as AUSNUT2007 contains no information about the GI. In short, the four steps involved in the assignment process are as follows:
Step 1. Determine if the item has < 5 g of carbohydrates (CHO) per 100 g. If yes, a GI value of 0 was assigned to that item. If no;
Step 2. Determine if there is a ‘closely related food item’ or an exact match in the four databases used. If yes, assign that GI value. If no;
Step 3. Determine if the median GI value of the food subgroup is available. If yes, assign the median GI value of the subgroup. If no;
Step 4. Determine if the item is a ‘top carbohydrate contributor’(Reference Louie, Flood and Turner17).
If yes, assign a GI value of 50 or a GI value of an appropriate closest matched item as decided by the research nutritionists. If no, a GI value of 0 is assigned.
Recipes in the original 2007ANCNPAS database were recorded as individual ingredients, and GI values were assigned to the individual ingredients rather than the recipe as an item. It was not envisaged to significantly affect the final mean dietary GI or GL of the participants. This is because the majority of these recipes (n 3332) were sandwiches, where the GI of the recipe was not anticipated to be significantly different from the main CHO component(s) in the recipe.
There were a total of 3418 different food items recorded in the 24 h recalls. Items with similar expected glycaemic effects and nutritional properties were grouped together for this analysis. The details of food groupings used in this analysis can be found in Table S1 of the supplementary material (available online at http://www.journals.cambridge.org/bjn).
Following the aforementioned method, 1075 (31·5 %) food items that contained < 5 g of CHO per 100 g were assigned a GI of 0 (step 1), whereas 1829 (53·5 %) items were assigned the GI value of a ‘closely related’ food item or an exact match in the GI databases used (step 2). A total of 239 (7·0 %) items were assigned the median GI of their corresponding food subgroups (step 3), while 275 (8·0 %) items were assigned a GI of 0 because they were not the ‘top carbohydrate contributors’, and ten items in the ‘top carbohydrate contributors’ list (0·3 %) were assigned a GI of 50 (step 4).
Calculation of the glycaemic load and dietary glycaemic index
The GL of each food item was calculated as the corresponding GI (%) × amount (g) of available CHO in a serve of that food. The daily dietary GL of each subject was calculated as ΣGL, and the dietary GI was obtained by (dietary GL/total available CHO intake in the day) × 100 %.
Statistical analysis
In order to increase the representativeness of the analyses, specific sample weighting was applied for all statistical analyses to account for over sampling in the age group of 2–3 years and in South Australia. Nutrient intakes and dietary GI and GL were analysed as the mean of the two 24 h recalls. One-way ANOVA was used to test for differences in dietary GI and GL between the groups. Age- and sex-specific energy-adjusted nutrients/dietary GI residuals were calculated by linear regression, with nutrient of interest/dietary GI as the dependent variable and daily energy intake as the independent variable. Pearson's χ2 test was used to test for differences in numbers of male participants, indigenous participants and participants from urban area across the age groups. Trends for mean dietary GI and GL across age groups were tested by linear regression, with age in years (continuous) as the independent variable and GI and GL residuals as the dependent variable. Pearson's correlation coefficients (by age group) between dietary GI and percentage energy from macronutrients and Ca were calculated: model 1 included adjustment for sex and BMI Z-score; model 2 calculated the correlation between the age- and sex-specific energy-adjusted residuals of dietary GI and percentage energy from macronutrients as well as from Ca, with additional adjustment for BMI Z-score. Per consumer analysis included only subjects who had reported the consumption of food item(s) in the food groups tested. The Kruskal–Wallis test was used to test for differences in the GL contribution between boys and girls, as these were not normally distributed. A P value < 0·05 was considered to indicate statistical significance. All statistical analyses were carried out using Statistical Packages for Social Science version 17.0 (SPSS Australasia Private Limited, North Sydney, NSW, Australia).
Results
Subjects excluded from the analysis (n 460) were older (11·1 v. 8·3 years; P <0·001) and had higher BMI (22·5 v. 18·3 kg/m2; P <0·001) than the included subjects. There was also a higher proportion of girls among the excluded subjects (57·8 v. 48·9 %; P <0·001).
The weighted mean dietary GI and GL of the study population were 54·1 (sd 4·7) and 135·6 (sd 43·9), respectively. No significant difference by sex was detected for dietary GI, but boys had a significantly higher dietary GL than girls (145·5 (sd 47·4) v. 124·9 (sd 37·0); P <0·001). There was no significant difference by state of residence (data not shown). Table 2 outlines the mean daily intake of select macro- and micronutrients by age groups of the participants. The age-dependent increase in total energy intake was accompanied by an increase in percentage energy from starch, as well as a decrease in percentage energy from sugars and CHO. There was no significant trend in GI or GL as age increased, but children aged 2–3 years had significantly lower GI and GL than their older counterparts. Fibre density showed significant decreasing trends.
Table 2 Daily intake of selected macronutrients, fibre and demographics of the subjects by age group
(Weighted mean values and standard deviations)

GI, glycaemic index; GL, glycaemic load.
* P values represent P for trend except for male (%), urban (%) and indigenous (%), which were tested by χ2 test, and for BMI that was tested by one-way ANOVA.
† ↑ and ↓ preceding P for trend indicate the direction of trend.
Table 3 shows Pearson's correlation coefficients between dietary GI and selected macro- and micronutrients, stratified by age groups. Energy from non-glycaemic macronutrients was inversely associated with dietary GI. Among CHO, percentage energy from sugar was inversely associated with dietary GI, while percentage energy from starch was strongly positively associated with dietary GI. A weak inverse correlation between fibre density and dietary GI was also found for all age groups, but not for the age group of 2–3 years, and Ca was found to be strongly inversely associated with dietary GI. Percentage energy from saturated fat was also negatively associated with dietary GI.
Table 3 Pearson's correlation coefficient between dietary glycaemic index (GI) and selected nutrient intakes of the subjects by age group

GL, glycaemic load.
* Correlation between dietary GI and the nutrient variables, adjusted for sex, BMI Z-score.
† Correlation between dietary GI and the nutrient variables (as age-, sex- and energy-adjusted residuals), adjusted for BMI Z-score.
‡ Correlation coefficient values were not statistically significant.
Table 4 shows the number of consumers for the top twenty GL-contributing food groups by age groups, while Table 5 shows the mean GL contribution by the top twenty GL-contributing food groups stratified by age groups. At age 2–3 years, the main contributors to the GL were fruit, breakfast cereals and white bread, while at age 14–16 years, white bread, breakfast cereals, cakes, pastries and doughnuts as well as soft drinks ranked among the top contributors. There were significant increases in percentage GL contributed by energy-dense and/or nutrient-poor foods such as soft drinks, white breads, high-fat potatoes and fast foods across age groups, while that for nutrient-dense foods such as fruit, milk and yogurt was found to be decreasing across age groups.
Table 4 Number of consumers for the top twenty glycaemic load-contributing food groups by age group

* Energy-dense and/or nutrient-poor food groups.
Table 5 Glycaemic load (GL) contribution by the top twenty GL-contributing food groups by age group
(Mean values and standard deviations)

* See Table 4 for the number of each cell.
† ↑ and ↓ preceding P for trend indicate the direction of trend.
‡ Energy-dense and/or nutrient-poor food groups.
Per consumer analyses found that for all food groups, except fruit and milk, the daily absolute dietary GL contribution was increasing across age groups. Notably, the daily dietary GL contributed by white breads and that by energy-dense and/or nutrient-poor items such as cakes, pastry and doughnut, salty snacks and soft drinks, etc. in subjects aged 14–16 years was nearly or more than doubled that of their 2–3-year counterparts. Increases in nutrient-dense food groups across age group were more modest. When examined by sex (Table 6), per consumer analysis revealed that girls across all age groups had a significantly less percentage GL and daily GL from breakfast cereals, and older girls had less daily GL from confectionery and soft drinks than younger girls.
Table 6 Glycaemic load (GL) contribution by the top twenty GL-contributing food groups by age group and sex
(Mean values and standard deviations)

* See Table 4 for the number of each cell.
† ↑ and ↓ preceding p for trend indicate the direction of trend.
‡ Energy-dense and/or nutrient-poor food groups.
§ Mean values were significantly different from those of boys in the same age group (P < 0·05).
Discussion
The present study is the first to investigate the dietary GI and GL of a nationally representative sample of children and adolescents. We have shown that Australian children and adolescents were having a significant proportion of their dietary GL from energy-dense and/or nutrient-poor food items, and these proportions were found to increase with age. The present results also suggest that Australian children and adolescents were having a high-GI dietary pattern characterised by high starch and low Ca intake. Apart from low Ca intake, overall, the nutritional intake of 2007ANCNPAS participants appeared to be satisfactory, with most of the participants meeting the estimated average requirement for key micronutrients(18).
As expected, GI correlates positively with GL of which it is a component (model 1). Correlations are enhanced once potential variations due to differences in energy intake are accounted for (model 2), i.e. when GL can no longer vary due to increases or decreases in CHO. With this adjustment, GL variations are theoretically reduced to those attributable to exchanging CHO for protein or fat intake and to variations in GI itself, which explains the high correlation between this energy-adjusted GL and GI.
The weak negative correlation between percentage energy from sugar and GI suggested that the popular belief that high intake of sugary foods increases the dietary GI may be incorrect, at least among Australian children and adolescents. Fibre density, unlike Buyken et al. (Reference Buyken, Dettmann and Kersting19) who showed a strong significant negative trend across GI tertiles, was only modestly correlated with GI in an inverse fashion. While soluble or viscous fibre is able to reduce the digestion rate of CHO by limiting the access of enzyme to the CHO (hence lowering the GI), insoluble fibre, when finely milled, e.g. in wholemeal flour, has no or minimal effect on glucose absorption rate because it does not block the access of enzymes to the CHO. Therefore, foods high in finely milled insoluble fibre may still have a high GI, e.g. wholemeal breads, and the consumption of these foods seemed to be higher in the 2007ANCNPAS population than those in the DONALD study. The AUSNUT2007 database used for this analysis unfortunately did not allow for the separation of the two types of fibre, thus disallowing further analysis.
There have been few investigations into the dietary GI and GL among children and adolescents in large samples or national surveys. A Canadian study of 4936 adolescents aged 9–17 years has reported a mean GI and GL of 55 and 144(Reference Forbes, Storey and Fraser10). A small Italian study of 105 children aged 8 years, which assessed the subjects' diets with a validated FFQ, has produced similar findings to the present study. The mean dietary GI and GL were found to be 58 and 145, respectively, and boys were found to have a higher GL but not a higher GI than girls. A Danish study(Reference Nielsen, Bjornsbo and Tetens12) of 849 children aged 10 and 16 years has also found that boys were having a higher dietary GL than girls. However, the dietary GL reported (mean GL for boys 231 (sd 67)) was much higher than that found in the present study for the 9–16-year-olds, and only 1 d of recall was obtained. The DONALD study(Reference Buyken, Cheng and Gunther4) found the dietary GI and GL of children at 2 years to be 52 and 63, and these were shown to have increased to 56 and 113 in 5 years, respectively, though not statistically significant. The present results showed that although there was no trend for GI or GL as age increases, older ( ≥ 4 years) children had a significantly higher GI and GL than their 2–3-year counterparts. Higher percentage GL from dairy foods and fruits, both having low GI, among participants aged 2–3 years may explain such a difference. The concurrent increase in dietary GI with age also suggests that older respondents were selecting more high/higher-GI foods in their diet.
A decrease in percentage energy from sugars with age was found, which seemed contradictory to the fact that daily GL from energy-dense and/or nutrient-poor foods, which were mostly sugary in nature, was increasing with age. Indeed, the DONALD study(Reference Buyken, Cheng and Gunther4) has shown that the percentage energy from added sugars increased from 9·5 to 14·2 % in 5 years. Unfortunately, the AUSNUT2007 database used in this analysis did not allow the separation of added sugars from naturally occurring sugars.
An increase in percentage energy from starch with age was also observed, and this may partly explain the increase in dietary GI with age, as most starchy foods, especially those that are highly processed, are of high GI. Most of the subjects' dietary GL was from white breads and other energy-dense and/or nutrient-poor foods, such as soft drinks, high-fat potatoes, high-sugar, low-fibre breakfast cereals, cordial, etc. These foods have a moderate/high GI, and provide a small amount of fibre/nutrients or are high in energy, or both, making them poor dietary choices. Age-stratified analyses revealed that older respondents were having more of their dietary GL from energy-dense and/or nutrient-poor foods, and less from fruits.
Very few data are available for the food types contributing to the dietary GL among children and adolescents. The present findings differed from that of the German DONALD study(Reference Buyken, Dettmann and Kersting19), which showed that German children in the study had high relative contributions to dietary GL by bread and rolls, as well as by milk and dairy products, and that contributions by breakfast cereals and cereal grains were lower than that of the Australian children in the present study. This suggests that the contribution to GL by different foods is specific to the food habits of the respective countries. However, ‘tolerated food groups’ (similar to the energy-dense and/or nutrient-poor foods described in the present study) contributed to a significant proportion of the subjects' GL in both studies, which raised concerns that the diets of these children were suboptimal.
Both high dietary GI and GL have recently been found to be associated with higher risk for chronic diseases(Reference Barclay, Petocz and McMillan-Price1–Reference Liu and Chou3, Reference Liu, Willett and Stampfer20). The development of chronic diseases usually occurs slowly over a long period, and it has been suggested that long-term dietary behaviour could be shaped during childhood and adolescence(Reference Mikkilä, Räsänen and Raitakari21–Reference Pérez-Rodrigo and Aranceta23). Lowering the dietary GL may therefore be an effective, yet simple and easy strategy for Australian children and adolescents to reduce the risk of developing these chronic diseases in later life. In theory, by reducing the GI of the food items and/or the amount of CHO eaten could reduce the dietary GL. However, reducing the amount of CHO eaten could be difficult for people who follow a traditionally high-CHO diet, e.g. Asians. Utilising low- or lower-GI alternatives of their traditional staple foods, e.g. basmati rice for jasmine rice, is therefore a more suitable strategy to be employed(Reference Liu24).
A particular strength of the present study is the use of a published method for assigning GI values to the food items in the 2007ANCNPAS food database. Based on this method, we assigned GI values to around 85 % of the food items in the first two steps, which utilised the current best available sources of GI values, therefore increasing the reliability of the GI values assigned. The use of a nationally representative sample (through sample weighting) also increased the generalisability of the findings.
However, there are several limitations to the present study. Despite being a suitable dietary assessment method to be used for a large number of subjects, the evidence to support the use of a 24 h dietary recall in children is limited, and it has been argued that an accurate dietary assessment among children is especially difficult(Reference Livingstone and Robson25). Parental recall of food intake, as in the case for children aged 8 years or below in the present study, had been suggested to be unreliable, especially when the reporting parent was away from home for more than 4 h/d (e.g. working)(Reference Livingstone and Robson25). This is likely to result in under-reporting and may hence contribute to inaccuracy at an individual level(Reference Klesges, Klesges and Brown26–Reference Baranowski, Sprague and Baranowski29). Children who reported their own food intake were also likely to inaccurately recall their food intake, due to incorrect identification of foods(Reference Samuelson30, Reference Emmons and Hayes31) and unfamiliarity of the food(Reference Warren, Henry and Livingstone32), which may lead to misreporting, as well as information overload (e.g. large number of foods to report) that is likely to result in under-reporting(Reference Baranowski, Dworkin and Henske33).
By using the Goldberg cut-off for a specific PAL method(Reference Goldberg, Black and Jebb16), we have excluded under- and over-reporters based on a scientifically accepted methodology, which is likely to increase the plausibility of the present findings. However, we were unable to detect incorrectly recalled foods in the database based on this method, which could possibly affect the calculated GI and GL, e.g. a low-GI mixed-grain bread may have been reported as ‘brown bread’, which could be subsequently coded as wholemeal bread, a high-GI food. We were also unable to exclude the possibility of residual error.
In addition, dietary intake may vary from day to day, and therefore data obtained from two 24 h recalls may not be representative of the habitual intake of an individual(Reference Livingstone and Robson25, Reference Biro, Hulshof and Ovesen34). A 7 d food record would be ideal, but the large-scale nature of the 2007ANCNPAS made this impractical. It is also highly demanding on the participants and may result in a low response rate, i.e. data are not representative any more. Also, while the two 24 h recalls for the same participant were collected only 7–21 d apart, the data collection for the whole study population spanned across autumn and winter in Australia, which may have arguably affected the consumption patterns of seasonal foods. Importation may have reduced the likelihood of unavailability of seasonal fruit and vegetables, though the higher price associated with it may still affect the consumption patterns. However, there are no notable differences in the availability of fruit and vegetables between autumn and winter in Australia(35).
In conclusion, the present results suggest that Australian children and adolescents were having a high-GI dietary pattern characterised by high starchy food intake and low Ca intake. A significant proportion of their dietary GL was from energy-dense and/or nutrient-poor foods. Effort should be made to encourage the replacement of high-GI, energy-dense and/or nutrient-poor food items for nutrient-dense low-GI foods to simultaneously lower the dietary GI and GL and improve the dietary quality of Australian children and adolescents.
Acknowledgements
The original data of the 2007ANCNPAS were collected by the Australian Commonwealth Scientific and Industrial Research Organization and the University of South Australia. The authors would like to thank the Australian Commonwealth Department of Health and Ageing for providing the survey data via the Australian Social Science Data Archive. The authors declare that those who carried out the original analysis and collection of the data bear no responsibility for further analysis or interpretation included in the manuscript. The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. J. C. Y. L., A. E. B. and V. M. F. contributed to the conception of the study. J. C. Y. L. and K. H. assigned the GI values with input from V. M. F.; J. C. Y. L. and K. H. performed the statistical analyses under the guidance of A. E. B. and V. M. F.; A. E. B., V. M. F. and J. C. Y. L. interpreted the data. J. C. Y. L. drafted the manuscript. All authors were involved in the subsequent edits of the manuscript, and read and approved the final manuscript. The authors declare they have no competing interest.








