Dietary consumption leading to an energy imbalance is among the most proximal drivers of obesity(Reference Swinburn, Sacks and Hall1). Diets today, especially in low-income, urban communities of colour, are often characterized by high intakes of refined carbohydrates, added sugars, fats and salt due to high consumption of energy-dense, processed foods(Reference Kirkpatrick, Dodd and Reedy2, Reference Martinez Steele, Baraldi and Louzada3). Analyses of nationally representative surveys have demonstrated increased intake of high-energy-dense foods, such as sugar-sweetened beverages(Reference Bleich, Wang and Wang4) and snacks(Reference Piernas and Popkin5), in the past three decades among US adults. Despite recent findings showing improvement in dietary quality from 1999 to 2012 among the overall adult population(Reference Wilson, Reedy and Krebs-Smith6), African-American and Hispanic adults continue to have the lowest dietary quality in the country(Reference Rehm, Penalvo and Afshin7). These disparities in diet quality are likely influenced by racial and ethnic residential segregations and inequalities in availability, access and affordability of nutrient-dense foods and resources(Reference Black, Moon and Baird8–Reference Gordon-Larsen, Nelson and Page11).
In view of the multifactorial aetiology of weight gain, efforts that simultaneously address multiple levels of the food system are recommended(Reference Lobstein, Jackson-Leach and Moodie12). One example of such efforts are multilevel multicomponent community-based interventions, in which different levels of influence are targeted to change the food environment surrounding the individual and to promote behavioural change(Reference Roberto, Swinburn and Hawkes13). Despite recognizing the importance of the various levels of influence outlined in socio-ecological models (i.e. individual, household, organizational, community, policy)(Reference McLeroy, Bibeau and Steckler14), most multilevel childhood obesity prevention interventions have primarily delivered nutrition education in school settings, yielding mixed results(Reference Hung, Tidwell and Hall15, Reference Gittelsohn and Kumar16), with limited activities to modify the out-of-school environment and for engaging families(Reference Bleich, Segal and Wu17). Furthermore, insufficient evaluation of the impact of multilevel community-based childhood obesity prevention trials on diet and food behaviours in children and their caregivers exists(Reference Waters, de Silva-Sanigorski and Hall18).
Childhood obesity prevention interventions that also engaged adult caregivers have shown more positive child-related outcomes than child-only interventions(Reference Heim, Bauer and Stang19, Reference Golan and Crow20). However, few child-focused interventions have reported impacts on caregiver behavioural outcomes(Reference Coffield, Nihiser and Sherry21), due to limited assessment of nutrition behaviours among this group(Reference Haerens, Deforche and Maes22). Understanding the impact of childhood obesity prevention on caregivers is important because families’ eating practices, rules and support influence children to initiate and sustain positive dietary changes, while providing opportunities for social learning(Reference Pollard, Zachary and Wingert23). Therefore, we evaluated the secondary impact of a child-focused community intervention on youths’ adult caregivers’ food acquisition, preparation, and fruit and vegetable (FV) consumption.
B’More Healthy Communities for Kids (BHCK) was a community-based multilevel multicomponent childhood obesity prevention intervention that sought to modify the food environment outside of school for low-income 9–15-year-old youths in Baltimore, MD, USA(Reference Gittelsohn, Steeves and Mui24). We hypothesized that caregivers would have improved food-related behaviours in part due to the environmental changes of the BHCK intervention and educational activities through social media and texting. For instance, BHCK improved availability and promotion of healthful foods and beverages in small food stores (i.e. corner stores/carry-out restaurants) that were frequented by youths outside school hours and located in the neighbourhoods where BHCK families lived(Reference Gittelsohn, Trude and Poirier25). Caregivers may also have been exposed to or attended community nutrition education sessions given that intervention activities in intervention neighbourhoods were public and available to all community members(Reference Trude, Kharmats and Jones-Smith26). In addition, caregivers could have also been exposed to flyers and giveaways that were brought home by youths attending BHCK activities in the after-school nutrition education sessions for youths. Lastly, BHCK social media and text-message intervention components targeted adult caregivers, in which the content aimed to reinforce health-related messages utilized at other BHCK intervention components.
Multilevel multicomponent interventions are implemented as synergistic interventions with components reinforcing one another at different levels(Reference Mikkelsen, Novotny and Gittelsohn27); however, this limits the researcher’s ability to identify which specific component was more successful in influencing behaviour change. Another consideration for multilevel multicomponent community-based interventions concerns the extent to which intervention components are implemented with sufficient intensity(Reference Glasgow, Klesges and Dzewaltowski28). One approach to identifying the intervention component that led to behaviour change in multilevel multicomponent interventions is to conduct treatment-on-the-treated effect (TTE) as a secondary impact analysis, in which study participants are analysed according to the treatment received, instead of the original treatment assigned (i.e. average treatment effects (ATE))(Reference Trude, Kharmats and Jones-Smith26). Although causality cannot be inferred, this analysis may provide information about the dose–response relationship between level of exposure to the intervention and behavioural change, and may identify specific intervention components that are more likely to influence the outcomes(Reference Sedgwick29).
Therefore, the present paper aimed to answer the following questions:
1. What was the impact of the multilevel BHCK intervention on food-related behaviours (purchasing of healthier and unhealthier food items, food preparation and FV consumption) among adult caregivers?
2. Was the change in food-related behaviours associated with caregivers’ exposure level (‘dose received’) to the BHCK intervention?
3. What component of the multilevel BHCK intervention was correlated with changes in food-related behaviours among caregivers?
Methods
Study design
BHCK employed a group-randomized controlled trial design with two intervention arms (random allocation to treatment on a 1:1 basis), implemented in two rounds (waves). A detailed description of the formative research, trial design and sample size calculation has been published elsewhere(Reference Gittelsohn, Steeves and Mui24).
The intervention integrated different levels of an ecological model and multiple intervention components into a food systems approach from wholesalers, to small food stores and to families that promoted access to nutritious foods and balanced diets. Using a socio-ecological model for health promotion, the BHCK intervention tapped into the dynamic interplay among individual, behavioural, household, environmental and policy levels(Reference McLeroy, Bibeau and Steckler14). Individual-level components were based in community recreation centres, using youth leaders (college and high-school trained mentors) to provide education and nutrition skills to youths (9–15 years old). The family level included social media and texting. Social media (Facebook and Instagram) were used to integrate the different levels of BHCK to inform family-level nutrition behaviours. Recipes, news and BHCK-specific activities were featured in these communication channels. Text messages (sent three times per week) and social media platforms also targeted mainly youths’ caregivers by guiding them to set and achieve goals to healthier behaviours for themselves and their families, as well as promoting BHCK community activities. An example of a goal setting text message was as follows: ‘Does your child have a sweet tooth? Try offering them granola bars or fruit as an alternative to candy 1 time this week.’ Intervention flyers and promotion of the intervention were mailed to caregivers and youths twice per month at the end of Wave 2 only. An overview of the intervention is presented in Table 1.
Table 1 Description of the B’More Healthy Communities for Kids (BHCK) intervention as implemented
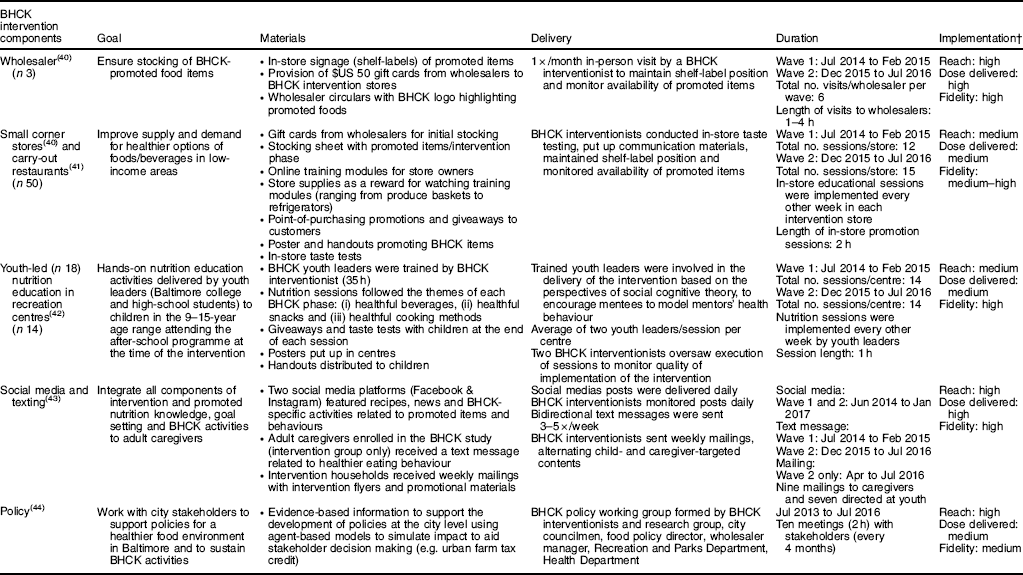
† Implementation (process evaluation) definitions: Reach = number of people in the target audience participating in each intervention activity. Dose delivered = units of intervention materials/activities (e.g. nutrition sessions, posters, flyers) provided by BHCK interventionists. Fidelity = quality of intervention component implementation, based on reactions to or engagement with the programme. High (≥100 %),medium (50–99·9 %) or low (<50 %) refers to a priori set standards.
The BHCK intervention promoted healthful foods/beverages and behaviours in three sequential phases, each lasting two months: (i) healthier beverages (i.e. lower-sugar fruit drinks (25–75 % less sugar than the original version), sugar-free drink mixes, zero-calorie flavoured water, diet or low-sugar soda, and water); (ii) healthier snacks (i.e. low-fat yoghurt, low-fat popcorn, fresh fruits, fresh vegetables, low-sugar granola bars, and mixed fruits in 100 % fruit juice); and (iii) healthier cooking methods (i.e. cooking ingredients, such as low-sugar cereals, low-fat milk, 100 % whole-wheat bread, fresh/canned/frozen vegetables). A fourth phase, intended to review main messages covered in the previous phases, was implemented in Wave 2 only.
Setting
The trial took place in thirty low-income, predominantly African-American neighbourhood zones in Baltimore, with low access to healthy foods. Zones were defined as an area of radius 2·4 km (1·5 mile) around a recreation centre (nucleus). Eligibility criteria for BHCK zones were: (i) predominantly African-American (>50 %); (ii) low-income (>20 % of residents living below the poverty line); (iii) ≥5 small (<3 aisles, no seating) food sources (e.g. corner stores and carry-out restaurants); and (iv) having a recreation centre more than 0·8 km (0·5 miles) away from a supermarket(Reference Walker, Keane and Burke30). The thirty zones were randomized into intervention (n 14) and comparison (n 16) groups, with recreation centres as the main unit of randomization. Wave 1 was implemented from July 2014 to February 2015 (seven intervention and seven comparison zones) and Wave 2 from December 2015 to July 2016 (seven intervention and nine comparison zones).
Participants
After randomly selecting BHCK zones, a sample of adult caregivers and their children was recruited in the recreation centres and around the stores within the 2·4 km (1·5 mile) buffer zone. Eligibility for the adult caregiver and child participants was determined at the household level. Household eligibility criteria were as follows: (i) being a caregiver (>18 years old) of at least one child aged 9–15 years; (ii) living in the same location for at least one month; and (iii) not anticipating a move in the next two years. Children and caregivers received $US 30 and $US 20 gift cards, respectively, after each of the pre- and post-intervention interviews.
Training of interventionists and data collectors
BHCK interventionists were graduate students, public health educators, dietitians or youth leaders trained in nutrition and health education, and were not masked to the group (zone) assignment. Data collectors were graduate students and staff who were intensively trained, including through role plays and observations. They were masked after assignment to intervention to reduce information bias.
Measures
Caregiver data collection
Baseline data were collected from June 2013 to June 2014 (Wave 1) in a total of 298 adult caregivers, and from April to November 2015 (Wave 2) in 235 caregivers. A post-evaluation was conducted from March 2015 to March 2016 (Wave 1) and from August 2016 to January 2017 (Wave 2), taking place immediately after implementation of the intervention to one year (Wave 1) or up to six months (Wave 2). We did not analyse participants who reported living in unstable housing arrangements such as in shelters or transitional housing (n 2), lived more than 2·4 km (1·5 miles) away from a BHCK recreation centre (n 5), had incomplete dietary intake data (n 14) or were considered an outlier (>10 servings/d, or >99·5th percentile) for fruit and vegetable intake (n 7), yielding a total of 373 participants with complete baseline and follow-up information for the analytical sample (Fig. 1).

Fig. 1 (colour online) CONSORT (Consolidated Standards of Reporting Trials) flowchart of the randomization and course of the B’More Healthy Communities for Kids (BHCK) intervention. *Analyses accounted for missing data and selection bias using the inverse probability weighted (IPW) method, with the probability of being observed at follow-up a function of the characteristics of the caregiver (age, sex and income) and study wave; final imputed sample size in the multilevel analysis, n 516 (FV, fruit and vegetables)
Fruit and vegetable consumption
The National Cancer Institute’s (NCI) FV Screener was used to collect usual consumption of ten categories of FV intake in adult caregivers over the past month. It is a short dietary assessment instrument consisting of fourteen questions and is a modified version of the FV screener from the Eating at America’s Table Study(Reference Subar, Thompson and Kipnis31). The screener inquired about frequency of intake of fruits, 100 % fruit juice and vegetables (lettuce, greens, potatoes and legumes) consumed on a monthly, weekly or daily basis. The amount of each food item was estimated as cups or servings and self-reported by the participant. We calculated the total number of both fruit servings and vegetable servings consumed daily using the 2005 MyPyramid definition of cup equivalents. For each food group, we multiplied the average frequency (daily) by the cup equivalent. The instrument has been validated, presents high correlations with 24 h dietary recall and is less burdensome compared with other instruments(Reference Yaroch, Tooze and Thompson32). Food models were used to improve accuracy of serving size information. The NCI FV Screener was added to the data collection protocol after the Wave 1 intervention had begun and was first administered during Wave 1 post-intervention. Therefore, the effect of the intervention on FV intake of adults was calculated only using the BHCK Wave 2 sample with pre- and post-evaluation data (n 196), as this instrument was not used during Wave 1 baseline data collection.
Household food preparation
Adult caregivers reported their frequency of meal preparation (cooking methods) for the household in the previous 30 d from the interview(Reference Suratkar, Gittelsohn and Song33). In addition, respondents ranked the top three most common cooking methods used when they prepared chicken, turkey (including ground turkey and turkey bacon), pork (including bacon), ground beef, fish, eggs, greens (excluding lettuce) and potatoes. The survey was adapted form an instrument used in a similar study(Reference Suratkar, Gittelsohn and Song33) and on the basis of formative research(Reference Gittelsohn, Franceschini and Rasooly34).
We created a healthful cooking score using similar methods previously reported in the literature(Reference Vedovato, Surkan and Jones-Smith35). Cooking methods were assigned values based on the amount of fat used, as follows: deep fry or pan-fried with oil (−2); pan-fried, drained or use of cooking spray (−1); not prepared in the last 30 d (0); pan-fried, drained and rinsed with hot water (+1); broiled/baked, or grilled, or steamed, or boiled, or raw, or microwaved (+2). The scores were separately calculated for each food, weighted according to the most commonly reported method to estimate the healthiness of the cooking preparation: 60 % (first method most commonly used), 30 % (second method) and 10 % (third method). For example, if chicken was most commonly pan-fried, second most commonly grilled and third most commonly cooked with cooking spray, the score was calculated as (0·60 × −2)+(0·30 × 2)+(0·1 × −1) as an indicator of the overall healthiness of chicken preparation. Then, the scores for all eight foods were summed to obtain the overall household food preparation score (mean −0·07 (se 0·88; range: −1 to 2·1).
Frequency of food acquisition
Caregivers reported the number of times they acquired foods from different food sources in the previous 30 d from the interview date (e.g. ‘How many times did you get these foods?’). Food acquisition included all the following: foods/beverages that were purchased with cash purchased with food safety net programme benefits (SNAP, WIC) and food that was obtained for free (i.e. from pantries or donated by family/friends)(Reference Gittelsohn, Anliker and Sharma36).
A list of thirty-three BHCK-promoted healthier foods and beverages and twenty-one less healthful foods and beverages was provided, and respondents reported the number of times they had acquired each food in the specified time frame. Prepared foods acquired from delis, vendors or restaurants were not included, as this instrument was designed to measure foods purchased for consumption in the home environment rather than for immediate consumption. The list was designed on the basis of formative research conducted with the community(Reference Suratkar, Gittelsohn and Song33) and reflected foods promoted during the BHCK intervention. Face and content validity of the questionnaire were assessed on fifteen randomly selected adult caregivers during the pilot phase(Reference Suratkar, Gittelsohn and Song33). The healthful and less healthful food acquisition variables were additive items based on the acquisition frequency of thirty-three healthful and twenty-one less healthful foods for each respondent and divided by 30 to yield a daily frequency score, respectively. Additive daily healthful food acquisition frequency ranged from 0·6 to 4·8 with a mean of 0·9 (sd 0·6), and less healthful food acquisition frequency from 0·1 to 10·2 with a mean of 1·3 (sd 1·1).
Exposure score
The key variables for assessing exposure (‘dose received’) were obtained using the twenty-nine-item Intervention Exposure Questionnaire (IEQ) collected as part of the post-intervention assessment for intervention and comparison groups. The IEQ measured participants’ self-reported viewing of BHCK communication materials (posters, handouts, giveaway), participation in food environment intervention activities (i.e. taste tests, seeing educational displays, redesigned carry-out restaurants’ menus, store promotional shelf-labels), enrolment in social media/viewing of media posts and receiving the text messaging programme(Reference Trude, Kharmats and Jones-Smith26). In addition, eight red herring questions were used to address response bias, and included materials used in previous studies conducted at other sites. We classified individuals into tertiles of red herring responses, where selecting 0–2 red herring answers was considered truthful, 3–5 moderate and 6–8 untruthful responses, and kept only individuals in the tertile with the least number of red herring responses. No respondent answered positively to >3 (one-third or more) of the red herring questions; thus, none of the caregivers with complete responses were excluded from the analysis.
We calculated exposure scores for each component of the BHCK intervention to which adults could be exposed (communication materials, food environment intervention, social media, texting) and an overall BHCK exposure score. Detailed description of the formation of the exposure score is presented in Table 2 and published elsewhere(Reference Trude, Kharmats and Jones-Smith26). For each intervention component, points were assigned for exposure to study materials/activities and then scaled into proportions (0–1 range), yielding an overall BHCK exposure score of 11 points (possible highest score). A total of 370 adult caregivers had complete exposure data information.
Table 2 Formation of exposure scores by B’More Healthy Communities for Kids (BHCK) intervention

† P value based on two-tailed t test comparing mean scores between intervention and comparison groups.
‡ We asked participants the number of places where they saw the BHCK logo or saw a BHCK shelf-label at a corner store with four possible answers (none; 1–2 places; 3–5 places; 6 or more). When coding, we chose the average number in the range of places they reported seeing the intervention materials (i.e. 0, 1·5, 4 and 6, respectively). Then, we re-scaled the points to range from 0 to 1 to make all the intervention materials exposure score equivalent before summing by exposure components (communication materials, food environment, social media and text messages).
Covariates
Caregivers were assessed on demographics and household socio-economic information, namely age, sex, caregiver education level (categorized into <high school, completed high school and >high school), employment status, household income ($US 0–10 000, $US 10 001–20 000, $US 20 001–30 000, >$US 30 000), housing arrangement (owned, rent, shared with family or other arrangement (group housing, transitional housing)) and household participation in food assistance programmes. These included receiving the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) or Supplemental Nutrition Assistance Program (SNAP) benefits in the past year. Caregivers also had their anthropometric measures taken (height using a stadiometer and weight using a portable scale) after removing shoes and heavy clothing. BMI was calculated as weight divided by height squared (kg/m2).
Data analysis
All analyses were conducted using the statistical software package Stata version 13.1. Descriptive statistics were performed to characterize the study sample at baseline by study group assignment. Continuous variables were tested for differences between intervention and comparison groups with independent two-tailed t tests. The χ 2 test for proportions was used for categorical variables. Variable and model residual distributions were examined for normality and extreme values (outliers) using quantile–quantile plots and goodness-of-fit tests (Kolmogorov–Smirnov).
The ATE on the change in diet, food preparation and food acquisition behaviours among adult caregivers were assessed by the difference between the mean change of the outcome in the intervention group compared with the control group. We tested the intervention effect on adult caregivers’ food-related behaviours using a multilevel linear mixed-effect model fit by maximum likelihood. Random effects accounted for variation at the BHCK zone and at the caregiver level (repeated measures).
Due to the 24·9 % attrition rate, we used inverse probability weighting to address potential bias due to loss to follow-up and to correct for the effects of missing data(Reference Fitzmaurice, Laird and Ware37). Using all available data, we estimated weights for every missing outcome of interest fitting a logistic regression model. We treated the categorical indicator of response at follow-up as the outcome variable and performed the regression v. the baseline response for intake, preparation or acquisition, with age, sex, income and wave (predictive of dropout) as covariates. Once the weights were determined, they were incorporated in the multilevel linear mixed-effect analysis using the pweight option for the mixed command in Stata. Results of the ATE analysis using only completed cases without the inverse probability weighting method are shown in the online supplementary material, Supplemental Table 1.
We also conducted a TTE analysis, in which study participants were analysed according to the treatment received(Reference Sedgwick29), as estimated by their exposure scores. We conducted multiple linear regression models to analyse the association between the change in caregivers’ food behaviours (intake, preparation and acquisition) and caregivers’ exposure levels (total exposure score and by exposure to intervention components), adjusted for age, sex, income and household size. We used a bootstrap method with 2000 repetitions and bias-corrected CI to account for the within-individual correlation of the data, clustered on the BHCK zone(Reference Guan38, Reference Amemiya39). For the significant results, we estimated the proportion of variability explained (effect size) with ω 2 after fitting the multivariate models. A sensitivity analysis using multiple logistic regression on the correlation between the categorical change in food-related behaviour (no change v. positive change) and the exposure scores (low (if 0) v. high (if above 0)) was also conducted to estimate the standardized effect size given by the OR. Given the time frame for follow-up data collection differed by wave, we conducted tests of homogeneity to explore if the effect of exposure was moderated by the two BHCK waves.
For all analyses, we reported the 95 % CI. Statistical significance was defined by a P value of <0·05.
Results
Implementation of each component of the BHCK intervention was evaluated through detailed process evaluation reported elsewhere(Reference Schwendler, Shipley and Budd40–Reference Nam, Ross and Ruggiero44). Table 1 illustrates implementation quality of each BHCK component. The intervention was implemented with overall moderate-to-high reach, dose delivered and fidelity(Reference Ruggiero, Poirier and Trude45).
On average, caregivers presented an overall BHCK exposure score mean of 1·38 (sd 1·2) points (range: 0–6·9), a BHCK communication materials exposure score mean of 0·6 (observed range: 0·0–3·1; highest possible score: 4), a food environment exposure score mean of 0·3 (observed range: 0·0–3·1; highest possible score: 5), a social media exposure score mean of 0·2 (observed range: 0·0–2; highest possible score: 2); and a text messaging exposure score mean (based on the frequency of BHCK text messages received per week) of 1·10 (observed range: 0–3).
When comparing the overall exposure scores between the groups, adult caregivers in the intervention group demonstrated significantly higher mean exposure scores than adult caregivers in the comparison group (intervention: mean 1·90 (sd 0·08); comparison: mean 0·82 (sd 0·07), P < 0·001; Table 2). Even though the comparison group was exposed to the BHCK intervention components, the intervention group had significantly higher exposure scores than the comparison group for the communication materials, food environment and text message components (P < 0·001). Social media exposure scores were not statistically significantly different when comparing group means (P = 0·06). Reported exposure level to the BHCK intervention was low among caregivers.
Characteristics of the baseline BHCK evaluation sample
The vast majority of our study sample self-identified as African-American (96·6 %), and 49·0 % of caregivers were either overweight or obese (Table 3). Most caregivers were female (93·2 %) and from a household that received SNAP (70·8 %). Significant differences were found between treatment groups with respect to caregiver’s age (P = 0·01), being higher in the comparison group.
Table 3 Baseline characteristics of the B’More Healthy Communities for Kids adult caregiver sample (n 516)

SNAP, Supplemental Nutrition Assistance Program; WIC, The Special Supplemental Nutrition Program for Women, Infants, and Children.
* Intervention and comparison groups are statistically different (P < 0·05) when comparing the proportion of adult characteristics using the χ 2 test or means with the two-tailed t test.
† Food security classified according to the measure of the US Department of Agriculture, Economic Research Service. Food-secure households encompassed high food security and marginal food security. Food-insecure households were either low food secure or very low food secure.
Impact of BHCK intervention on food-related behaviours of caregivers
In the ATE analysis, we did not find a significant effect of the intervention on the food acquisition, home food preparation and daily consumption of FV among intervention adult caregivers compared with their counterparts (Table 4).
Table 4 Impact of the B’More Healthy Communities for Kids (BHCK) intervention on food-related behaviours among low-income African-American adult caregivers: average-treatment-effects analysis

† Multilevel models were conducted using the Stata version 13.1 statistical software package with the maximum likelihood option and corrected missing data using the inverse probability weighted method (n 516 for purchasing and n 226 for consumption). Multilevel models are good approach to be used under the missing-at-random assumption, as they model both the means and the random effect jointly.
‡ In all models: treatment group was coded as comparison (0) and intervention (1); time was coded as baseline (0) and post-intervention (1); standard errors were corrected for clustering for repeated measures from the same individual and BHCK neighbourhood (from 1 to 30).
§ Mean difference in change over time for intervention compared with control adult caregiver.
║ Food acquisition frequency (daily) was estimated via a predefined list containing 100 % fruit juice, apples, bananas, oranges, other fresh fruits, frozen fruits, canned fruits, fresh vegetables, frozen vegetables, and canned vegetables (excluding potatoes). Adults reported frequency of purchasing these items in the previous 30 d.
¶ Fruit and vegetable intakes were estimated via the Quick Fruit and Vegetable Screener from the National Cancer Institute’s Eating at America’s Table Study. Sample size, n 226.
Associations between food-related behaviours and exposure to the BHCK intervention
The results of the TTE analysis are presented in Table 5 (overall exposure score) and Table 6 (BHCK components exposure scores). For each one-point increase in exposure score, there was a 0·24 increase in mean daily fruit serving intake over time (0·24 (se 0·11); 95 % CI 0·04, 0·47). There was no statistical difference in the effect of exposure moderated by the two BHCK waves (see online supplementary material, Supplemental Table 2).
Table 5 Association between exposure to the B’More Healthy Communities for Kids (BHCK) intervention and change in food-related behaviours and fruit and vegetable consumption among low-income African-American adult caregivers: treatment-on-the-treated-effect analysis

se, bootstrapped standard error; CI, bias-corrected confidence interval.
* Statistically significant at P < 0·05.
† Change from pre- to post-intervention evaluation, n 370.
‡ Multiple linear regression models with bootstrap variance (2000 replications) and clustered by BHCK zone, controlled for adult caregiver’s age, sex, income and household size.
§ Mean exposure score = 1·1 (observed range: 0–6·7).
║ Fruit and vegetable intakes were estimated via the Quick Fruit and Vegetable Screener from the National Cancer Institute’s Eating at America’s Table Study. Sample size, n 184.
Table 6 Association between exposure to B’More Healthy Communities for Kids (BHCK) intervention components and change in food-related behaviours and fruit and vegetable consumption among low-income African-American adult caregivers: treatment-on-the-treated-effect analysis
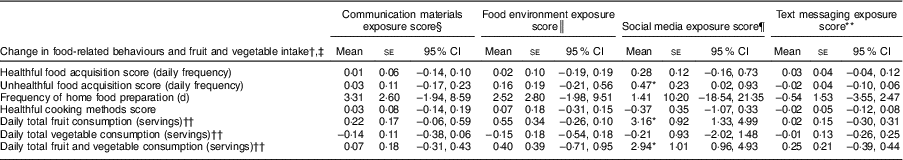
se, bootstrapped standard error; CI, bias-corrected confidence interval.
* Statistically significant behavioural change at P < 0·05; ω 2 estimates of the proportion of variance in unhealthful food acquisition, fruit, and fruit and vegetable intake which is due to variance in the social media exposure score (effect size) = 0·005, 0·04 and 0·02, respectively.
† Change from pre- to post-intervention evaluation, n 370.
‡ Multiple linear regression models with bootstrap variance (2000 replications) and clustered by BHCK zone, controlled for adult caregiver’s age, sex, income and household size.
§ Communication materials exposure score mean = 0·6 (observed range: 0–3·1).
║ Food environment exposure score mean = 0·3 (observed range: 0–3·1).
¶ Social media exposure score mean = 0·2 (observed range: 0–2).
** Text messaging exposure score mean = 1·1 (observed range: 0–3).
†† Fruit and vegetable intakes were estimated via the Quick Fruit and Vegetable Screener from the National Cancer Institute’s Eating at America’s Table Study. Sample size, n 184.
When exploring the exposure score by intervention component, we found a positive change in food-related behaviours among adult caregivers correlated with a greater exposure to the BHCK social media component. For each one-point increase in social media exposure score (e.g. following an additional social media account or seeing an additional post online), there was an increased three servings of daily fruit intake (3·16 (se 0·92); 95 % CI 1·33, 4·99) and daily FV intake (2·94 (se 1·01); 95 % CI 0·96, 4·93). A higher social media exposure score was also associated with increased unhealthful daily food acquisition score (0·47 (se 0·23); 95 % CI 0·02, 0·93). Effect sizes estimated by ω 2 showed a higher proportion of the variance in fruit intake explained by the variance in the social media exposure score (ω 2 = 0·04) than the effect size of unhealthful food acquisition (ω 2 = 0·0005; Table 6 and online supplementary material, Supplemental Table 3). Our sensitivity analysis conducted with multivariate logistic regression models showed that the direction of the association and the estimated effect sizes given by standardized OR were similar to those from the linear regression models (Supplemental Table 3).
Discussion
BHCK tested a 6- to 8-month community-based intervention designed for low-income African-American families to improve access to and consumption of healthful foods. The ATE analysis did not show evidence of significant improvement in food acquisition, preparation and FV consumption among adult caregivers. However, the TTE analysis (‘dose received’) showed a statistically significant increase in daily intake of fruits among participants who reported higher exposure to the intervention. In addition, we used the exposure score to partition out the change in food-related behaviours influenced by different BHCK intervention components and found that the social media component had a positive correlation with improved daily fruit intake, daily FV intake, and unexpectedly with higher frequency of unhealthful food acquisition.
Mixed results have been observed among the few childhood obesity interventions that assessed behavioural change at the caregiver level, mainly due to differences in level of caregiver participation in the intervention, varied quality of outcome measurements and quality of intervention implementation. The Screen-Time Weight-loss Intervention, delivered face-to-face in households by community workers to youths (9–12 years old) and their caregivers, did not find an impact on BMI nor physical activity levels of primary caregivers(Reference Maddison, Marsh and Foley46). Authors attributed the null effects due to low adherence to the fidelity of the initial implementation protocol(Reference Maddison, Marsh and Foley46, Reference Epstein, Roemmich and Robinson47). The Shape Up Somerville community-based participatory research reported decreases in BMI among intervention caregivers; however, height and weight were self-reported, and no behavioural outcome was assessed(Reference Coffield, Nihiser and Sherry21).
The null impact of BHCK on caregivers’ behaviour may be attributed to: (i) the low intervention exposure experienced by caregivers; and/or (ii) the contamination of the intervention activities among comparison caregivers, thus attenuating the average effect towards the null in the ATE analysis(Reference Hull, Buchowski and Canedo48). Other community-based interventions have also attributed limited effects resulting from an ATE approach to the low level of engagement informed by TTE analysis. The Switch what you Do, View, and Chew intervention observed greater change in weekly FV intake among youth who were more involved in the intervention, compared with those who were less involved(Reference Gentile, Welk and Eisenmann49). Another community-based childhood obesity prevention intervention – The Healthy Families Study – found positive health-related outcomes among families with higher exposure to the intervention (TTE) and null results with ATE analyses(Reference Hull, Buchowski and Canedo48). Authors attributed the null effects from the primary impact analysis to low participation in community classes(Reference Hull, Buchowski and Canedo48).
In our study, low exposure might be explained by the fact that the BHCK study sample was not required to attend community-based activities (i.e. taste tests, point-of-purchase promotions and nutrition education sessions in corner stores, carry-out restaurants and recreation centres). Furthermore, we did not expect the intervention study sample to receive the same dose of the intervention across all components. Conversely, only adult caregivers in the intervention arm were asked to join the text messaging programme at study enrolment and were given directions on how to follow BHCK social media platforms. However, both social media platforms were public, meaning that any individual could follow the social media accounts (Facebook and Instagram), which increased the likelihood of exposure contamination among participants in the control group, and that may have attenuated differences between study arms. On the other hand, the usage of a tailored approach may help explain behaviour changes observed among only those with higher levels of exposure to the social media component. The social media and text messaging component employed goal-setting bidirectional communication strategies. Social media pages were public accounts with daily posts that mirrored the content of text messaging and other BHCK components, and participants were encouraged to share online achievement, barriers, tips and resources. The higher reach and intensity of the social media component may help explain the positive correlation with food-related behaviours, compared with the other intervention components.
The increase in fruit intake was driven by a one-point increase in social media exposure, which corresponds to following at least one of the study social media accounts or seeing four or more posts. Similar to our findings, The Food Hero study – a social media campaign targeted at SNAP-eligible families with children – found increased positive beliefs about FV among participants(Reference Tobey and Manore50). Although previous studies have tested social media approaches for behavioural interventions(Reference O’Brien and Palfai51–Reference George, Roberts and Beasley54), to our knowledge, BHCK was the first study to combine these strategies into a multilevel multicomponent community-based nutrition intervention. The use of social media to provide a platform for actionable information and social support for families with children has been recommended in the obesity prevention literature(Reference George, Roberts and Beasley54–Reference Medairos, Kang and Aboubakare56) and is being further tested in ongoing community-based trials(Reference Gittelsohn, Jock and Redmond57, Reference Tomayko, Prince and Cronin58).
Given the low consumption of FV among the US population(59), especially among low-income African-American individuals(Reference Robinson60, Reference Di Noia, Monica and Cullen61), it is necessary to explore innovative strategies to promote healthier dietary intake. Although we found a positive association between self-reported exposure to the BHCK social media component and FV, the main increase in intake was in fruits, and not vegetables. Fruits are sweeter, often do not require any preparation (consumed raw), and generally are consumed and accepted as a snack, drink and dessert(Reference Burg, de Vet and de Nooijer62), whereas vegetables often require cooking and are more typically consumed as part of meals(Reference Anderson, Cox and McKellar63). Future studies should consider the impact of the intervention on fruits and vegetables as separate and different food types(Reference Appleton, Hemingway and Saulais64, Reference Glasson, Chapman and James65).
Unexpectedly, we found that an increased frequency of unhealthful food acquisition was associated with greater exposure to the BHCK social media component. One potential reason for this may be that adults exposed to BHCK social media may have also been exposed to online advertising for energy-dense, nutrient-poor foods and mobile marketing food campaigns(Reference Zimmerman66, Reference Montgomery, Chester and Grier67). Prior studies have demonstrated a negative effect of online food advertisements on youths’ consumption of healthful foods(Reference Harris, Speers and Schwartz68, Reference Boyland, Nolan and Kelly69), and similar trends were found for adult caregivers(Reference Dixon, Scully and Wakefield70, Reference Pettigrew, Tarabashkina and Roberts71). More research needs to be conducted to examine the relationship between public health social media campaigns and advertising exposure.
Limitations of the present study should be noted. The survey was administered to self-identified caregivers, under the assumption that they acquire most of the food and cook for their family members. However, some caregivers may not be the primary food purchasers in their households. Also, our measure of frequency of food purchased did not take into consideration the quality or quantity of the acquired food/beverage. Future child-focused interventions should conduct more comprehensive food and nutrient assessments of adult caregivers. The loss of observations over the course of the study is also a limitation, despite our efforts to avoid dropouts during the course of the study (e.g. eligibility criteria included intent to stay within the study areas over the next two years; multiple attempts were made to contact the families over the phone – and if not possible to reach over the phone, household visits were done to conduct follow-up surveys). Thus, to address potential selection bias, inverse probability weighting was employed in the analysis to correct for the effects of missing data(Reference Fitzmaurice, Laird and Ware37). Another study limitation might be the risk of social desirability bias by treatment assignment, reflected in the self-reported intervention exposure questionnaire. However, our questionnaire included red herring questions to improve validity, and data collectors were masked to intervention treatment assignment. We were not able to directly assess individuals’ social media participation, as individuals often display nicknames instead of names used on their profile pages, which precluded our efforts to cross-check the self-reported information. In addition, although we utilized a computer software to manage our text messaging programme, some people may have not received the texts (because of low credit balance on their phone) or may have not read the text sent.
BHCK was an intervention that sought to modify the out-of-school community food environment and engage families through social media, but it did not implement a component to improve the household food environment. Therefore, future studies aiming at preventing childhood obesity among underserved communities should consider intervening in both community and household food environments. Lastly, although multilevel, multicomponent interventions have broader reach than single-level approaches, they have the additional challenge of achieving low exposure(Reference Heerman, JaKa and Berge72). Hence, conducting a detailed process evaluation during implementation is essential for understanding to what extent the target population is receiving the programme.
Conclusions
The BHCK intervention is one of the few child-focused obesity prevention interventions to measure treatment effects at the caregiver level in terms of food acquisition, preparation and FV consumption, and the first study to attempt to evaluate a dose–response relationship in terms of exposure level to the different intervention components. Although our ATE analysis including all trial participants demonstrated no effect of BHCK on food-related behaviours, we were able to demonstrate that a higher level of exposure to the BHCK intervention was associated with improvements in daily fruit intake among adult caregivers, particularly among those with higher exposures to the social media component. Our study highlights the importance of optimal dose and intensity of community-based intervention activities to achieve intended behavioural changes, and the possibility of intervention contamination between intervention and comparison participants in community-based behaviour interventions. Future multilevel multicomponent community-based interventions should engage caregivers more in the intervention, enrol larger samples, as well as assess engagement and exposure to intervention activities during the trial to enhance likelihood of intervention effectiveness. Social media (Facebook, Instagram) may be a promising tool to improve reach and engage caregiver participants in multilevel childhood obesity interventions.
Acknowledgements
Acknowledgements: The authors would like to thank the families interviewed and the following students, staff and volunteers who assisted in the BHCK data collection, including: Anna Kharmats, Cara F. Ruggiero, Kelleigh Eastman, Melissa Sattler, JaWanna Henry, Jenny Brooks, Selma Pourzal, Teresa Schwendler, Gabriela Vedovato, Sarah Rastatter, Kate Perepezko, Lisa Poirier, Thomas Eckmann, Maria Jose Mejia, Yeeli Mui, Priscila Sato, Bengucan Gunen, Ivory Loh, Courtney Turner, Whitney Kim, Shruti Patel, Ellen Sheehan, Ryan Wooley, Gabrielle Headrick, Donna Dennis, Elizabeth Chen, Kiara James, Latecia Williams, Harmony Farner, Rena Hamzey, Nandita Krishnan and Alexandra Ross. Financial support: Research reported in this publication was supported by the Global Obesity Prevention Center (GOPC) at Johns Hopkins, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the Office of the Director, National Institutes of Health (OD; award number U54HD070725). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the Office of Behavioral and Social Sciences Research. This work was also funded by the Centers for Disease Control and Prevention (grant number 1U48DP000040, SIP 14-027). A.C.B.T. is supported by a doctoral fellowship from the Brazilian National Council for Scientific and Technological Development (CNPq; grant number GDE: 249316/2013-7). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: A.C.B.T. formulated the research questions. J.G. designed the intervention. A.C.B.T. and E.A.S. implemented and evaluated the research study. A.C.B.T. conducted the data analysis, wrote the first draft of the paper, and had the primary responsibility for the final content. P.J.S., E.A.S., K.P.P. and J.G. contributed to the interpretation of the results and revised the article critically. All authors edited drafts of the manuscript, read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board (IRB #00004203). Written informed consent was obtained from all subjects.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018003038









